Quality-by-design approaches for mRNA manufacturing to control capping, tailing and sequence integrity
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Manufacturing Evolution and QbD Objectives
The evolution of mRNA manufacturing has undergone significant transformation since its inception in the early 1990s. Initially, mRNA production was limited to small-scale laboratory settings primarily for research purposes. The manufacturing process was characterized by inconsistent quality, low yields, and limited scalability. The breakthrough came in the mid-2000s when improved in vitro transcription (IVT) methods enabled more efficient production of functional mRNA molecules.
The COVID-19 pandemic served as a catalyst for unprecedented advancement in mRNA manufacturing technologies. The urgent need for billions of vaccine doses accelerated the development of large-scale production capabilities, pushing the industry to overcome critical challenges in purification, stability, and quality control. This period saw the emergence of continuous manufacturing processes and automated systems that significantly enhanced production efficiency and consistency.
Current mRNA manufacturing involves a complex multi-step process including plasmid preparation, linearization, IVT, purification, and formulation. Each step presents unique challenges for maintaining product quality and consistency. The industry has gradually shifted from a traditional quality-by-testing approach to a more proactive quality-by-design (QbD) methodology, recognizing the limitations of end-product testing for such complex biologics.
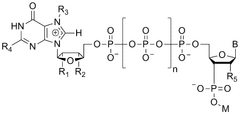
Quality-by-design represents a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding based on sound science and quality risk management. For mRNA manufacturing, QbD objectives specifically target three critical quality attributes: capping efficiency, poly(A) tail integrity, and sequence fidelity.
Efficient 5' capping is essential for mRNA stability, translation efficiency, and reduced immunogenicity. QbD approaches aim to optimize capping reactions and implement analytical methods that can accurately quantify capping efficiency. Similarly, controlling poly(A) tail length and homogeneity is crucial as these parameters directly impact mRNA half-life and protein expression levels.
Sequence integrity presents perhaps the most significant challenge, as even minor nucleotide modifications or truncations can affect functionality or safety. QbD objectives include developing robust methods to detect and minimize sequence variants throughout the manufacturing process, implementing in-process controls, and establishing appropriate specifications based on clinical relevance.
The ultimate goal of QbD implementation in mRNA manufacturing is to establish a design space within which process parameters can be adjusted while consistently delivering product meeting predefined quality attributes. This approach enables more flexible manufacturing, facilitates continuous improvement, and supports regulatory compliance while ensuring patient safety and product efficacy.
The COVID-19 pandemic served as a catalyst for unprecedented advancement in mRNA manufacturing technologies. The urgent need for billions of vaccine doses accelerated the development of large-scale production capabilities, pushing the industry to overcome critical challenges in purification, stability, and quality control. This period saw the emergence of continuous manufacturing processes and automated systems that significantly enhanced production efficiency and consistency.
Current mRNA manufacturing involves a complex multi-step process including plasmid preparation, linearization, IVT, purification, and formulation. Each step presents unique challenges for maintaining product quality and consistency. The industry has gradually shifted from a traditional quality-by-testing approach to a more proactive quality-by-design (QbD) methodology, recognizing the limitations of end-product testing for such complex biologics.
Quality-by-design represents a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding based on sound science and quality risk management. For mRNA manufacturing, QbD objectives specifically target three critical quality attributes: capping efficiency, poly(A) tail integrity, and sequence fidelity.
Efficient 5' capping is essential for mRNA stability, translation efficiency, and reduced immunogenicity. QbD approaches aim to optimize capping reactions and implement analytical methods that can accurately quantify capping efficiency. Similarly, controlling poly(A) tail length and homogeneity is crucial as these parameters directly impact mRNA half-life and protein expression levels.
Sequence integrity presents perhaps the most significant challenge, as even minor nucleotide modifications or truncations can affect functionality or safety. QbD objectives include developing robust methods to detect and minimize sequence variants throughout the manufacturing process, implementing in-process controls, and establishing appropriate specifications based on clinical relevance.
