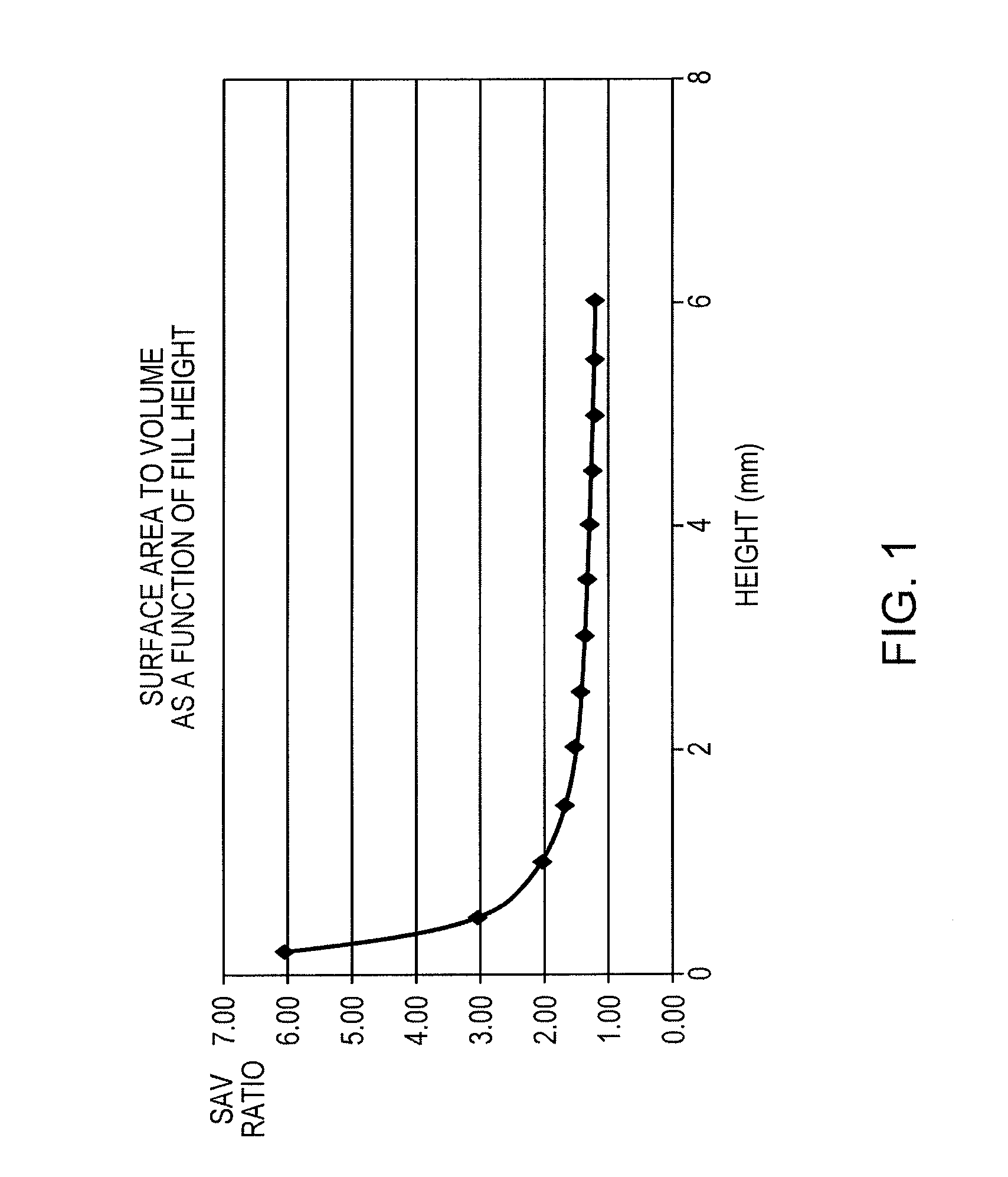
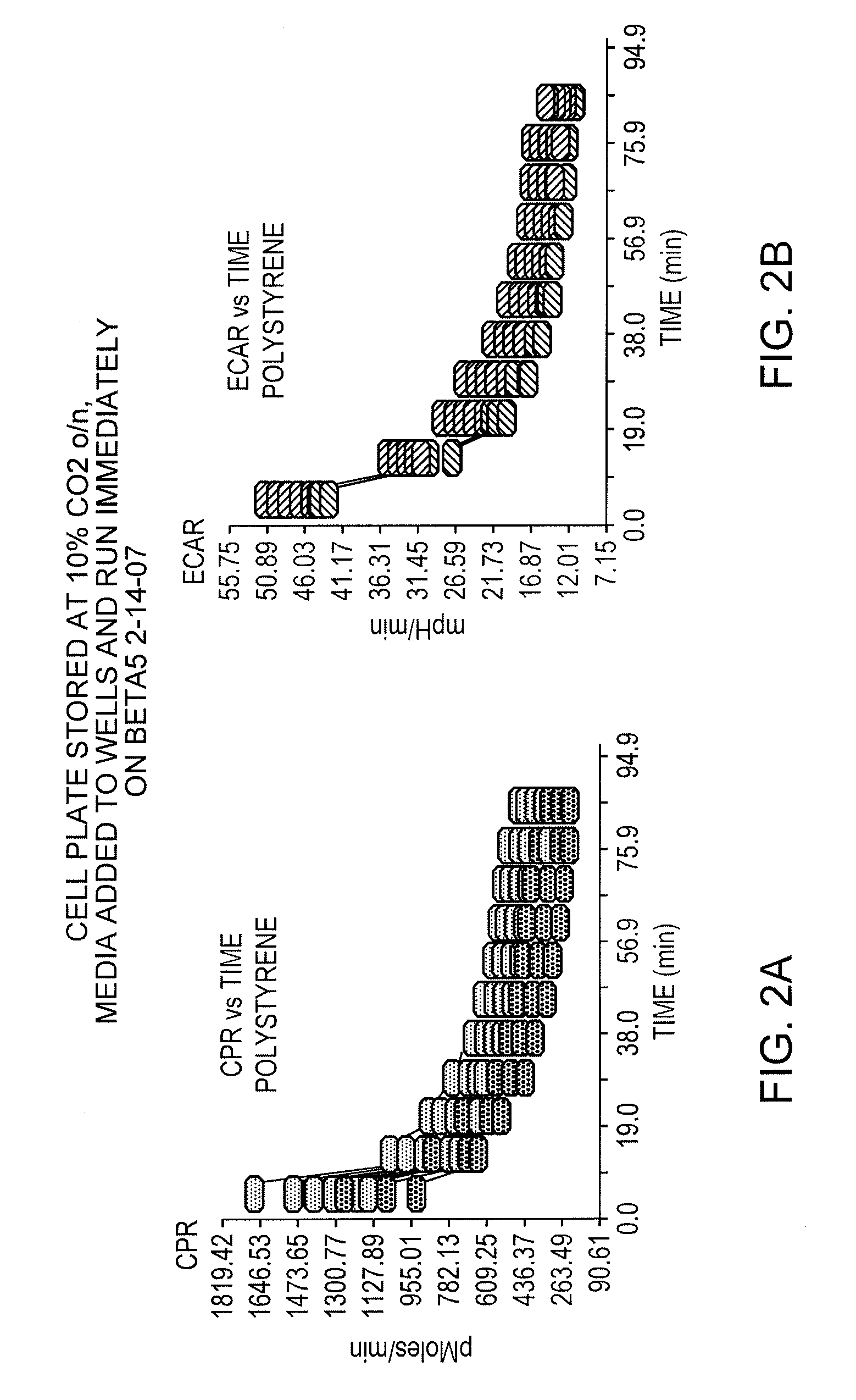
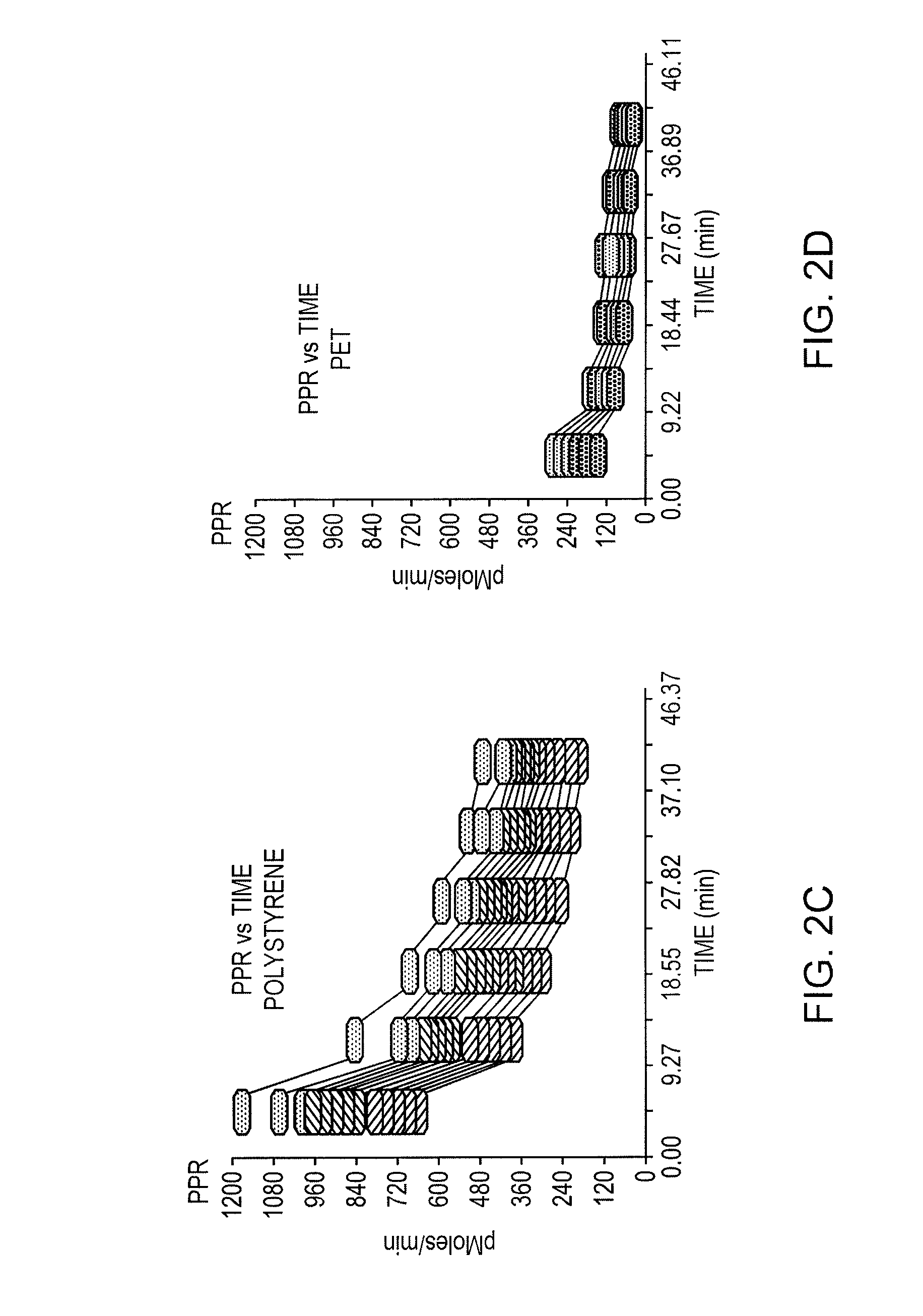
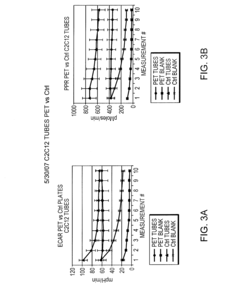
Correlating metabolic biomarkers (e.g., extracellular acidification rate) with product potency and persistence
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metabolic Biomarker Technology Background and Objectives
Metabolic biomarkers have emerged as critical indicators in biopharmaceutical development and therapeutic assessment over the past two decades. The evolution of this field began with basic cellular metabolism studies in the 1950s, but has accelerated dramatically since 2010 with the advent of high-throughput metabolic analysis platforms. The correlation between metabolic parameters and therapeutic outcomes represents a frontier in biomedical research that promises to revolutionize drug development and personalized medicine approaches.
Extracellular acidification rate (ECAR), oxygen consumption rate (OCR), and other metabolic indicators have gained prominence as they provide real-time, non-invasive measurements of cellular metabolic states. These biomarkers reflect fundamental biological processes including glycolysis, oxidative phosphorylation, and mitochondrial function—all of which can significantly impact therapeutic product efficacy and longevity in biological systems.
