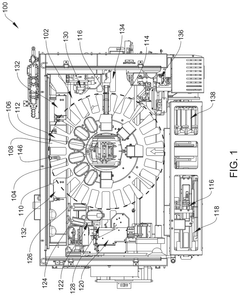
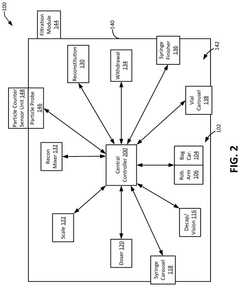
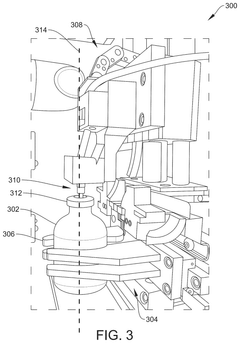
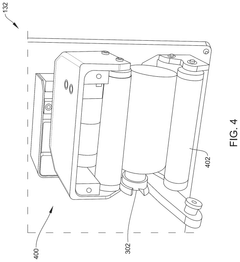
Robotics for aseptic handling: reducing operator variability in dose preparation workflows
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Aseptic Robotics Background and Objectives
Aseptic handling in pharmaceutical and healthcare environments has traditionally relied heavily on manual processes performed by trained operators. The evolution of this field traces back to the early 20th century when sterile techniques were first formalized for medical procedures. Over subsequent decades, increasingly stringent regulatory frameworks emerged, including FDA's aseptic processing guidelines and EU GMP Annex 1, establishing rigorous standards for contamination control in sterile product manufacturing.
The technological trajectory has progressed from basic laminar flow workbenches in the 1960s to sophisticated isolators and restricted access barrier systems (RABS) in recent decades. Despite these advancements, the human element has remained central to aseptic operations, introducing inherent variability and contamination risks. Studies indicate that 65-80% of contamination events in aseptic processing can be attributed to human interventions.
Robotics technology has evolved significantly since its industrial introduction in the 1960s. Early robotic systems were rigid, programmed for repetitive tasks with limited adaptability. Modern robotic platforms incorporate advanced sensors, machine vision, artificial intelligence, and collaborative capabilities that make them increasingly suitable for complex, precision-oriented tasks in controlled environments.
The convergence of robotics with aseptic handling represents a natural technological progression aimed at addressing persistent challenges in sterile processing. Current objectives for aseptic robotics focus on developing systems capable of performing precise manipulations of sterile components while maintaining environmental integrity and eliminating human-source contamination.
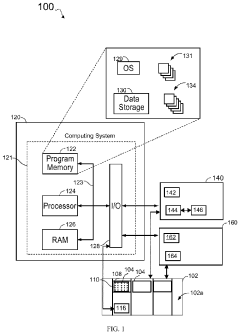
Key technical goals include achieving six-axis manipulation with sterility-compatible materials, implementing real-time environmental monitoring, developing validated cleaning and decontamination protocols for robotic components, and creating intuitive programming interfaces that allow adaptation to various dose preparation workflows.
The market is increasingly driven by escalating regulatory pressures, growing demand for personalized medicines requiring complex aseptic manipulations, and persistent challenges in maintaining consistent sterile practices across global manufacturing networks. Particularly in oncology, where hazardous drug handling presents additional operator safety concerns, robotic solutions offer dual benefits of contamination reduction and worker protection.
The ultimate objective is to establish robotic systems as a new standard in aseptic processing—creating reproducible, validated workflows that significantly reduce process variability while enhancing product quality, operator safety, and manufacturing efficiency. This represents not merely an incremental improvement but a paradigm shift in how sterile products are prepared and handled across pharmaceutical and healthcare settings.
The technological trajectory has progressed from basic laminar flow workbenches in the 1960s to sophisticated isolators and restricted access barrier systems (RABS) in recent decades. Despite these advancements, the human element has remained central to aseptic operations, introducing inherent variability and contamination risks. Studies indicate that 65-80% of contamination events in aseptic processing can be attributed to human interventions.
Robotics technology has evolved significantly since its industrial introduction in the 1960s. Early robotic systems were rigid, programmed for repetitive tasks with limited adaptability. Modern robotic platforms incorporate advanced sensors, machine vision, artificial intelligence, and collaborative capabilities that make them increasingly suitable for complex, precision-oriented tasks in controlled environments.