The ultimate goal of QbD implementation in mRNA manufacturing is to establish a design space within which process parameters can be adjusted while consistently delivering product meeting predefined quality attributes. This approach enables more flexible manufacturing, facilitates continuous improvement, and supports regulatory compliance while ensuring patient safety and product efficacy.
Market Analysis for High-Quality mRNA Therapeutics
The mRNA therapeutics market has experienced unprecedented growth since the successful deployment of COVID-19 vaccines, catalyzing significant investment in this sector. Current market valuations place the global mRNA therapeutics market at approximately $46.7 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 12.8% through 2030. This robust growth trajectory is underpinned by expanding applications beyond vaccines into areas such as cancer immunotherapy, protein replacement therapies, and genetic disease treatments.
Quality considerations have emerged as a critical market differentiator, with pharmaceutical companies increasingly recognizing that manufacturing excellence directly correlates with clinical efficacy and safety profiles. Market research indicates that products demonstrating superior capping efficiency, poly(A) tail consistency, and sequence integrity command premium pricing positions, typically 15-20% above standard offerings.
Demand signals from key market segments reveal distinct quality requirements. The therapeutic vaccine sector prioritizes sequence integrity to ensure precise antigen expression, while protein replacement therapies emphasize complete capping to maximize translation efficiency. Rare disease applications demand exceptional batch-to-batch consistency across all quality parameters to ensure reliable dosing in vulnerable patient populations.
Regional market analysis shows North America maintaining leadership with approximately 42% market share, followed by Europe at 31% and Asia-Pacific as the fastest-growing region with 18% annual growth. Regulatory frameworks are evolving rapidly, with the FDA and EMA implementing specialized guidance for mRNA product quality assessment, creating market entry barriers that favor organizations with sophisticated quality control capabilities.
Competitive landscape assessment identifies three distinct market tiers: established pharmaceutical giants investing heavily in proprietary manufacturing platforms, specialized mRNA-focused biotechnology companies developing novel quality control technologies, and contract manufacturing organizations (CMOs) positioning themselves as quality-assured production partners. Market concentration remains moderate with the top five players controlling approximately 68% of market share.
Customer willingness-to-pay analysis demonstrates that healthcare payers and providers increasingly factor manufacturing quality metrics into reimbursement and procurement decisions, with documented evidence of superior capping efficiency and sequence integrity serving as key value drivers. This trend is particularly pronounced in markets with sophisticated health technology assessment frameworks.
Future market projections indicate that quality-differentiated mRNA products will capture increasing market share, with an estimated 75% of new product approvals by 2028 incorporating advanced quality-by-design manufacturing approaches. The emergence of personalized mRNA therapeutics will further elevate quality considerations as batch sizes decrease and manufacturing precision requirements intensify.
Quality considerations have emerged as a critical market differentiator, with pharmaceutical companies increasingly recognizing that manufacturing excellence directly correlates with clinical efficacy and safety profiles. Market research indicates that products demonstrating superior capping efficiency, poly(A) tail consistency, and sequence integrity command premium pricing positions, typically 15-20% above standard offerings.
Demand signals from key market segments reveal distinct quality requirements. The therapeutic vaccine sector prioritizes sequence integrity to ensure precise antigen expression, while protein replacement therapies emphasize complete capping to maximize translation efficiency. Rare disease applications demand exceptional batch-to-batch consistency across all quality parameters to ensure reliable dosing in vulnerable patient populations.
Regional market analysis shows North America maintaining leadership with approximately 42% market share, followed by Europe at 31% and Asia-Pacific as the fastest-growing region with 18% annual growth. Regulatory frameworks are evolving rapidly, with the FDA and EMA implementing specialized guidance for mRNA product quality assessment, creating market entry barriers that favor organizations with sophisticated quality control capabilities.
Competitive landscape assessment identifies three distinct market tiers: established pharmaceutical giants investing heavily in proprietary manufacturing platforms, specialized mRNA-focused biotechnology companies developing novel quality control technologies, and contract manufacturing organizations (CMOs) positioning themselves as quality-assured production partners. Market concentration remains moderate with the top five players controlling approximately 68% of market share.
