Manufacturing considerations for self-amplifying mRNA therapeutics: potency vs safety tradeoffs
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
saRNA Therapeutics Background and Development Goals
Self-amplifying mRNA (saRNA) therapeutics represent a significant evolution in the field of nucleic acid-based medicine, building upon the foundation established by conventional mRNA technologies. Unlike standard mRNA which encodes only the target protein, saRNA contains additional genetic elements that enable the RNA to replicate itself within cells, potentially offering higher and more sustained protein expression from lower doses.
The development of saRNA therapeutics can be traced back to the early 2000s, with research initially focused on alphavirus-derived replicons. However, significant acceleration in this field occurred following the COVID-19 pandemic, which demonstrated the clinical viability and manufacturing scalability of mRNA platforms. This catalyzed increased investment and research interest in saRNA as a potentially more potent alternative.
The technological evolution of saRNA has progressed through several key phases: initial proof-of-concept studies demonstrating self-amplification mechanisms, optimization of RNA stability and translation efficiency, development of delivery systems compatible with the larger molecular size of saRNA, and most recently, engineering approaches to enhance safety profiles while maintaining potency.
Current technical goals in saRNA development center around addressing several critical challenges. Foremost is the optimization of the potency-safety balance - a fundamental consideration in manufacturing. Higher potency through self-amplification mechanisms must be carefully balanced against potential safety concerns related to prolonged expression and immune responses to replication machinery.
Another key objective involves improving manufacturing consistency and scalability. The larger size of saRNA molecules (typically 9-12kb compared to 2-4kb for conventional mRNA) presents unique challenges in production, purification, and quality control processes that must be overcome for commercial viability.
Researchers are also focused on enhancing the stability profile of saRNA formulations, as the larger molecular structure can present challenges for storage and distribution. Development goals include achieving thermostability comparable to conventional mRNA products while maintaining functional integrity.
The field is additionally working toward optimizing delivery systems specifically designed for saRNA's unique characteristics. Current lipid nanoparticle (LNP) formulations require adaptation to accommodate the larger molecular size and potentially different cellular trafficking requirements of self-amplifying constructs.
Long-term development goals include expanding therapeutic applications beyond vaccines into protein replacement therapies, gene editing, and cancer immunotherapy - areas where sustained expression from saRNA could provide significant advantages over conventional mRNA approaches.
The development of saRNA therapeutics can be traced back to the early 2000s, with research initially focused on alphavirus-derived replicons. However, significant acceleration in this field occurred following the COVID-19 pandemic, which demonstrated the clinical viability and manufacturing scalability of mRNA platforms. This catalyzed increased investment and research interest in saRNA as a potentially more potent alternative.
The technological evolution of saRNA has progressed through several key phases: initial proof-of-concept studies demonstrating self-amplification mechanisms, optimization of RNA stability and translation efficiency, development of delivery systems compatible with the larger molecular size of saRNA, and most recently, engineering approaches to enhance safety profiles while maintaining potency.
Current technical goals in saRNA development center around addressing several critical challenges. Foremost is the optimization of the potency-safety balance - a fundamental consideration in manufacturing. Higher potency through self-amplification mechanisms must be carefully balanced against potential safety concerns related to prolonged expression and immune responses to replication machinery.
Another key objective involves improving manufacturing consistency and scalability. The larger size of saRNA molecules (typically 9-12kb compared to 2-4kb for conventional mRNA) presents unique challenges in production, purification, and quality control processes that must be overcome for commercial viability.
Researchers are also focused on enhancing the stability profile of saRNA formulations, as the larger molecular structure can present challenges for storage and distribution. Development goals include achieving thermostability comparable to conventional mRNA products while maintaining functional integrity.
The field is additionally working toward optimizing delivery systems specifically designed for saRNA's unique characteristics. Current lipid nanoparticle (LNP) formulations require adaptation to accommodate the larger molecular size and potentially different cellular trafficking requirements of self-amplifying constructs.