The convergence of robotics with aseptic handling represents a natural technological progression aimed at addressing persistent challenges in sterile processing. Current objectives for aseptic robotics focus on developing systems capable of performing precise manipulations of sterile components while maintaining environmental integrity and eliminating human-source contamination.
Key technical goals include achieving six-axis manipulation with sterility-compatible materials, implementing real-time environmental monitoring, developing validated cleaning and decontamination protocols for robotic components, and creating intuitive programming interfaces that allow adaptation to various dose preparation workflows.
The market is increasingly driven by escalating regulatory pressures, growing demand for personalized medicines requiring complex aseptic manipulations, and persistent challenges in maintaining consistent sterile practices across global manufacturing networks. Particularly in oncology, where hazardous drug handling presents additional operator safety concerns, robotic solutions offer dual benefits of contamination reduction and worker protection.
The ultimate objective is to establish robotic systems as a new standard in aseptic processing—creating reproducible, validated workflows that significantly reduce process variability while enhancing product quality, operator safety, and manufacturing efficiency. This represents not merely an incremental improvement but a paradigm shift in how sterile products are prepared and handled across pharmaceutical and healthcare settings.
Market Demand for Automated Dose Preparation
The global pharmaceutical industry is experiencing a significant shift towards automation in dose preparation workflows, driven by the critical need for precision, consistency, and contamination control. The market for robotic aseptic handling systems is projected to grow substantially, with healthcare facilities increasingly recognizing the limitations of manual dose preparation processes.
Current market research indicates that medication errors cost healthcare systems billions annually, with a significant portion attributable to manual preparation inconsistencies. These errors not only impact patient safety but also result in substantial financial losses through wasted medications, extended hospital stays, and potential litigation. The demand for automated solutions is particularly acute in oncology departments, where cytotoxic drug preparation requires meticulous handling to ensure both patient safety and operator protection.
Hospital pharmacies and compounding centers represent the primary market segments actively seeking robotic solutions for aseptic handling. These facilities typically process hundreds to thousands of doses daily, making them ideal candidates for automation technologies that can maintain consistent quality while increasing throughput. The COVID-19 pandemic has further accelerated this demand, highlighting vulnerabilities in traditional manual preparation methods during staff shortages and increased workloads.
Regulatory pressures are also driving market growth, with agencies worldwide implementing stricter guidelines for aseptic processing. The United States Pharmacopeia (USP) chapters <797> and <800>, along with European GMP Annex 1, have established rigorous standards that are difficult to consistently meet with manual processes alone. Healthcare facilities are increasingly turning to automation to ensure compliance with these evolving regulations.
Market analysis reveals that hospitals and pharmaceutical companies are willing to make significant capital investments in robotic systems that demonstrate clear return on investment through error reduction, increased throughput, and improved staff utilization. The potential for redeploying skilled pharmacy staff from repetitive preparation tasks to more clinically focused activities represents an additional value proposition driving market demand.
Regional market variations exist, with North America and Europe currently leading adoption rates due to higher labor costs and stricter regulatory environments. However, rapidly growing healthcare markets in Asia-Pacific regions are expected to show the highest growth rates in the coming years as healthcare infrastructure development accelerates and quality standards harmonize globally.
The market increasingly demands solutions that can integrate with existing hospital information systems and electronic medical records, creating seamless workflows from prescription to administration. This connectivity requirement represents both a challenge and opportunity for robotics developers seeking to establish market leadership in this growing sector.
Current market research indicates that medication errors cost healthcare systems billions annually, with a significant portion attributable to manual preparation inconsistencies. These errors not only impact patient safety but also result in substantial financial losses through wasted medications, extended hospital stays, and potential litigation. The demand for automated solutions is particularly acute in oncology departments, where cytotoxic drug preparation requires meticulous handling to ensure both patient safety and operator protection.
Hospital pharmacies and compounding centers represent the primary market segments actively seeking robotic solutions for aseptic handling. These facilities typically process hundreds to thousands of doses daily, making them ideal candidates for automation technologies that can maintain consistent quality while increasing throughput. The COVID-19 pandemic has further accelerated this demand, highlighting vulnerabilities in traditional manual preparation methods during staff shortages and increased workloads.