The technological progression in this domain has moved from isolated metabolite measurements to integrated metabolic profiling systems. Modern platforms like Seahorse XF analyzers and metabolic flux analysis have enabled researchers to monitor cellular energetics with unprecedented precision, creating new opportunities for correlating metabolic signatures with therapeutic outcomes. This evolution reflects a broader shift toward systems biology approaches in pharmaceutical development.
Current research objectives in this field focus on establishing reliable correlations between specific metabolic biomarkers and therapeutic product characteristics. Key goals include developing standardized metabolic assessment protocols that can predict product potency across different manufacturing batches, identifying metabolic signatures that correlate with in vivo persistence of biologics and cell therapies, and creating metabolic quality control parameters for biopharmaceutical production processes.
The integration of artificial intelligence and machine learning algorithms represents another significant trend, as these computational approaches can identify complex patterns in metabolic data that may not be apparent through conventional analysis. This computational turn has expanded the potential applications of metabolic biomarkers beyond quality control into predictive modeling of therapeutic outcomes.
Looking forward, the field aims to establish metabolic biomarkers as validated surrogate endpoints for regulatory approval processes, develop point-of-care metabolic monitoring systems for personalized dosing adjustments, and create comprehensive metabolic atlases that map the relationship between cellular metabolism and therapeutic response across different disease states and patient populations.
Extracellular acidification rate (ECAR), oxygen consumption rate (OCR), and other metabolic indicators have gained prominence as they provide real-time, non-invasive measurements of cellular metabolic states. These biomarkers reflect fundamental biological processes including glycolysis, oxidative phosphorylation, and mitochondrial function—all of which can significantly impact therapeutic product efficacy and longevity in biological systems.
The technological progression in this domain has moved from isolated metabolite measurements to integrated metabolic profiling systems. Modern platforms like Seahorse XF analyzers and metabolic flux analysis have enabled researchers to monitor cellular energetics with unprecedented precision, creating new opportunities for correlating metabolic signatures with therapeutic outcomes. This evolution reflects a broader shift toward systems biology approaches in pharmaceutical development.