Long-term development goals include expanding therapeutic applications beyond vaccines into protein replacement therapies, gene editing, and cancer immunotherapy - areas where sustained expression from saRNA could provide significant advantages over conventional mRNA approaches.
Market Analysis for Self-Amplifying mRNA Products
The self-amplifying mRNA (saRNA) therapeutics market is experiencing rapid growth, driven by the success of conventional mRNA vaccines during the COVID-19 pandemic. Current market projections indicate that the global mRNA therapeutics market, which includes saRNA products, is expected to reach $37 billion by 2030, with a compound annual growth rate of 28.4% from 2022 to 2030.
The primary advantage of saRNA over conventional mRNA is its ability to self-replicate, requiring significantly lower doses to achieve similar or enhanced immune responses. This dose-sparing effect translates directly to cost efficiency in manufacturing and potentially wider accessibility, particularly in resource-limited settings. Market analysis suggests that saRNA products could reduce manufacturing costs by 30-60% compared to conventional mRNA products.
Healthcare systems worldwide are increasingly focused on cost-effective preventative measures, creating a favorable environment for saRNA vaccines. The potential for multi-valent applications, where a single saRNA construct can express multiple antigens, represents a substantial market opportunity estimated to be worth $12 billion by 2028.
Geographically, North America currently dominates the mRNA therapeutics market with approximately 45% market share, followed by Europe at 30%. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years due to increasing healthcare expenditure and expanding manufacturing capabilities in countries like China, India, and South Korea.
Investor confidence in saRNA technology is evidenced by recent funding rounds, with biotechnology companies focused on saRNA platforms securing over $3 billion in investments since 2020. This capital influx is accelerating clinical development pipelines across multiple therapeutic areas.
The competitive landscape includes established pharmaceutical companies expanding into saRNA through acquisitions and partnerships, as well as specialized biotechnology firms focused exclusively on saRNA platform development. Key market segments include infectious diseases (currently representing 65% of the pipeline), oncology (20%), and rare genetic disorders (15%).
Regulatory pathways for saRNA products are still evolving, with agencies like the FDA and EMA developing specific guidelines. This regulatory uncertainty represents both a challenge and an opportunity for early market entrants who can help shape the regulatory framework.
Consumer acceptance of mRNA technology has improved significantly following COVID-19 vaccines, creating a more receptive market for saRNA products. However, public education regarding the safety profile of self-amplifying technology remains crucial for market penetration.
The primary advantage of saRNA over conventional mRNA is its ability to self-replicate, requiring significantly lower doses to achieve similar or enhanced immune responses. This dose-sparing effect translates directly to cost efficiency in manufacturing and potentially wider accessibility, particularly in resource-limited settings. Market analysis suggests that saRNA products could reduce manufacturing costs by 30-60% compared to conventional mRNA products.
Healthcare systems worldwide are increasingly focused on cost-effective preventative measures, creating a favorable environment for saRNA vaccines. The potential for multi-valent applications, where a single saRNA construct can express multiple antigens, represents a substantial market opportunity estimated to be worth $12 billion by 2028.
Geographically, North America currently dominates the mRNA therapeutics market with approximately 45% market share, followed by Europe at 30%. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years due to increasing healthcare expenditure and expanding manufacturing capabilities in countries like China, India, and South Korea.
Investor confidence in saRNA technology is evidenced by recent funding rounds, with biotechnology companies focused on saRNA platforms securing over $3 billion in investments since 2020. This capital influx is accelerating clinical development pipelines across multiple therapeutic areas.
The competitive landscape includes established pharmaceutical companies expanding into saRNA through acquisitions and partnerships, as well as specialized biotechnology firms focused exclusively on saRNA platform development. Key market segments include infectious diseases (currently representing 65% of the pipeline), oncology (20%), and rare genetic disorders (15%).
Regulatory pathways for saRNA products are still evolving, with agencies like the FDA and EMA developing specific guidelines. This regulatory uncertainty represents both a challenge and an opportunity for early market entrants who can help shape the regulatory framework.
Consumer acceptance of mRNA technology has improved significantly following COVID-19 vaccines, creating a more receptive market for saRNA products. However, public education regarding the safety profile of self-amplifying technology remains crucial for market penetration.