Regulatory pressures are also driving market growth, with agencies worldwide implementing stricter guidelines for aseptic processing. The United States Pharmacopeia (USP) chapters <797> and <800>, along with European GMP Annex 1, have established rigorous standards that are difficult to consistently meet with manual processes alone. Healthcare facilities are increasingly turning to automation to ensure compliance with these evolving regulations.
Market analysis reveals that hospitals and pharmaceutical companies are willing to make significant capital investments in robotic systems that demonstrate clear return on investment through error reduction, increased throughput, and improved staff utilization. The potential for redeploying skilled pharmacy staff from repetitive preparation tasks to more clinically focused activities represents an additional value proposition driving market demand.
Regional market variations exist, with North America and Europe currently leading adoption rates due to higher labor costs and stricter regulatory environments. However, rapidly growing healthcare markets in Asia-Pacific regions are expected to show the highest growth rates in the coming years as healthcare infrastructure development accelerates and quality standards harmonize globally.
The market increasingly demands solutions that can integrate with existing hospital information systems and electronic medical records, creating seamless workflows from prescription to administration. This connectivity requirement represents both a challenge and opportunity for robotics developers seeking to establish market leadership in this growing sector.
Current Challenges in Aseptic Handling Technology
Despite significant advancements in pharmaceutical manufacturing, aseptic handling in dose preparation workflows continues to face substantial challenges. The current manual processes rely heavily on human operators, introducing variability that can compromise product quality and patient safety. Studies indicate that human errors account for approximately 60-80% of deviations in aseptic processing environments, highlighting the critical need for technological intervention.
The most pressing challenge is maintaining sterility throughout the preparation process. Traditional cleanrooms require extensive environmental controls, yet human operators remain the primary source of contamination. Even with proper gowning and training, human movements generate particles and potentially introduce microorganisms into critical areas. Current monitoring systems can detect contamination only after it occurs, rather than preventing it proactively.
Operator technique variability presents another significant hurdle. Research demonstrates that even among experienced technicians, variations in handling techniques can lead to inconsistencies in dose accuracy ranging from 2-15%. This variability becomes particularly problematic for high-potency or narrow therapeutic index medications where precision is paramount. The industry currently lacks standardized quantitative metrics to evaluate operator performance beyond basic qualification assessments.
Ergonomic limitations of current aseptic workstations contribute to operator fatigue and subsequent errors. Traditional isolators and laminar flow hoods restrict natural movement, requiring operators to work in uncomfortable positions for extended periods. Studies show that after four hours of continuous work in these environments, error rates increase by approximately 30%, directly impacting product quality.
Documentation and traceability remain challenging in manual processes. Current paper-based or hybrid systems are prone to transcription errors and provide limited real-time visibility into process deviations. Regulatory bodies increasingly demand complete process transparency, yet existing systems struggle to provide comprehensive audit trails that connect operator actions with specific preparation steps.
The economic burden of maintaining aseptic handling capabilities is substantial. Training and retraining operators requires significant investment, with industry averages suggesting 6-8 months before personnel achieve full competency. High turnover rates in pharmaceutical manufacturing (approximately 15-20% annually) exacerbate this challenge, creating a continuous cycle of recruitment and training that impacts operational efficiency.
Cross-contamination risks persist despite procedural controls, particularly in facilities handling multiple products. Current cleaning validation methodologies cannot always detect residual product at the microscopic levels required for certain applications, creating potential safety risks that are difficult to quantify and mitigate consistently.
The most pressing challenge is maintaining sterility throughout the preparation process. Traditional cleanrooms require extensive environmental controls, yet human operators remain the primary source of contamination. Even with proper gowning and training, human movements generate particles and potentially introduce microorganisms into critical areas. Current monitoring systems can detect contamination only after it occurs, rather than preventing it proactively.
Operator technique variability presents another significant hurdle. Research demonstrates that even among experienced technicians, variations in handling techniques can lead to inconsistencies in dose accuracy ranging from 2-15%. This variability becomes particularly problematic for high-potency or narrow therapeutic index medications where precision is paramount. The industry currently lacks standardized quantitative metrics to evaluate operator performance beyond basic qualification assessments.
Ergonomic limitations of current aseptic workstations contribute to operator fatigue and subsequent errors. Traditional isolators and laminar flow hoods restrict natural movement, requiring operators to work in uncomfortable positions for extended periods. Studies show that after four hours of continuous work in these environments, error rates increase by approximately 30%, directly impacting product quality.