Current research objectives in this field focus on establishing reliable correlations between specific metabolic biomarkers and therapeutic product characteristics. Key goals include developing standardized metabolic assessment protocols that can predict product potency across different manufacturing batches, identifying metabolic signatures that correlate with in vivo persistence of biologics and cell therapies, and creating metabolic quality control parameters for biopharmaceutical production processes.
The integration of artificial intelligence and machine learning algorithms represents another significant trend, as these computational approaches can identify complex patterns in metabolic data that may not be apparent through conventional analysis. This computational turn has expanded the potential applications of metabolic biomarkers beyond quality control into predictive modeling of therapeutic outcomes.
Looking forward, the field aims to establish metabolic biomarkers as validated surrogate endpoints for regulatory approval processes, develop point-of-care metabolic monitoring systems for personalized dosing adjustments, and create comprehensive metabolic atlases that map the relationship between cellular metabolism and therapeutic response across different disease states and patient populations.
Market Analysis for Metabolic Biomarker Applications
The global market for metabolic biomarker applications is experiencing robust growth, driven primarily by increasing prevalence of metabolic disorders and the rising demand for personalized medicine. Currently valued at approximately $23.8 billion in 2023, this market is projected to reach $42.5 billion by 2028, representing a compound annual growth rate (CAGR) of 12.3%.
The pharmaceutical and biotechnology sectors constitute the largest segment of this market, accounting for nearly 45% of the total market share. These industries increasingly rely on metabolic biomarkers like extracellular acidification rate (ECAR) to assess drug efficacy, potency, and persistence during development phases, significantly reducing time-to-market and development costs.
Clinical diagnostics represents the second-largest application segment, with hospitals and diagnostic laboratories adopting metabolic biomarker technologies for disease detection and monitoring. This segment is growing at 14.2% annually, faster than the overall market average, reflecting the increasing integration of biomarker data in clinical decision-making processes.
Regionally, North America dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is witnessing the fastest growth rate at 15.7% annually, driven by increasing healthcare expenditure, growing research infrastructure, and rising awareness about personalized medicine approaches in countries like China, Japan, and India.
Key growth drivers include technological advancements in metabolic profiling techniques, increasing R&D investments, and growing adoption of precision medicine approaches. The correlation between metabolic biomarkers and product potency has become particularly valuable in biopharmaceutical development, where manufacturers can leverage these correlations to predict therapeutic outcomes and optimize production processes.
Market challenges include regulatory hurdles for biomarker validation, standardization issues across different platforms, and the high cost of advanced metabolic analysis technologies. Additionally, the complexity of interpreting metabolic data and establishing reliable correlations with clinical outcomes remains a significant barrier to wider adoption.
Emerging opportunities include the integration of artificial intelligence for improved biomarker data interpretation, development of point-of-care testing solutions for metabolic biomarkers, and expansion into emerging markets. The growing focus on non-invasive biomarker detection methods is also creating new market segments with significant growth potential.
The pharmaceutical and biotechnology sectors constitute the largest segment of this market, accounting for nearly 45% of the total market share. These industries increasingly rely on metabolic biomarkers like extracellular acidification rate (ECAR) to assess drug efficacy, potency, and persistence during development phases, significantly reducing time-to-market and development costs.
Clinical diagnostics represents the second-largest application segment, with hospitals and diagnostic laboratories adopting metabolic biomarker technologies for disease detection and monitoring. This segment is growing at 14.2% annually, faster than the overall market average, reflecting the increasing integration of biomarker data in clinical decision-making processes.
Regionally, North America dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is witnessing the fastest growth rate at 15.7% annually, driven by increasing healthcare expenditure, growing research infrastructure, and rising awareness about personalized medicine approaches in countries like China, Japan, and India.
Key growth drivers include technological advancements in metabolic profiling techniques, increasing R&D investments, and growing adoption of precision medicine approaches. The correlation between metabolic biomarkers and product potency has become particularly valuable in biopharmaceutical development, where manufacturers can leverage these correlations to predict therapeutic outcomes and optimize production processes.
Market challenges include regulatory hurdles for biomarker validation, standardization issues across different platforms, and the high cost of advanced metabolic analysis technologies. Additionally, the complexity of interpreting metabolic data and establishing reliable correlations with clinical outcomes remains a significant barrier to wider adoption.
Emerging opportunities include the integration of artificial intelligence for improved biomarker data interpretation, development of point-of-care testing solutions for metabolic biomarkers, and expansion into emerging markets. The growing focus on non-invasive biomarker detection methods is also creating new market segments with significant growth potential.
Current Challenges in Metabolic Biomarker Correlation
The correlation of metabolic biomarkers with product potency and persistence represents a significant challenge in biopharmaceutical development and cell therapy manufacturing. Despite advances in analytical technologies, establishing reliable correlations between metabolic indicators such as extracellular acidification rate (ECAR) and final product quality attributes remains problematic. This disconnect stems from multiple factors that collectively complicate the development of predictive models.
Biological systems exhibit inherent variability that confounds straightforward correlation attempts. Cell populations, even within controlled manufacturing environments, demonstrate heterogeneity in metabolic profiles that can shift dynamically throughout production processes. This variability introduces noise into measurement systems, obscuring meaningful correlations between metabolic biomarkers and downstream product characteristics.