Customer willingness-to-pay analysis demonstrates that healthcare payers and providers increasingly factor manufacturing quality metrics into reimbursement and procurement decisions, with documented evidence of superior capping efficiency and sequence integrity serving as key value drivers. This trend is particularly pronounced in markets with sophisticated health technology assessment frameworks.
Future market projections indicate that quality-differentiated mRNA products will capture increasing market share, with an estimated 75% of new product approvals by 2028 incorporating advanced quality-by-design manufacturing approaches. The emergence of personalized mRNA therapeutics will further elevate quality considerations as batch sizes decrease and manufacturing precision requirements intensify.
Current Challenges in mRNA Capping, Tailing and Sequence Integrity
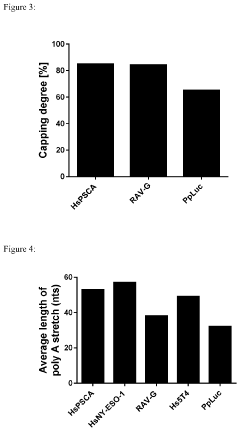
Despite significant advancements in mRNA technology, manufacturing processes face substantial challenges in ensuring the quality and integrity of critical structural elements. The capping process, essential for mRNA stability and translation efficiency, continues to present technical difficulties in achieving complete and proper cap formation. Current manufacturing methods struggle to consistently achieve capping efficiencies above 80%, with the remaining uncapped or improperly capped mRNAs potentially triggering immune responses or exhibiting reduced functionality.
Poly(A) tailing represents another critical challenge area, where controlling the precise length and homogeneity of poly(A) tails remains problematic. Conventional enzymatic methods using poly(A) polymerase often produce heterogeneous tail lengths, ranging from 50 to 250 nucleotides. This variability significantly impacts mRNA stability, translation efficiency, and ultimately, protein expression levels in target cells. The industry lacks standardized approaches to consistently produce uniform tail lengths optimized for specific therapeutic applications.
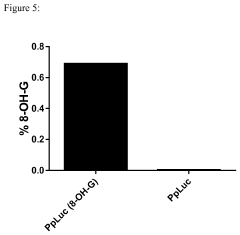
Sequence integrity preservation throughout the manufacturing process presents multifaceted challenges. RNA is inherently unstable and susceptible to degradation by ubiquitous RNases, requiring stringent manufacturing conditions. Furthermore, the occurrence of sequence errors during in vitro transcription, particularly with longer mRNA constructs, can introduce point mutations or deletions that compromise therapeutic efficacy or safety. Current quality control methods struggle to detect these subtle sequence variations at scale.
Contaminant control represents a persistent manufacturing challenge, with residual DNA templates, aberrant RNA species, and process-related impurities potentially triggering immunogenicity. The industry lacks standardized analytical methods capable of detecting and quantifying these impurities at the sensitivity levels required for therapeutic applications. This analytical gap hampers the implementation of robust quality control frameworks.
The scalability of high-quality manufacturing processes presents additional complications. Methods that work effectively at laboratory scale often encounter significant challenges when scaled to commercial production volumes. Maintaining consistent capping efficiency, poly(A) tail homogeneity, and sequence integrity becomes increasingly difficult with larger batch sizes, creating bottlenecks in manufacturing capacity.
Regulatory frameworks for mRNA quality standards remain in development, with evolving expectations regarding acceptable levels of impurities, structural variants, and process controls. This regulatory uncertainty complicates the establishment of validated manufacturing processes and quality control strategies that can reliably ensure product consistency across multiple production batches.
Poly(A) tailing represents another critical challenge area, where controlling the precise length and homogeneity of poly(A) tails remains problematic. Conventional enzymatic methods using poly(A) polymerase often produce heterogeneous tail lengths, ranging from 50 to 250 nucleotides. This variability significantly impacts mRNA stability, translation efficiency, and ultimately, protein expression levels in target cells. The industry lacks standardized approaches to consistently produce uniform tail lengths optimized for specific therapeutic applications.