Technical Challenges in saRNA Manufacturing
The manufacturing of self-amplifying mRNA (saRNA) therapeutics presents significant technical challenges that must be addressed to ensure both efficacy and safety. Unlike conventional mRNA, saRNA contains additional genetic elements that enable self-replication within cells, creating unique manufacturing complexities.
RNA synthesis represents the first major hurdle in saRNA production. The substantially larger size of saRNA molecules (typically 8-12kb compared to 1-5kb for conventional mRNA) creates challenges for in vitro transcription (IVT) processes. Longer templates are more prone to truncation, abortive transcription, and sequence errors, potentially generating heterogeneous products with varying degrees of functionality and safety profiles.
Purification processes for saRNA face considerable obstacles due to the molecule's size and structural complexity. Conventional chromatography techniques often yield lower recovery rates for saRNA compared to standard mRNA. The presence of double-stranded regions in saRNA intermediates can trigger innate immune responses if not properly removed, necessitating more stringent purification protocols that may further reduce yield.
Formulation stability presents another critical challenge. saRNA's larger size requires specialized lipid nanoparticle (LNP) formulations with optimized lipid compositions and manufacturing processes. These LNPs must protect the saRNA from degradation while enabling efficient cellular uptake and endosomal escape. The balance between protection and delivery efficiency directly impacts the potency-safety profile of the final product.
Quality control for saRNA products requires advanced analytical methods beyond those used for conventional mRNA. Techniques must verify not only sequence integrity but also replication functionality, which is essential for saRNA's therapeutic mechanism. Current analytical limitations make it difficult to fully characterize saRNA products, potentially allowing suboptimal variants to reach clinical testing.
Scale-up considerations further complicate saRNA manufacturing. The complexity of production processes, combined with stringent quality requirements, creates challenges for consistent large-scale manufacturing. Batch-to-batch variability can significantly impact both safety and efficacy profiles, making process optimization and control strategies particularly critical.
Regulatory frameworks for saRNA manufacturing remain evolving, with limited precedent for quality standards and safety assessments. This regulatory uncertainty adds another layer of complexity to manufacturing decisions, as companies must anticipate potential future requirements while developing current processes.
The inherent self-amplifying nature of saRNA creates a fundamental tension in manufacturing: higher purity may reduce potency by eliminating beneficial variants, while less stringent purification may increase potency at the cost of potential safety concerns. This potency-safety tradeoff represents perhaps the most significant technical challenge in saRNA manufacturing today.
RNA synthesis represents the first major hurdle in saRNA production. The substantially larger size of saRNA molecules (typically 8-12kb compared to 1-5kb for conventional mRNA) creates challenges for in vitro transcription (IVT) processes. Longer templates are more prone to truncation, abortive transcription, and sequence errors, potentially generating heterogeneous products with varying degrees of functionality and safety profiles.
Purification processes for saRNA face considerable obstacles due to the molecule's size and structural complexity. Conventional chromatography techniques often yield lower recovery rates for saRNA compared to standard mRNA. The presence of double-stranded regions in saRNA intermediates can trigger innate immune responses if not properly removed, necessitating more stringent purification protocols that may further reduce yield.
Formulation stability presents another critical challenge. saRNA's larger size requires specialized lipid nanoparticle (LNP) formulations with optimized lipid compositions and manufacturing processes. These LNPs must protect the saRNA from degradation while enabling efficient cellular uptake and endosomal escape. The balance between protection and delivery efficiency directly impacts the potency-safety profile of the final product.
Quality control for saRNA products requires advanced analytical methods beyond those used for conventional mRNA. Techniques must verify not only sequence integrity but also replication functionality, which is essential for saRNA's therapeutic mechanism. Current analytical limitations make it difficult to fully characterize saRNA products, potentially allowing suboptimal variants to reach clinical testing.
Scale-up considerations further complicate saRNA manufacturing. The complexity of production processes, combined with stringent quality requirements, creates challenges for consistent large-scale manufacturing. Batch-to-batch variability can significantly impact both safety and efficacy profiles, making process optimization and control strategies particularly critical.