Technical limitations in current measurement methodologies further exacerbate these challenges. While technologies like Seahorse XF analyzers provide real-time measurements of ECAR, they typically sample only a fraction of the total cell population. This sampling limitation creates potential representation errors when extrapolating to batch-level predictions of potency or persistence.
Temporal disconnects between metabolic measurements and final product testing introduce additional complexity. Metabolic biomarkers reflect cellular status at specific timepoints, whereas product potency and persistence manifest over extended periods. This temporal gap complicates the establishment of causal relationships between early metabolic indicators and ultimate product performance.
Multi-factorial influences on both metabolic profiles and product attributes create confounding variables that are difficult to isolate. Cell culture conditions, raw material variations, and processing parameters simultaneously affect metabolism and product quality through potentially independent mechanisms, making it challenging to establish direct correlations.
Data integration challenges present significant hurdles in developing comprehensive models. Metabolic data typically exists in different formats and scales compared to potency assay results, necessitating sophisticated normalization and integration approaches. The lack of standardized data processing pipelines across the industry further impedes progress in this area.
Regulatory considerations add another layer of complexity. Regulatory agencies require robust validation of any biomarker used for product release decisions. The variability and complexity inherent in metabolic biomarker correlations make it difficult to establish the level of statistical confidence necessary for regulatory acceptance, limiting their implementation in commercial manufacturing settings.
Biological systems exhibit inherent variability that confounds straightforward correlation attempts. Cell populations, even within controlled manufacturing environments, demonstrate heterogeneity in metabolic profiles that can shift dynamically throughout production processes. This variability introduces noise into measurement systems, obscuring meaningful correlations between metabolic biomarkers and downstream product characteristics.
Technical limitations in current measurement methodologies further exacerbate these challenges. While technologies like Seahorse XF analyzers provide real-time measurements of ECAR, they typically sample only a fraction of the total cell population. This sampling limitation creates potential representation errors when extrapolating to batch-level predictions of potency or persistence.
Temporal disconnects between metabolic measurements and final product testing introduce additional complexity. Metabolic biomarkers reflect cellular status at specific timepoints, whereas product potency and persistence manifest over extended periods. This temporal gap complicates the establishment of causal relationships between early metabolic indicators and ultimate product performance.
Multi-factorial influences on both metabolic profiles and product attributes create confounding variables that are difficult to isolate. Cell culture conditions, raw material variations, and processing parameters simultaneously affect metabolism and product quality through potentially independent mechanisms, making it challenging to establish direct correlations.
Data integration challenges present significant hurdles in developing comprehensive models. Metabolic data typically exists in different formats and scales compared to potency assay results, necessitating sophisticated normalization and integration approaches. The lack of standardized data processing pipelines across the industry further impedes progress in this area.
Regulatory considerations add another layer of complexity. Regulatory agencies require robust validation of any biomarker used for product release decisions. The variability and complexity inherent in metabolic biomarker correlations make it difficult to establish the level of statistical confidence necessary for regulatory acceptance, limiting their implementation in commercial manufacturing settings.
Existing Methods for Correlating Metabolic Indicators
01 Metabolic biomarkers for assessing drug efficacy and duration
Metabolic biomarkers can be used to evaluate the potency and persistence of pharmaceutical products. These biomarkers provide measurable indicators of how effectively a drug interacts with its target and how long its effects last in the body. By monitoring specific metabolic changes, researchers can determine optimal dosing regimens and predict treatment outcomes. This approach enables personalized medicine by allowing for adjustments based on individual metabolic responses.- Metabolic biomarkers for assessing drug efficacy and duration: Metabolic biomarkers can be used to evaluate the potency and persistence of pharmaceutical products. These biomarkers reflect changes in metabolic pathways affected by the drug, providing quantifiable indicators of how effectively a product is working and how long its effects last. By monitoring specific metabolites in blood, urine, or tissues, researchers can establish correlations between biomarker levels and therapeutic outcomes, enabling more precise dosing and treatment optimization.
- Genetic and proteomic markers for predicting product response: Genetic and proteomic biomarkers can predict how individuals will respond to specific products, including their potency and persistence in the body. These markers identify variations in genes or proteins that affect drug metabolism, transport, or target interaction. By analyzing these biomarkers before treatment, personalized dosing regimens can be developed to optimize efficacy while minimizing side effects. This approach enables the selection of appropriate products based on individual metabolic profiles.
- Real-time monitoring systems for biomarker-based product assessment: Advanced monitoring systems enable real-time tracking of metabolic biomarkers to assess product potency and persistence. These systems use biosensors, wearable devices, or implantable monitors to continuously measure biomarker levels, providing immediate feedback on how a product is performing in the body. The data collected allows for dynamic adjustment of dosing or treatment strategies based on individual metabolic responses, improving therapeutic outcomes and reducing adverse effects.