Sequence integrity preservation throughout the manufacturing process presents multifaceted challenges. RNA is inherently unstable and susceptible to degradation by ubiquitous RNases, requiring stringent manufacturing conditions. Furthermore, the occurrence of sequence errors during in vitro transcription, particularly with longer mRNA constructs, can introduce point mutations or deletions that compromise therapeutic efficacy or safety. Current quality control methods struggle to detect these subtle sequence variations at scale.
Contaminant control represents a persistent manufacturing challenge, with residual DNA templates, aberrant RNA species, and process-related impurities potentially triggering immunogenicity. The industry lacks standardized analytical methods capable of detecting and quantifying these impurities at the sensitivity levels required for therapeutic applications. This analytical gap hampers the implementation of robust quality control frameworks.
The scalability of high-quality manufacturing processes presents additional complications. Methods that work effectively at laboratory scale often encounter significant challenges when scaled to commercial production volumes. Maintaining consistent capping efficiency, poly(A) tail homogeneity, and sequence integrity becomes increasingly difficult with larger batch sizes, creating bottlenecks in manufacturing capacity.
Regulatory frameworks for mRNA quality standards remain in development, with evolving expectations regarding acceptable levels of impurities, structural variants, and process controls. This regulatory uncertainty complicates the establishment of validated manufacturing processes and quality control strategies that can reliably ensure product consistency across multiple production batches.
Established QbD Methodologies for mRNA Production
01 mRNA capping technologies
Various enzymatic and chemical methods are employed for adding 5' cap structures to mRNA during manufacturing. These caps are crucial for mRNA stability, translation efficiency, and protection against exonucleases. Advanced capping technologies include co-transcriptional capping, post-transcriptional capping using cap analogs, and enzymatic capping systems that ensure high capping efficiency. These methods help maintain the structural integrity of mRNA and improve its functionality in therapeutic applications.- mRNA capping technologies for improved stability: Various capping technologies are employed in mRNA manufacturing to enhance stability and translation efficiency. These include co-transcriptional capping using cap analogs, post-transcriptional capping with capping enzymes, and the development of novel cap structures that provide protection against decapping enzymes. Proper capping is essential for mRNA stability, recognition by translation machinery, and protection against exonucleases, ultimately improving the efficacy of mRNA-based therapeutics.
- Poly(A) tailing methods for mRNA functionality: Poly(A) tailing is critical for mRNA functionality, influencing stability, translation efficiency, and nuclear export. Various methods are employed for adding poly(A) tails, including enzymatic polyadenylation using poly(A) polymerase, incorporation of poly(A) sequences during template preparation, and chemical synthesis approaches. The length and quality of the poly(A) tail significantly impact mRNA half-life and protein expression levels in therapeutic applications.
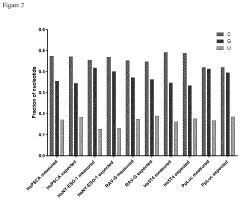
- Sequence integrity verification and quality control: Maintaining sequence integrity throughout the mRNA manufacturing process is crucial for safety and efficacy. Advanced analytical methods are employed for quality control, including next-generation sequencing, mass spectrometry, and chromatographic techniques. These methods help detect sequence errors, truncations, and modifications that could affect protein expression or trigger immunogenic responses. Comprehensive quality control strategies ensure batch-to-batch consistency and regulatory compliance for mRNA therapeutics.
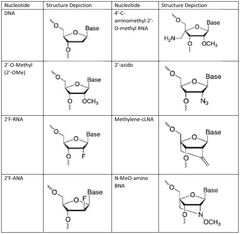
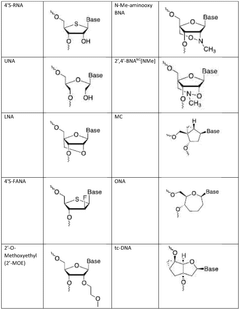
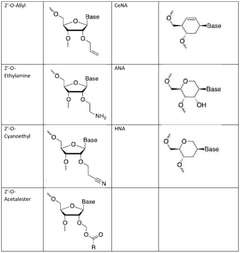
- Enzymatic and chemical modification strategies: Various enzymatic and chemical modification strategies are employed to enhance mRNA stability and reduce immunogenicity. These include incorporation of modified nucleosides such as pseudouridine and 5-methylcytidine, optimization of codon usage, and structural modifications to the 5' and 3' untranslated regions. These modifications help evade innate immune responses, improve translation efficiency, and extend the half-life of therapeutic mRNA molecules.