Regulatory frameworks for saRNA manufacturing remain evolving, with limited precedent for quality standards and safety assessments. This regulatory uncertainty adds another layer of complexity to manufacturing decisions, as companies must anticipate potential future requirements while developing current processes.
The inherent self-amplifying nature of saRNA creates a fundamental tension in manufacturing: higher purity may reduce potency by eliminating beneficial variants, while less stringent purification may increase potency at the cost of potential safety concerns. This potency-safety tradeoff represents perhaps the most significant technical challenge in saRNA manufacturing today.
Current Manufacturing Approaches for Potency Optimization
01 Optimization of self-amplifying mRNA design for potency and safety balance
The design of self-amplifying mRNA (saRNA) therapeutics involves careful optimization of sequence elements to balance potency and safety. This includes modifications to the replicase gene, untranslated regions, and coding sequences to enhance expression while minimizing immunogenicity. Strategic codon optimization and incorporation of modified nucleosides can improve translation efficiency and reduce innate immune responses, creating a more favorable potency-safety profile for therapeutic applications.- Optimization of self-amplifying mRNA design for potency enhancement: Self-amplifying mRNA (saRNA) therapeutics can be optimized through structural modifications to enhance potency while maintaining safety profiles. These modifications include codon optimization, sequence modifications to reduce immunogenicity, and incorporation of stabilizing elements. By carefully engineering the mRNA sequence and structure, researchers can achieve higher expression levels of the target protein while minimizing potential adverse effects, thus improving the therapeutic index of saRNA-based treatments.
- Delivery system innovations to balance efficacy and safety: Advanced delivery systems for self-amplifying mRNA therapeutics play a crucial role in balancing potency and safety. Lipid nanoparticles (LNPs), polymeric carriers, and targeted delivery approaches can enhance cellular uptake and protect the mRNA from degradation, leading to improved efficacy at lower doses. These delivery technologies can be engineered to reduce off-target effects and systemic toxicity while maximizing therapeutic activity at the intended site of action.
- Immunogenicity management strategies: Managing the immunogenicity of self-amplifying mRNA is essential for optimizing the potency-safety balance. Techniques include chemical modifications of nucleotides, purification methods to remove double-stranded RNA contaminants, and co-delivery with immunomodulatory agents. These approaches help minimize unwanted immune responses that could lead to safety concerns while preserving the therapeutic potency of the saRNA, enabling more effective treatments with reduced side effects.
- Dosing regimen optimization for therapeutic window expansion: Careful optimization of dosing regimens can significantly impact the potency-safety balance of self-amplifying mRNA therapeutics. Strategies include dose fractionation, extended-release formulations, and personalized dosing approaches based on patient characteristics. These methods aim to maintain therapeutic concentrations while minimizing peak levels that might cause toxicity, thereby expanding the therapeutic window and improving the overall benefit-risk profile of saRNA treatments.
- Regulatory considerations and safety assessment frameworks: Developing comprehensive safety assessment frameworks is crucial for advancing self-amplifying mRNA therapeutics. This includes establishing appropriate preclinical models, biomarkers for safety monitoring, and regulatory pathways that address the unique characteristics of saRNA. Standardized methods for evaluating biodistribution, persistence, and potential off-target effects help ensure that potency enhancements do not come at the expense of safety, facilitating the development of therapeutics with optimal benefit-risk profiles.