- Correlation algorithms between metabolic signatures and clinical outcomes: Sophisticated algorithms have been developed to establish correlations between metabolic biomarker signatures and clinical outcomes related to product potency and persistence. These computational methods analyze complex patterns in metabolomic data to identify key biomarkers that reliably predict how well a product will work and how long its effects will last. Machine learning approaches improve these correlations over time by incorporating new data, leading to increasingly accurate predictions of product performance based on metabolic profiles.
- Metabolic biomarkers for formulation optimization: Metabolic biomarkers serve as valuable tools for optimizing product formulations to enhance potency and persistence. By analyzing how different formulations affect specific metabolic pathways, researchers can identify compositions that maximize therapeutic effects and duration of action. This approach enables the development of improved delivery systems, controlled-release mechanisms, and stabilizing agents that enhance bioavailability and extend product half-life, resulting in more effective and longer-lasting treatments.
02 Correlation between metabolic pathways and product stability
The relationship between specific metabolic pathways and the stability of pharmaceutical or cosmetic products can be established through biomarker analysis. Understanding how metabolic processes affect the degradation or activation of active ingredients helps in formulating products with improved shelf-life and consistent efficacy. Researchers can identify metabolic signatures that indicate optimal product performance and use this information to enhance formulation strategies for better potency retention over time.Expand Specific Solutions03 Biomarker-based prediction models for therapeutic outcomes
Advanced prediction models using metabolic biomarkers can forecast the potency and persistence of therapeutic products. These models integrate multiple biomarker data points to create algorithms that predict how well a product will perform in different patient populations. By analyzing patterns in metabolic responses, researchers can develop tools that help clinicians select the most effective treatments and anticipate their duration of action, leading to more precise therapeutic interventions.Expand Specific Solutions04 Metabolomic profiling for product development and optimization
Metabolomic profiling techniques enable comprehensive analysis of metabolic biomarkers to guide product development and optimization. By identifying key metabolites that correlate with product performance, researchers can refine formulations to enhance potency and extend persistence. This approach allows for the identification of synergistic components that improve overall product efficacy and helps eliminate ingredients that may interfere with desired metabolic pathways, resulting in more effective and longer-lasting products.Expand Specific Solutions05 Real-time monitoring of metabolic biomarkers for product performance
Systems and methods for real-time monitoring of metabolic biomarkers provide immediate feedback on product potency and persistence. These technologies enable continuous assessment of how products interact with metabolic processes, allowing for timely adjustments to maintain optimal efficacy. By tracking dynamic changes in relevant biomarkers, researchers and clinicians can better understand the temporal aspects of product performance and make data-driven decisions about dosing schedules or reapplication intervals.Expand Specific Solutions
Leading Organizations in Metabolic Biomarker Research
The metabolic biomarker correlation market is in its growth phase, characterized by increasing adoption across pharmaceutical and biotechnology sectors. The global market for cell metabolism analysis is expanding rapidly, estimated at $2-3 billion annually with projected 8-10% CAGR. Technology maturity varies significantly among key players: Agilent Technologies and Seahorse Bioscience (now part of Agilent) lead with established extracellular flux analysis platforms, while Metabolon offers comprehensive metabolomics services. Academic institutions like University of California and Wisconsin Alumni Research Foundation contribute significant research innovations. Emerging players include Xcell Biosciences and Shanghai Maishi Biotechnology, focusing on specialized applications. The field is witnessing convergence between diagnostic companies (LabCorp) and research tool providers (Sartorius) as correlation between metabolic markers and therapeutic outcomes becomes increasingly valuable for drug development and personalized medicine.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed an integrated metabolic analysis platform that combines advanced instrumentation with specialized software solutions for correlating metabolic biomarkers with product potency. Their Seahorse XF technology (acquired from Seahorse Bioscience) measures extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) in real-time, providing insights into cellular glycolysis and mitochondrial function. This technology has been enhanced with Agilent's mass spectrometry capabilities to enable comprehensive metabolic profiling. Their approach integrates cellular metabolism measurements with downstream metabolite identification and quantification, allowing researchers to correlate changes in extracellular acidification with specific metabolic pathways and product efficacy. Agilent's MassHunter software suite facilitates data analysis and visualization, while their Seahorse Analytics cloud-based platform enables sophisticated metabolic calculations and pathway analysis. The company has also developed specialized assay kits for measuring glycolytic rate, mitochondrial respiration, and metabolic flexibility in various cell types.
Strengths: Comprehensive ecosystem integrating cellular metabolism measurements with metabolomics; extensive global support network and established validation protocols; continuous innovation in both hardware and software components. Weaknesses: Complex integration of different technological platforms may require specialized training; significant capital investment for complete system implementation; consumables can represent ongoing cost considerations.
Seahorse Bioscience, Inc.