Documentation and traceability remain challenging in manual processes. Current paper-based or hybrid systems are prone to transcription errors and provide limited real-time visibility into process deviations. Regulatory bodies increasingly demand complete process transparency, yet existing systems struggle to provide comprehensive audit trails that connect operator actions with specific preparation steps.
The economic burden of maintaining aseptic handling capabilities is substantial. Training and retraining operators requires significant investment, with industry averages suggesting 6-8 months before personnel achieve full competency. High turnover rates in pharmaceutical manufacturing (approximately 15-20% annually) exacerbate this challenge, creating a continuous cycle of recruitment and training that impacts operational efficiency.
Cross-contamination risks persist despite procedural controls, particularly in facilities handling multiple products. Current cleaning validation methodologies cannot always detect residual product at the microscopic levels required for certain applications, creating potential safety risks that are difficult to quantify and mitigate consistently.
Current Robotic Solutions for Dose Preparation
01 Robotic systems for aseptic pharmaceutical handling
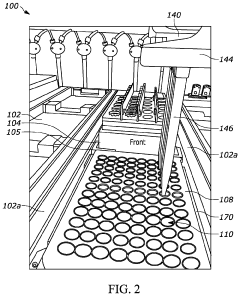
Robotic systems designed specifically for pharmaceutical applications that maintain aseptic conditions during drug preparation, filling, and packaging. These systems minimize human intervention, reducing contamination risks and operator variability. They incorporate isolator technology, sterile transfer mechanisms, and automated quality control to ensure consistent aseptic processing in compliance with regulatory standards.- Robotic systems for aseptic pharmaceutical handling: Robotic systems designed specifically for pharmaceutical applications that minimize human intervention in aseptic processes. These systems include automated filling lines, robotic arms for vial handling, and integrated isolator technologies that maintain sterile conditions while reducing operator variability. The automation helps ensure consistent performance in critical aseptic operations where human factors could introduce contamination risks.
- Operator variability reduction through automation interfaces: Advanced human-machine interfaces and control systems that standardize operator interactions with aseptic equipment. These technologies include guided workflow systems, error-proofing mechanisms, and standardized operating procedures embedded in control software. By constraining operator actions to validated parameters, these systems reduce the variability inherent in manual operations while maintaining process integrity.
- Robotic isolator and barrier systems: Specialized robotic systems operating within isolators or barrier systems that physically separate the aseptic processing area from human operators. These systems incorporate glove ports, transfer mechanisms, and robotic manipulators that can be operated remotely, eliminating direct human contact with sterile environments. The combination of physical barriers and automation significantly reduces contamination risks associated with operator variability.
- Validation and monitoring systems for robotic aseptic processes: Integrated monitoring technologies that continuously validate robotic aseptic handling performance. These systems employ sensors, vision systems, and data analytics to detect deviations from established parameters in real-time. By providing continuous verification of critical process attributes, these technologies ensure consistent performance regardless of which operator is supervising the automated system, effectively standardizing quality outcomes.
- Training and simulation systems for aseptic robotics: Virtual and augmented reality systems designed to train operators in the proper use of aseptic robotic equipment. These technologies allow operators to practice complex procedures in simulated environments before performing them in actual clean rooms. The training systems help standardize operator knowledge and technique, reducing variability in how different personnel interact with robotic systems while maintaining aseptic conditions.