- Manufacturing process optimization for integrity preservation: Optimizing manufacturing processes is essential for preserving mRNA integrity throughout production. This includes developing controlled in vitro transcription conditions, implementing purification strategies that minimize degradation, and establishing appropriate storage and formulation methods. Process parameters such as temperature, pH, buffer composition, and the presence of RNase inhibitors are carefully controlled to prevent degradation and maintain the structural integrity of mRNA from synthesis through final formulation.
02 Poly(A) tailing strategies
Poly(A) tailing is essential for mRNA stability and translation efficiency. Manufacturing processes incorporate various approaches for adding poly(A) tails, including enzymatic polyadenylation using poly(A) polymerases, template-encoded poly(A) tails, and chemical synthesis methods. The length and quality of the poly(A) tail significantly impact mRNA half-life and protein expression levels. Optimized tailing processes ensure consistent tail length distribution and improve overall mRNA performance.Expand Specific Solutions03 Sequence integrity verification methods
Maintaining sequence integrity throughout the mRNA manufacturing process is critical for safety and efficacy. Advanced analytical techniques are employed to verify sequence fidelity, including next-generation sequencing, mass spectrometry, and specialized PCR methods. These techniques can detect point mutations, deletions, insertions, and other sequence alterations that might occur during synthesis or processing. Quality control protocols incorporate multiple orthogonal methods to ensure comprehensive sequence verification before release of the final mRNA product.Expand Specific Solutions04 Integrated manufacturing platforms
Integrated manufacturing platforms combine capping, tailing, and sequence verification into streamlined processes. These platforms utilize automated systems, microfluidic technologies, and continuous processing to improve efficiency and reduce contamination risks. The integration of multiple steps allows for real-time quality monitoring and reduces batch-to-batch variability. Advanced platforms incorporate in-line analytics and process analytical technology (PAT) to ensure consistent quality attributes throughout the manufacturing process.Expand Specific Solutions05 Purification and stability enhancement
Specialized purification techniques are essential for removing impurities and ensuring the integrity of capped and tailed mRNA. Chromatographic methods, tangential flow filtration, and precipitation techniques are optimized to preserve the structural features of mRNA while removing process-related impurities. Stability enhancement strategies include formulation with lipids, polymers, and other excipients that protect the mRNA structure. These approaches extend shelf-life and maintain the functional integrity of the mRNA during storage and delivery.Expand Specific Solutions
Leading Companies in mRNA Manufacturing and QbD Implementation
The mRNA manufacturing quality-by-design landscape is currently in a growth phase, with the market expanding rapidly following COVID-19 vaccine successes. Technical maturity varies significantly among key players, with established companies like Moderna, CureVac, and Translate Bio leading innovation in capping, tailing, and sequence integrity control. Emerging competitors including Quantoom Biosciences, Cisterna Biologics, and Regis Biotechnology are developing proprietary technologies to address manufacturing challenges. Chinese companies such as Nanjing Genscript and Jiangsu Synthgene are increasingly competitive in providing cost-effective solutions. The industry is characterized by strategic partnerships between technology developers and pharmaceutical giants like Sanofi and Daiichi Sankyo, indicating the technology's transition toward standardization while still requiring significant optimization.