02 Delivery systems for self-amplifying mRNA therapeutics
Delivery systems play a crucial role in determining both the potency and safety of self-amplifying mRNA therapeutics. Lipid nanoparticles (LNPs) and other advanced delivery vehicles can be engineered to enhance cellular uptake and protect the mRNA from degradation, thereby increasing potency. However, these delivery systems must be carefully designed to minimize toxicity, immunogenicity, and off-target effects. The composition, size, and surface properties of delivery vehicles significantly impact the biodistribution and safety profile of saRNA therapeutics.Expand Specific Solutions03 Immunogenicity management in saRNA therapeutics
Managing the immunogenicity of self-amplifying mRNA therapeutics is essential for balancing potency and safety. While the inherent immunostimulatory properties of saRNA can be beneficial for vaccine applications, they may cause adverse effects in other therapeutic contexts. Strategies to modulate immunogenicity include incorporating modified nucleosides, optimizing the RNA sequence to reduce recognition by pattern recognition receptors, and co-delivering immunomodulatory molecules. These approaches help achieve the desired therapeutic effect while minimizing unwanted immune responses.Expand Specific Solutions04 Dosing strategies to optimize therapeutic window
Developing appropriate dosing strategies is critical for maximizing the therapeutic window of self-amplifying mRNA treatments. Due to the self-amplifying nature of these therapeutics, lower initial doses may achieve sufficient potency while reducing safety concerns. Controlled release formulations and sequential dosing regimens can help maintain therapeutic levels while minimizing peak concentrations associated with adverse effects. Personalized dosing based on patient characteristics and biomarkers may further optimize the potency-safety balance.Expand Specific Solutions05 Regulatory considerations and safety assessment frameworks
Regulatory frameworks for evaluating the safety and efficacy of self-amplifying mRNA therapeutics are evolving to address their unique characteristics. Comprehensive safety assessment includes evaluating potential genotoxicity, biodistribution, persistence, and long-term effects. Standardized methods for potency determination help establish appropriate dosing while ensuring safety margins. Regulatory guidance emphasizes the importance of characterizing the risk-benefit profile through appropriate preclinical models and clinical trial designs that can detect both immediate and delayed adverse effects.Expand Specific Solutions
Key Industry Players in saRNA Therapeutics
The self-amplifying mRNA therapeutics market is currently in an early growth phase, characterized by rapid technological advancement and expanding clinical applications. The global market size is projected to reach significant value in the coming years, driven by increasing investment in RNA-based medicine. Technologically, the field faces critical manufacturing challenges balancing potency and safety. Leading players like BioNTech, Moderna, and CureVac are advancing manufacturing processes with proprietary platforms, while emerging companies such as Nutcracker Therapeutics and Amplitude Therapeutics are developing innovative microfluidic and biochip-based systems. Academic institutions including MIT and Boston University collaborate with industry partners to address formulation challenges. The competitive landscape shows pharmaceutical giants (GlaxoSmithKline, Merck) investing alongside specialized biotechs, indicating growing market maturity.
CureVac SE
Technical Solution: CureVac's manufacturing approach for saRNA therapeutics leverages their RNAoptimizer® platform, which employs unmodified nucleotides combined with sequence optimization to balance immunogenicity and translation efficiency. Their production process features proprietary in vitro transcription methods that minimize double-stranded RNA formation through controlled reaction kinetics and optimized buffer systems. CureVac has developed specialized purification techniques using tangential flow filtration and chromatography to remove truncated transcripts while preserving the functional integrity of the replicase encoding regions. Their manufacturing platform incorporates real-time monitoring of critical quality attributes including 5' cap incorporation efficiency and poly(A) tail distribution. CureVac's approach to the potency-safety balance includes engineered mutations in the replicase gene that limit cytopathic effects while maintaining sufficient amplification capacity, with proprietary analytical methods to quantify replication kinetics as a release criterion.
Strengths: Unique expertise with unmodified mRNA that potentially reduces manufacturing complexity; established large-scale production capabilities; proprietary sequence optimization technology. Weaknesses: Potentially higher innate immune activation with unmodified nucleotides; challenges in balancing immunostimulatory properties with safety profile; more complex quality control requirements for replicase functionality.