02 Automated aseptic handling in medical procedures
Robotic systems that assist in medical procedures requiring aseptic conditions, such as surgery, sample handling, and patient care. These systems help maintain sterility while performing precise manipulations that would otherwise be subject to human error. The automation reduces variability in technique, improves procedural consistency, and minimizes infection risks associated with manual handling.Expand Specific Solutions03 Robotic interfaces and control systems for aseptic operations
Advanced control systems and interfaces that enable precise operation of robotic systems in aseptic environments. These technologies include haptic feedback mechanisms, intuitive user interfaces, and automated decision-making algorithms that compensate for potential operator variability. The systems can adapt to different operational parameters while maintaining aseptic integrity throughout the process.Expand Specific Solutions04 Validation and monitoring systems for aseptic robotic processes
Systems designed to validate, monitor, and document aseptic conditions during robotic operations. These technologies include real-time environmental monitoring, process verification tools, and data analytics that ensure compliance with aseptic standards. They help identify potential contamination risks from operator variability and provide documentation for regulatory compliance.Expand Specific Solutions05 Specialized end-effectors and tools for aseptic handling
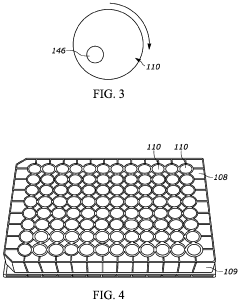
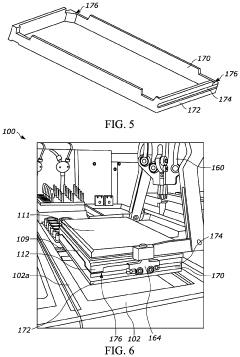
Purpose-built robotic end-effectors and tools designed specifically for aseptic applications. These components feature materials and designs that facilitate sterilization, prevent particle generation, and maintain sterility during operation. The specialized tools compensate for the limitations of human operators in maintaining aseptic technique, providing consistent performance regardless of operator skill level or fatigue.Expand Specific Solutions
Key Industry Players in Aseptic Automation
The robotics for aseptic handling market is currently in a growth phase, driven by increasing demand for precision and contamination control in pharmaceutical dose preparation. The global market is estimated to reach $1.5-2 billion by 2025, with a CAGR of approximately 12-15%. Technology maturity varies across applications, with companies like YASKAWA Electric and Omnicell leading in industrial automation, while Equashield Medical and Tofflon Science & Technology Group specialize in pharmaceutical-specific solutions. Newer entrants like Swisslog Italia and Deenova are advancing integrated logistics systems. Major pharmaceutical companies including Amgen, Genentech, and Roche are increasingly adopting these technologies to reduce human error and ensure compliance with stringent regulatory requirements for sterile manufacturing processes.
Omnicell, Inc.
Technical Solution: Omnicell has developed the IVX Workflow solution, an advanced robotic system for aseptic handling in pharmacy operations. The system incorporates barcode scanning, gravimetric measurement, and image recognition to guide technicians through the IV preparation process. Their technology includes robotic arms that operate within controlled environments to manipulate sterile components with precision beyond human capability. The system features real-time verification of each preparation step, including automated checks of drug vials, diluents, and final preparations. Omnicell's platform integrates with hospital information systems to receive orders electronically and document the entire preparation process, creating a comprehensive audit trail. Their robotic systems maintain consistent sterile technique regardless of operator experience level, effectively standardizing the preparation process across all shifts and personnel.
Strengths: Comprehensive integration with existing pharmacy systems; gravimetric verification provides accuracy to 0.1g; reduces preparation errors by over 97% compared to manual processes. Weaknesses: Requires significant initial capital investment; needs regular maintenance and calibration; limited flexibility for handling unusual container types or preparation methods.
Equashield Medical Ltd.
Technical Solution: Equashield has pioneered the Equashield Pro, a robotic system specifically designed for hazardous drug compounding in aseptic environments. Their technology features a fully automated closed system that eliminates direct human contact with hazardous drugs during preparation. The robot operates within a negative pressure isolator and utilizes proprietary Closed System Transfer Device (CSTD) technology to prevent environmental contamination and exposure. The system employs dual robotic arms with six degrees of freedom that mimic human movement but with greater precision and consistency. Equashield's solution incorporates machine vision systems for real-time verification of drug vials, syringes, and IV bags, ensuring accurate dose preparation. The platform includes built-in gravimetric verification that weighs components before and after drug transfer to confirm precise dosing within 0.2ml accuracy. Their system maintains electronic records of all preparations, creating complete traceability from prescription to administration.
Strengths: Specialized focus on hazardous drug handling provides superior protection for operators; closed-system design virtually eliminates environmental contamination; achieves 99.9% accuracy in dose preparation. Weaknesses: Higher cost compared to semi-automated alternatives; requires specialized consumables compatible with the robotic system; limited throughput capacity for high-volume facilities.
Critical Technologies in Aseptic Handling Robotics
Systems and approaches for drug processing
PatentPendingUS20240103025A1
Innovation
- A robotic-based drug processing system with a workstation, agitating member, liquid handler, and plate mover, which includes a vortex plate for mixing and a six-tip member for simultaneous fluid addition/removal, along with a visual identification system and adapter bracket to securely hold filter plates, enabling automated reconstitution and concentration of drug substances.