CureVac SE
Technical Solution: CureVac has pioneered a differentiated approach to mRNA manufacturing quality control through their RNAoptimizer® platform. Their technology focuses on sequence engineering and manufacturing process optimization to enhance mRNA stability and translation efficiency. For capping, CureVac employs a proprietary co-transcriptional capping method that achieves over 90% capping efficiency while minimizing cap analog incorporation errors[1]. Their approach includes the use of specially designed cap analogs that promote correct orientation during incorporation. For controlling poly(A) tail length, CureVac utilizes a DNA template-encoded approach rather than enzymatic polyadenylation, which provides more consistent tail lengths between 120-150 nucleotides[2]. To ensure sequence integrity, they implement a comprehensive nucleotide purification system prior to in vitro transcription and utilize modified nucleotides that reduce immunogenicity while enhancing stability. CureVac's manufacturing process incorporates multiple in-process controls including capillary electrophoresis, HPLC analysis, and mass spectrometry to monitor critical quality attributes throughout production[3]. Their QbD approach includes design space mapping for critical process parameters affecting capping efficiency and sequence integrity.
Strengths: Proprietary RNActive® technology enhances stability at standard refrigeration temperatures; extensive experience with unmodified mRNA platforms; manufacturing process requires less specialized equipment. Weaknesses: Clinical efficacy results have been mixed compared to competitors; manufacturing scale-up challenges have affected consistent quality control; relatively lower translation efficiency compared to some competitor platforms.
Nanjing Genscript Biotechnology Co., Ltd.
Technical Solution: GenScript has developed a comprehensive mRNA manufacturing platform with integrated quality control systems specifically designed to ensure capping efficiency, tailing consistency, and sequence integrity. Their approach incorporates a systematic QbD framework that begins with raw material qualification and extends through final product testing. For 5' capping, GenScript employs both post-transcriptional and co-transcriptional capping strategies, with their proprietary GenCap™ technology achieving capping efficiencies of 92-98%[1]. Their process includes specialized reaction conditions that minimize the formation of undesired cap structures and ensure correct orientation. For poly(A) tailing, they utilize a hybrid approach combining template-encoded tails with enzymatic polyadenylation refinement, allowing precise control of tail length between 100-150 nucleotides with minimal heterogeneity[2]. To ensure sequence integrity, GenScript implements a multi-tiered purification strategy including chromatographic methods and tangential flow filtration, coupled with comprehensive analytics including next-generation sequencing, capillary electrophoresis, and LC-MS to verify sequence accuracy and identify potential modifications. Their manufacturing process incorporates real-time monitoring systems with defined acceptance criteria for critical quality attributes at each production stage.
Strengths: Comprehensive contract development and manufacturing capabilities; integrated platform from gene synthesis to final mRNA production; competitive pricing structure for commercial scale production. Weaknesses: Less clinical validation data compared to leading pharmaceutical companies; manufacturing capacity limitations for large-scale commercial production; relatively newer entrant to therapeutic mRNA manufacturing.
Critical Quality Attributes and Process Parameters for mRNA Integrity
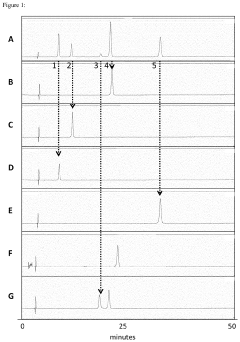
Methods of simultaneously identifying or quantifying capping and tailing modifications of messenger RNA
PatentWO2024256674A1
Innovation
- A multi-attribute method using liquid chromatography with UV detection (LC-UV), liquid chromatography coupled to mass spectrometry (LC-MS), or liquid chromatography coupled with UV and mass spectrometry (LC-UV-MS) to simultaneously identify and quantify mRNA capping and tailing modifications, including poly A tail length and polydispersity, in a single sample, employing oligonucleotide probes and nucleases to cleave RNA into cap and tail fragments for analysis.
RNA analysis by total hydrolysis and quantification of released nucleosides
PatentActiveUS11920174B2
Innovation
- A method involving total hydrolysis of RNA followed by HPLC detection, where hydrolysates are analyzed to determine RNA identity, integrity, capping efficiency, polyadenylation, oxidation status, and incorporation of modified nucleotides, using specific nucleosides and cap analogues to quantify and calculate these parameters.