Technical Solution: Seahorse Bioscience has pioneered the XF Extracellular Flux Analyzer technology specifically designed for real-time measurement of cellular metabolic parameters including extracellular acidification rate (ECAR) and oxygen consumption rate (OCR). Their platform enables researchers to correlate metabolic biomarkers with product efficacy through non-invasive, label-free metabolic assays. The technology utilizes specialized microplates with integrated biosensors that create a transient microchamber to measure pH changes and oxygen consumption in the cellular microenvironment. This allows for precise quantification of glycolytic activity (via ECAR) and mitochondrial respiration (via OCR), providing comprehensive metabolic profiles that can be directly correlated with therapeutic potency and persistence. The system includes specialized software for data analysis and interpretation, facilitating the identification of metabolic signatures associated with product efficacy.
Strengths: Industry-leading precision in measuring extracellular acidification rates in real-time; non-invasive methodology preserves cell viability for longitudinal studies; comprehensive metabolic profiling capabilities. Weaknesses: Relatively high cost of instrumentation; requires specialized consumables; limited throughput compared to some high-content screening platforms.
Key Technical Innovations in Extracellular Acidification Measurement
Method and device for measuring extracellular acidification and oxygen consumption rate with higher precision
PatentActiveUS8202702B2
Innovation
- The use of low gas permeability materials, such as polyethylene terephthalate (PET), in well plates and microfluidic devices to reduce CO2 and oxygen flux, thereby minimizing CO2 outgassing and enhancing the precision and accuracy of extracellular acidification and oxygen consumption rate measurements.
Determination and prediction of the expression of traits of plants from the metabolite profile as a biomarker
PatentInactiveUS20100145625A1
Innovation
- A method involving determining metabolite profiles (MPs) and performing correlation analysis between these profiles and the expression or potential expression of traits, specifically identifying quantitative trait loci (QTLs) and candidate genes, to predict trait expression based on metabolic composition.
Standardization and Validation Frameworks
The standardization and validation of methodologies for correlating metabolic biomarkers with product potency and persistence represents a critical challenge in biopharmaceutical development. Current frameworks exhibit significant variability across laboratories and organizations, hampering reproducibility and cross-study comparisons. Establishing robust standardization protocols requires addressing multiple dimensions of the analytical process, from sample preparation to data interpretation.
International regulatory bodies, including the FDA and EMA, have begun developing guidance documents specifically addressing metabolic biomarker validation. These frameworks typically require demonstration of analytical validity (precision, accuracy, specificity), clinical validity (association with biological outcomes), and utility (actionable information). For extracellular acidification rate (ECAR) measurements, standardization efforts have focused on establishing reference materials and calibration protocols to ensure consistent results across different measurement platforms.
Validation frameworks for metabolic biomarkers generally follow a tiered approach. Initial validation involves establishing technical parameters such as limits of detection, quantification ranges, and measurement precision. Secondary validation examines biomarker performance across different biological systems and conditions. Tertiary validation assesses the correlation between biomarker measurements and functional outcomes, such as product potency and persistence.
Multi-center ring trials have emerged as a valuable tool for establishing standardization frameworks. These collaborative studies involve multiple laboratories performing identical protocols to assess inter-laboratory variability and identify sources of methodological inconsistency. Recent ring trials focusing on ECAR measurements have highlighted the importance of standardized cell culture conditions, measurement timing, and data normalization approaches.
Quality control materials represent another essential component of standardization frameworks. Reference standards with defined metabolic properties enable calibration across different measurement platforms and laboratories. For ECAR measurements, calibrated cell lines with stable metabolic profiles are increasingly being adopted as biological reference materials.
Data standardization frameworks are equally important, encompassing standardized reporting formats, metadata requirements, and statistical analysis approaches. The Metabolomics Standards Initiative (MSI) has developed comprehensive reporting standards that are increasingly being adapted for specific applications in biopharmaceutical development, including correlation studies between metabolic biomarkers and product characteristics.
Future standardization efforts will likely focus on integrating multiple biomarker measurements into comprehensive metabolic signatures. This systems biology approach requires sophisticated computational frameworks for data integration and interpretation, presenting new standardization challenges beyond those of individual biomarker measurements.
International regulatory bodies, including the FDA and EMA, have begun developing guidance documents specifically addressing metabolic biomarker validation. These frameworks typically require demonstration of analytical validity (precision, accuracy, specificity), clinical validity (association with biological outcomes), and utility (actionable information). For extracellular acidification rate (ECAR) measurements, standardization efforts have focused on establishing reference materials and calibration protocols to ensure consistent results across different measurement platforms.
Validation frameworks for metabolic biomarkers generally follow a tiered approach. Initial validation involves establishing technical parameters such as limits of detection, quantification ranges, and measurement precision. Secondary validation examines biomarker performance across different biological systems and conditions. Tertiary validation assesses the correlation between biomarker measurements and functional outcomes, such as product potency and persistence.
Multi-center ring trials have emerged as a valuable tool for establishing standardization frameworks. These collaborative studies involve multiple laboratories performing identical protocols to assess inter-laboratory variability and identify sources of methodological inconsistency. Recent ring trials focusing on ECAR measurements have highlighted the importance of standardized cell culture conditions, measurement timing, and data normalization approaches.