Systems and methods for parallel preparation processing
PatentActiveUS12125574B2
Innovation
- An automated dosing device with multiple stations and a central controller that uses a robotic arm and bag carousel to perform parallel processing, iteratively assigning tasks and directing the transport of medication containers between stations to create dosed medication delivery containers, while maintaining sterility and cleanliness through controlled environments and continuous quality monitoring.
Regulatory Compliance for Pharmaceutical Robotics
The regulatory landscape for pharmaceutical robotics is complex and multifaceted, requiring strict adherence to various international standards and guidelines. For aseptic handling robots in dose preparation workflows, compliance with FDA's current Good Manufacturing Practices (cGMP) and the EU's GMP Annex 1 for sterile medicinal products is mandatory. These regulations emphasize contamination control, validation protocols, and quality assurance systems.
Robotic systems must meet the requirements outlined in USP <797> for sterile compounding and USP <800> for handling hazardous drugs. Additionally, ISO 14644 standards for cleanroom environments and ISO 13485 for medical device quality management systems apply to robotic equipment operating in aseptic environments.
Regulatory bodies increasingly recognize the potential of robotics to enhance compliance through reduced human intervention. The FDA's Emerging Technology Program and similar initiatives in Europe provide frameworks for evaluating novel technologies like aseptic handling robots. These programs offer manufacturers pathways to demonstrate compliance while implementing innovative solutions.
Documentation requirements for robotic systems are extensive, including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols. Manufacturers must maintain comprehensive records of system performance, maintenance activities, and any deviations from expected operation. Computer system validation following GAMP 5 guidelines is essential for software components controlling robotic functions.
Risk management frameworks such as ICH Q9 must be applied throughout the development and implementation of pharmaceutical robotics. This includes identifying potential failure modes, implementing appropriate controls, and establishing continuous monitoring systems to ensure ongoing compliance.
Regulatory considerations extend to data integrity aspects, with robotic systems required to comply with 21 CFR Part 11 for electronic records and signatures. This ensures that data generated during automated dose preparation processes remains attributable, legible, contemporaneous, original, and accurate (ALCOA principles).
The validation of cleaning and decontamination procedures for robotic components presents unique challenges. Regulatory expectations include demonstration of effective cleaning methods that prevent cross-contamination while maintaining the integrity of sensitive electronic and mechanical components.
As regulatory frameworks evolve to accommodate technological advancements, manufacturers of aseptic handling robots must maintain vigilance regarding changing requirements and emerging standards. Establishing proactive relationships with regulatory authorities through scientific advice meetings and participation in industry working groups can facilitate smoother approval processes and implementation strategies.
Robotic systems must meet the requirements outlined in USP <797> for sterile compounding and USP <800> for handling hazardous drugs. Additionally, ISO 14644 standards for cleanroom environments and ISO 13485 for medical device quality management systems apply to robotic equipment operating in aseptic environments.
Regulatory bodies increasingly recognize the potential of robotics to enhance compliance through reduced human intervention. The FDA's Emerging Technology Program and similar initiatives in Europe provide frameworks for evaluating novel technologies like aseptic handling robots. These programs offer manufacturers pathways to demonstrate compliance while implementing innovative solutions.
Documentation requirements for robotic systems are extensive, including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols. Manufacturers must maintain comprehensive records of system performance, maintenance activities, and any deviations from expected operation. Computer system validation following GAMP 5 guidelines is essential for software components controlling robotic functions.
Risk management frameworks such as ICH Q9 must be applied throughout the development and implementation of pharmaceutical robotics. This includes identifying potential failure modes, implementing appropriate controls, and establishing continuous monitoring systems to ensure ongoing compliance.
Regulatory considerations extend to data integrity aspects, with robotic systems required to comply with 21 CFR Part 11 for electronic records and signatures. This ensures that data generated during automated dose preparation processes remains attributable, legible, contemporaneous, original, and accurate (ALCOA principles).
The validation of cleaning and decontamination procedures for robotic components presents unique challenges. Regulatory expectations include demonstration of effective cleaning methods that prevent cross-contamination while maintaining the integrity of sensitive electronic and mechanical components.