BioNTech SE
Technical Solution: BioNTech's manufacturing strategy for self-amplifying mRNA therapeutics employs their RiboCure™ platform, which integrates proprietary sequence optimization algorithms to enhance stability and translation efficiency. Their approach focuses on minimizing dsRNA formation during in vitro transcription through optimized reaction conditions and nucleotide ratios. BioNTech has developed a purification cascade that selectively removes truncated transcripts and dsRNA impurities while preserving the integrity of the replicase encoding region. Their saRNA constructs incorporate sequence modifications in the replicase gene to reduce potential cytotoxicity while maintaining amplification efficiency. The company has implemented automated manufacturing processes with in-line monitoring to ensure batch-to-batch consistency, addressing a key challenge in saRNA production. BioNTech's platform includes proprietary analytical methods to quantify replication efficiency in cellular models as a critical quality attribute.
Strengths: Proven expertise in mRNA sequence optimization and modification chemistry; established regulatory success with mRNA products; advanced automation in manufacturing processes. Weaknesses: Potential challenges with scale-up of longer RNA constructs; higher production costs compared to traditional vaccines; complexity in balancing replicase activity with safety profile.
Critical Patents and Innovations in saRNA Safety Controls
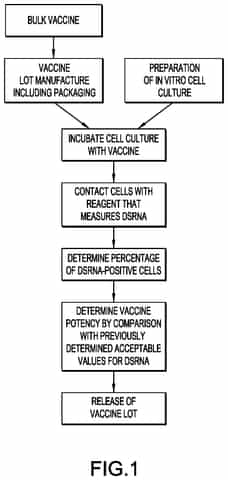
Release assay for determining potency of self-amplifying RNA drug product and methods for using
PatentPendingUS20240272143A1
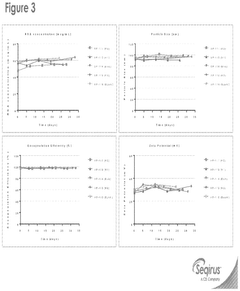
Innovation
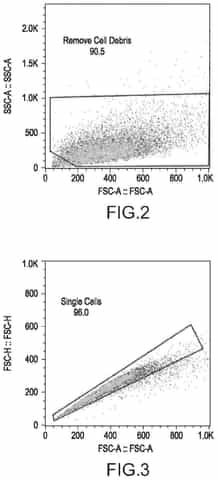
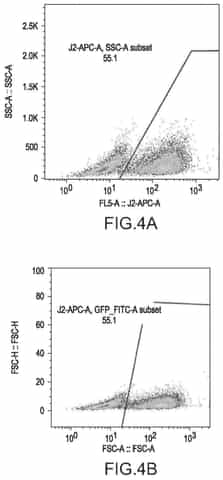
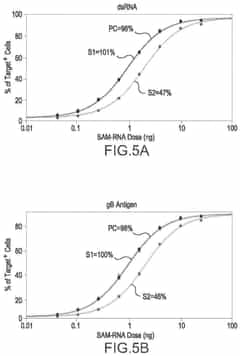
- A cell-based in vitro potency assay that measures the accumulation of double-stranded RNA (dsRNA) in cells transfected with SAM, allowing for antigen-agnostic assessment of SAM vaccine potency using a non-viral delivery system like lipid nanoparticles, eliminating the need for expensive animal-based assays and specific antibodies.
Enhancement of self-amplifying mRNA molecules within lipid nanoparticles
PatentActiveUS12083175B1
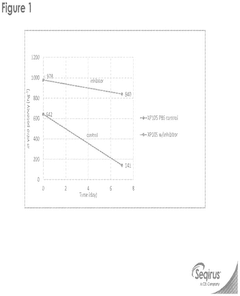
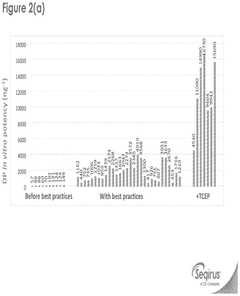
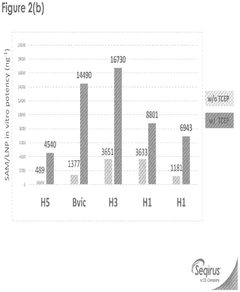
Innovation
- The incorporation of reducing agents like tris(2-carboxyethyl)phosphine (TCEP) during the formulation process of SAM/LNPs enhances their stability, potency, and shelf-life by reducing RNase degradation, allowing for improved encapsulation and delivery of influenza virus epitopes.