Regulatory Framework for mRNA-Based Therapeutics
The regulatory landscape for mRNA-based therapeutics has evolved significantly in response to the rapid advancement of this technology, particularly accelerated by the COVID-19 pandemic. Regulatory agencies worldwide, including the FDA, EMA, and NMPA, have established frameworks that specifically address the unique challenges associated with mRNA manufacturing quality control, with particular emphasis on capping, tailing, and sequence integrity.
The FDA's approach incorporates Quality-by-Design (QbD) principles through its Chemistry, Manufacturing, and Controls (CMC) guidelines, which require manufacturers to demonstrate thorough understanding of critical quality attributes (CQAs) for mRNA products. These guidelines specifically address the importance of 5' capping efficiency, poly(A) tail consistency, and sequence fidelity as key determinants of product safety and efficacy.
Similarly, the European Medicines Agency has developed the Advanced Therapy Medicinal Products (ATMP) framework, which includes specific provisions for mRNA therapeutics. The EMA emphasizes process validation and analytical method development to ensure consistent capping rates above 95%, appropriate poly(A) tail length distribution, and minimal sequence variants in the final product.
International Conference on Harmonisation (ICH) guidelines, particularly ICH Q8-Q11, provide the foundational quality management principles that regulatory agencies expect manufacturers to implement. These guidelines emphasize the importance of risk assessment throughout the manufacturing process, with special attention to the enzymatic reactions involved in capping and tailing.
Regulatory requirements typically mandate comprehensive characterization of mRNA products using orthogonal analytical methods. For capping analysis, manufacturers must employ techniques such as HPLC, mass spectrometry, and enzymatic assays to quantify capping efficiency. Poly(A) tail characterization requires techniques like electrophoresis, sequencing, and specialized PCR methods to verify length distribution.
Sequence integrity verification has become increasingly stringent, with regulatory bodies requiring next-generation sequencing (NGS) data to identify and quantify potential sequence variants. Acceptance criteria typically limit sequence variants to less than 1% of the total mRNA population, with specific mutations in coding regions facing even stricter limitations.
Recent regulatory trends indicate movement toward continuous manufacturing processes with real-time monitoring capabilities, allowing for immediate detection and correction of quality deviations in capping, tailing, and sequence parameters. This approach aligns with the QbD philosophy of building quality into the process rather than testing it into the final product.
The FDA's approach incorporates Quality-by-Design (QbD) principles through its Chemistry, Manufacturing, and Controls (CMC) guidelines, which require manufacturers to demonstrate thorough understanding of critical quality attributes (CQAs) for mRNA products. These guidelines specifically address the importance of 5' capping efficiency, poly(A) tail consistency, and sequence fidelity as key determinants of product safety and efficacy.
Similarly, the European Medicines Agency has developed the Advanced Therapy Medicinal Products (ATMP) framework, which includes specific provisions for mRNA therapeutics. The EMA emphasizes process validation and analytical method development to ensure consistent capping rates above 95%, appropriate poly(A) tail length distribution, and minimal sequence variants in the final product.
International Conference on Harmonisation (ICH) guidelines, particularly ICH Q8-Q11, provide the foundational quality management principles that regulatory agencies expect manufacturers to implement. These guidelines emphasize the importance of risk assessment throughout the manufacturing process, with special attention to the enzymatic reactions involved in capping and tailing.
Regulatory requirements typically mandate comprehensive characterization of mRNA products using orthogonal analytical methods. For capping analysis, manufacturers must employ techniques such as HPLC, mass spectrometry, and enzymatic assays to quantify capping efficiency. Poly(A) tail characterization requires techniques like electrophoresis, sequencing, and specialized PCR methods to verify length distribution.
Sequence integrity verification has become increasingly stringent, with regulatory bodies requiring next-generation sequencing (NGS) data to identify and quantify potential sequence variants. Acceptance criteria typically limit sequence variants to less than 1% of the total mRNA population, with specific mutations in coding regions facing even stricter limitations.
Recent regulatory trends indicate movement toward continuous manufacturing processes with real-time monitoring capabilities, allowing for immediate detection and correction of quality deviations in capping, tailing, and sequence parameters. This approach aligns with the QbD philosophy of building quality into the process rather than testing it into the final product.