As regulatory frameworks evolve to accommodate technological advancements, manufacturers of aseptic handling robots must maintain vigilance regarding changing requirements and emerging standards. Establishing proactive relationships with regulatory authorities through scientific advice meetings and participation in industry working groups can facilitate smoother approval processes and implementation strategies.
Risk Assessment and Validation Methodologies
The implementation of robotic systems for aseptic handling in pharmaceutical dose preparation requires rigorous risk assessment and validation methodologies to ensure patient safety and regulatory compliance. Traditional risk assessment frameworks like Failure Mode and Effects Analysis (FMEA) and Hazard Analysis and Critical Control Points (HACCP) must be adapted specifically for robotic aseptic handling environments, with particular attention to contamination risks, mechanical failures, and software errors that could impact dose accuracy.
Risk assessment for robotic aseptic handling systems should follow a systematic approach beginning with hazard identification across all process steps. This includes evaluating potential failure points in robotic arm movements, sterile barrier maintenance, material transfer operations, and human-robot interaction zones. Quantitative risk scoring methodologies that consider severity, probability, and detectability factors provide objective measures for prioritizing mitigation strategies and establishing appropriate control measures.
Validation methodologies for aseptic robotic systems must address both hardware and software components through Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols. These should be supplemented with aseptic process simulations using microbial growth media in place of actual drug products to verify sterility maintenance throughout the entire preparation workflow. Environmental monitoring during these simulations provides critical data on the system's capability to maintain required cleanliness classifications.
Computer system validation represents a particularly challenging aspect of robotic implementation, requiring verification of algorithm accuracy, system redundancies, and fail-safe mechanisms. Automated vision systems used for inspection and verification must undergo separate validation protocols to ensure they can reliably detect contamination, particulates, or preparation errors with statistical confidence. Process analytical technology (PAT) integration enables real-time monitoring and creates additional validation requirements but offers enhanced process control capabilities.
Regulatory bodies including FDA, EMA, and PIC/S have established specific expectations for validation of automated systems in GMP environments. These include requirements for data integrity, audit trails, and electronic records compliance under 21 CFR Part 11 and Annex 11 guidelines. Validation master plans should incorporate these regulatory expectations while establishing clear acceptance criteria for each qualification stage.
Continuous process verification represents an evolution beyond traditional validation approaches, enabling ongoing assessment of robotic system performance through statistical process control methods. This approach allows for early detection of process drift and provides documented evidence of maintained system capability throughout the operational lifecycle, supporting both quality assurance and regulatory compliance objectives.
Risk assessment for robotic aseptic handling systems should follow a systematic approach beginning with hazard identification across all process steps. This includes evaluating potential failure points in robotic arm movements, sterile barrier maintenance, material transfer operations, and human-robot interaction zones. Quantitative risk scoring methodologies that consider severity, probability, and detectability factors provide objective measures for prioritizing mitigation strategies and establishing appropriate control measures.
Validation methodologies for aseptic robotic systems must address both hardware and software components through Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) protocols. These should be supplemented with aseptic process simulations using microbial growth media in place of actual drug products to verify sterility maintenance throughout the entire preparation workflow. Environmental monitoring during these simulations provides critical data on the system's capability to maintain required cleanliness classifications.
Computer system validation represents a particularly challenging aspect of robotic implementation, requiring verification of algorithm accuracy, system redundancies, and fail-safe mechanisms. Automated vision systems used for inspection and verification must undergo separate validation protocols to ensure they can reliably detect contamination, particulates, or preparation errors with statistical confidence. Process analytical technology (PAT) integration enables real-time monitoring and creates additional validation requirements but offers enhanced process control capabilities.
Regulatory bodies including FDA, EMA, and PIC/S have established specific expectations for validation of automated systems in GMP environments. These include requirements for data integrity, audit trails, and electronic records compliance under 21 CFR Part 11 and Annex 11 guidelines. Validation master plans should incorporate these regulatory expectations while establishing clear acceptance criteria for each qualification stage.
Continuous process verification represents an evolution beyond traditional validation approaches, enabling ongoing assessment of robotic system performance through statistical process control methods. This approach allows for early detection of process drift and provides documented evidence of maintained system capability throughout the operational lifecycle, supporting both quality assurance and regulatory compliance objectives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!