Regulatory Framework for saRNA Therapeutic Approval
The regulatory landscape for self-amplifying mRNA (saRNA) therapeutics remains in a developmental phase, with frameworks evolving as scientific understanding advances. Currently, saRNA products fall under the broader regulatory categories established for gene therapies and biological products, requiring manufacturers to navigate complex approval pathways across different jurisdictions.
In the United States, the FDA oversees saRNA therapeutics primarily through the Center for Biologics Evaluation and Research (CBER), applying risk-based approaches that consider both manufacturing processes and final product characteristics. The agency has established specific guidance for mRNA-based products that addresses critical quality attributes, including potency assessment methods, impurity profiles, and stability requirements.
European regulatory bodies, led by the European Medicines Agency (EMA), have developed the Advanced Therapy Medicinal Products (ATMP) framework, which encompasses saRNA therapeutics. The EMA emphasizes comprehensive characterization of starting materials and manufacturing intermediates, with particular attention to potential contaminants that may trigger immunogenicity concerns.
Regulatory requirements specifically addressing the potency-safety balance in saRNA manufacturing include validated analytical methods for replication competence testing, stringent quality control measures for double-stranded RNA contaminants, and comprehensive characterization of innate immune activation profiles. These requirements reflect the unique challenges posed by saRNA's self-amplifying nature.
Global harmonization efforts through the International Council for Harmonisation (ICH) have begun addressing nucleic acid-based therapeutics, though specific guidelines for saRNA products remain under development. This creates challenges for manufacturers seeking multi-regional approvals, as they must reconcile varying requirements across jurisdictions.
Accelerated approval pathways exist for saRNA therapeutics targeting serious conditions with unmet medical needs. However, these pathways typically require robust post-marketing surveillance plans to monitor long-term safety, particularly regarding potential delayed immune responses or unexpected biodistribution patterns unique to self-amplifying constructs.
Regulatory agencies increasingly employ adaptive licensing approaches for novel therapeutic modalities like saRNA, allowing staged approvals with expanding indications as safety data accumulates. This approach helps balance innovation with patient safety, particularly important given the relatively limited clinical experience with saRNA therapeutics compared to conventional mRNA products.
In the United States, the FDA oversees saRNA therapeutics primarily through the Center for Biologics Evaluation and Research (CBER), applying risk-based approaches that consider both manufacturing processes and final product characteristics. The agency has established specific guidance for mRNA-based products that addresses critical quality attributes, including potency assessment methods, impurity profiles, and stability requirements.
European regulatory bodies, led by the European Medicines Agency (EMA), have developed the Advanced Therapy Medicinal Products (ATMP) framework, which encompasses saRNA therapeutics. The EMA emphasizes comprehensive characterization of starting materials and manufacturing intermediates, with particular attention to potential contaminants that may trigger immunogenicity concerns.
Regulatory requirements specifically addressing the potency-safety balance in saRNA manufacturing include validated analytical methods for replication competence testing, stringent quality control measures for double-stranded RNA contaminants, and comprehensive characterization of innate immune activation profiles. These requirements reflect the unique challenges posed by saRNA's self-amplifying nature.
Global harmonization efforts through the International Council for Harmonisation (ICH) have begun addressing nucleic acid-based therapeutics, though specific guidelines for saRNA products remain under development. This creates challenges for manufacturers seeking multi-regional approvals, as they must reconcile varying requirements across jurisdictions.
Accelerated approval pathways exist for saRNA therapeutics targeting serious conditions with unmet medical needs. However, these pathways typically require robust post-marketing surveillance plans to monitor long-term safety, particularly regarding potential delayed immune responses or unexpected biodistribution patterns unique to self-amplifying constructs.
Regulatory agencies increasingly employ adaptive licensing approaches for novel therapeutic modalities like saRNA, allowing staged approvals with expanding indications as safety data accumulates. This approach helps balance innovation with patient safety, particularly important given the relatively limited clinical experience with saRNA therapeutics compared to conventional mRNA products.