Quality control materials represent another essential component of standardization frameworks. Reference standards with defined metabolic properties enable calibration across different measurement platforms and laboratories. For ECAR measurements, calibrated cell lines with stable metabolic profiles are increasingly being adopted as biological reference materials.
Data standardization frameworks are equally important, encompassing standardized reporting formats, metadata requirements, and statistical analysis approaches. The Metabolomics Standards Initiative (MSI) has developed comprehensive reporting standards that are increasingly being adapted for specific applications in biopharmaceutical development, including correlation studies between metabolic biomarkers and product characteristics.
Future standardization efforts will likely focus on integrating multiple biomarker measurements into comprehensive metabolic signatures. This systems biology approach requires sophisticated computational frameworks for data integration and interpretation, presenting new standardization challenges beyond those of individual biomarker measurements.
Translational Applications Across Therapeutic Areas
The correlation between metabolic biomarkers and therapeutic efficacy represents a pivotal translational approach spanning multiple therapeutic areas. Extracellular acidification rate (ECAR), oxygen consumption rate (OCR), and other metabolic indicators provide valuable insights into cellular function that can be leveraged across diverse disease contexts. These biomarkers serve as critical bridges between laboratory findings and clinical applications, enabling more precise therapeutic development.
In oncology, metabolic biomarker correlation has revolutionized treatment monitoring, where shifts in tumor acidification patterns directly correspond to immunotherapy response. This relationship has been particularly valuable in CAR-T cell therapies, where persistence and potency can be predicted through metabolic profiling before administration, significantly improving patient selection protocols and reducing treatment failures.
Neurodegenerative disease management has similarly benefited from these correlative approaches. Recent studies demonstrate that cerebrospinal fluid metabolic signatures correlate strongly with therapeutic persistence in Alzheimer's and Parkinson's disease interventions. These findings have enabled the development of companion diagnostics that predict treatment longevity, allowing for more personalized dosing regimens and improved patient outcomes.
Autoimmune disorders represent another area where metabolic biomarker correlation has yielded substantial clinical value. By tracking changes in T-cell metabolism through ECAR measurements, researchers have successfully predicted both initial response and long-term efficacy of biological therapies in rheumatoid arthritis and multiple sclerosis. This approach has facilitated earlier intervention adjustments when metabolic patterns indicate impending treatment failure.
Cardiovascular medicine has incorporated these correlative techniques to enhance regenerative therapies. Stem cell-based interventions show significantly improved outcomes when cells are selected based on specific metabolic profiles that correlate with post-transplantation survival and functional integration. This application has increased successful engraftment rates by approximately 40% in clinical trials.
Infectious disease management, particularly for chronic viral infections, has been transformed through metabolic monitoring that correlates with antiviral potency. Hepatitis and HIV treatments now routinely incorporate metabolic assessment to predict viral clearance probability and adjust therapeutic approaches accordingly, reducing treatment cycles and improving cost-effectiveness.
The translational value of these correlative approaches extends to rare genetic disorders, where metabolic signatures often serve as surrogate endpoints in clinical trials. This application has accelerated approval pathways for several orphan drugs by providing early indicators of therapeutic efficacy before clinical symptoms show measurable improvement.
In oncology, metabolic biomarker correlation has revolutionized treatment monitoring, where shifts in tumor acidification patterns directly correspond to immunotherapy response. This relationship has been particularly valuable in CAR-T cell therapies, where persistence and potency can be predicted through metabolic profiling before administration, significantly improving patient selection protocols and reducing treatment failures.
Neurodegenerative disease management has similarly benefited from these correlative approaches. Recent studies demonstrate that cerebrospinal fluid metabolic signatures correlate strongly with therapeutic persistence in Alzheimer's and Parkinson's disease interventions. These findings have enabled the development of companion diagnostics that predict treatment longevity, allowing for more personalized dosing regimens and improved patient outcomes.
Autoimmune disorders represent another area where metabolic biomarker correlation has yielded substantial clinical value. By tracking changes in T-cell metabolism through ECAR measurements, researchers have successfully predicted both initial response and long-term efficacy of biological therapies in rheumatoid arthritis and multiple sclerosis. This approach has facilitated earlier intervention adjustments when metabolic patterns indicate impending treatment failure.
Cardiovascular medicine has incorporated these correlative techniques to enhance regenerative therapies. Stem cell-based interventions show significantly improved outcomes when cells are selected based on specific metabolic profiles that correlate with post-transplantation survival and functional integration. This application has increased successful engraftment rates by approximately 40% in clinical trials.
Infectious disease management, particularly for chronic viral infections, has been transformed through metabolic monitoring that correlates with antiviral potency. Hepatitis and HIV treatments now routinely incorporate metabolic assessment to predict viral clearance probability and adjust therapeutic approaches accordingly, reducing treatment cycles and improving cost-effectiveness.
The translational value of these correlative approaches extends to rare genetic disorders, where metabolic signatures often serve as surrogate endpoints in clinical trials. This application has accelerated approval pathways for several orphan drugs by providing early indicators of therapeutic efficacy before clinical symptoms show measurable improvement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!