Quality Control Strategies for saRNA Manufacturing
Quality control in saRNA manufacturing represents a critical component that directly impacts both product efficacy and safety profiles. The implementation of robust quality control strategies must address the unique challenges posed by self-amplifying mRNA's complex structure and replication capabilities.
Analytical methods for saRNA quality assessment require significantly higher sensitivity compared to conventional mRNA therapeutics due to the amplification mechanism. Chromatographic techniques, particularly reversed-phase HPLC and anion-exchange chromatography, have been optimized to detect minute impurities that could trigger unwanted immune responses or affect replication fidelity.
RNA integrity analysis presents unique challenges for saRNA due to its larger size (typically 8-12kb compared to 1-5kb for conventional mRNA). Advanced capillary electrophoresis methods with specialized algorithms have been developed to accurately quantify full-length transcripts versus degradation products. This distinction is crucial as truncated saRNA molecules may retain partial functionality while exhibiting altered safety profiles.
Contaminant detection strategies must address both process-related impurities (residual DNA templates, enzymes) and product-related variants (truncated sequences, incorrect nucleotide incorporation). Next-generation sequencing approaches have emerged as powerful tools for comprehensive sequence verification, enabling detection of mutations that might affect replication efficiency or introduce unintended biological activities.
Potency assays for saRNA require standardization beyond conventional mRNA approaches. Cell-based reporter systems measuring both initial translation and subsequent amplification have been established, allowing manufacturers to correlate analytical parameters with functional outcomes. These dual-readout systems provide critical insights into the relationship between structural integrity and biological activity.
Stability testing protocols have been adapted to account for saRNA's unique degradation pathways, particularly focusing on the integrity of replicase-encoding regions. Accelerated stability studies under various stress conditions help establish appropriate storage conditions and shelf-life parameters that maintain the critical quality attributes.
Batch release specifications must balance stringency with manufacturing feasibility. Industry consensus is emerging around acceptable limits for key parameters such as dsRNA content (<0.05%), residual template DNA (<10 ng/mg saRNA), and sequence accuracy (>99.9% for replicase-coding regions). These specifications represent the current best compromise between manufacturing capability and safety requirements.
Analytical methods for saRNA quality assessment require significantly higher sensitivity compared to conventional mRNA therapeutics due to the amplification mechanism. Chromatographic techniques, particularly reversed-phase HPLC and anion-exchange chromatography, have been optimized to detect minute impurities that could trigger unwanted immune responses or affect replication fidelity.
RNA integrity analysis presents unique challenges for saRNA due to its larger size (typically 8-12kb compared to 1-5kb for conventional mRNA). Advanced capillary electrophoresis methods with specialized algorithms have been developed to accurately quantify full-length transcripts versus degradation products. This distinction is crucial as truncated saRNA molecules may retain partial functionality while exhibiting altered safety profiles.
Contaminant detection strategies must address both process-related impurities (residual DNA templates, enzymes) and product-related variants (truncated sequences, incorrect nucleotide incorporation). Next-generation sequencing approaches have emerged as powerful tools for comprehensive sequence verification, enabling detection of mutations that might affect replication efficiency or introduce unintended biological activities.
Potency assays for saRNA require standardization beyond conventional mRNA approaches. Cell-based reporter systems measuring both initial translation and subsequent amplification have been established, allowing manufacturers to correlate analytical parameters with functional outcomes. These dual-readout systems provide critical insights into the relationship between structural integrity and biological activity.
Stability testing protocols have been adapted to account for saRNA's unique degradation pathways, particularly focusing on the integrity of replicase-encoding regions. Accelerated stability studies under various stress conditions help establish appropriate storage conditions and shelf-life parameters that maintain the critical quality attributes.
Batch release specifications must balance stringency with manufacturing feasibility. Industry consensus is emerging around acceptable limits for key parameters such as dsRNA content (<0.05%), residual template DNA (<10 ng/mg saRNA), and sequence accuracy (>99.9% for replicase-coding regions). These specifications represent the current best compromise between manufacturing capability and safety requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!