Scalability Challenges in Quality-Controlled mRNA Production
The scaling of mRNA production while maintaining stringent quality control presents significant challenges for the biopharmaceutical industry. As mRNA therapeutics transition from clinical trials to commercial manufacturing, production volumes must increase by several orders of magnitude without compromising product quality attributes such as capping efficiency, poly(A) tail consistency, and sequence integrity.
Current manufacturing processes typically operate at laboratory or small pilot scales, utilizing batch processing methods that become increasingly inefficient at larger volumes. The enzymatic reactions central to mRNA synthesis—including in vitro transcription, capping, and polyadenylation—exhibit different kinetics and efficiency profiles when scaled up, often resulting in reduced yields and inconsistent quality parameters.
Equipment limitations further compound these challenges. Specialized bioreactors designed for optimal mixing conditions and temperature control during enzymatic reactions are not readily available at commercial scales. Additionally, the purification processes involving chromatography and tangential flow filtration require significant adaptation when transitioning from laboratory to industrial scale, as flow dynamics and separation efficiencies change substantially.
Quality testing represents another critical bottleneck. Current analytical methods for assessing capping efficiency, poly(A) tail length distribution, and sequence integrity are often time-consuming and difficult to implement as in-process controls. This creates delays between production and release, hampering manufacturing throughput and responsiveness to market demands.
Raw material supply chains present additional complexity. The enzymes, nucleotides, and other components required for mRNA production must be available in significantly larger quantities while maintaining consistent quality. Many of these materials currently have limited suppliers and production capacity, creating potential bottlenecks for large-scale manufacturing.
Regulatory considerations also impact scalability. As production volumes increase, regulatory agencies require more robust demonstration of process consistency and product comparability. Implementing quality-by-design approaches becomes more challenging at larger scales, where process parameters may have different impacts on critical quality attributes compared to small-scale operations.
Addressing these scalability challenges requires innovative approaches, including continuous manufacturing technologies, automated in-line quality monitoring systems, and novel purification strategies. Development of scalable analytical methods that can rapidly assess critical quality attributes will be essential for enabling real-time release testing and improving manufacturing efficiency while ensuring consistent product quality.
Current manufacturing processes typically operate at laboratory or small pilot scales, utilizing batch processing methods that become increasingly inefficient at larger volumes. The enzymatic reactions central to mRNA synthesis—including in vitro transcription, capping, and polyadenylation—exhibit different kinetics and efficiency profiles when scaled up, often resulting in reduced yields and inconsistent quality parameters.
Equipment limitations further compound these challenges. Specialized bioreactors designed for optimal mixing conditions and temperature control during enzymatic reactions are not readily available at commercial scales. Additionally, the purification processes involving chromatography and tangential flow filtration require significant adaptation when transitioning from laboratory to industrial scale, as flow dynamics and separation efficiencies change substantially.
Quality testing represents another critical bottleneck. Current analytical methods for assessing capping efficiency, poly(A) tail length distribution, and sequence integrity are often time-consuming and difficult to implement as in-process controls. This creates delays between production and release, hampering manufacturing throughput and responsiveness to market demands.
Raw material supply chains present additional complexity. The enzymes, nucleotides, and other components required for mRNA production must be available in significantly larger quantities while maintaining consistent quality. Many of these materials currently have limited suppliers and production capacity, creating potential bottlenecks for large-scale manufacturing.
Regulatory considerations also impact scalability. As production volumes increase, regulatory agencies require more robust demonstration of process consistency and product comparability. Implementing quality-by-design approaches becomes more challenging at larger scales, where process parameters may have different impacts on critical quality attributes compared to small-scale operations.
Addressing these scalability challenges requires innovative approaches, including continuous manufacturing technologies, automated in-line quality monitoring systems, and novel purification strategies. Development of scalable analytical methods that can rapidly assess critical quality attributes will be essential for enabling real-time release testing and improving manufacturing efficiency while ensuring consistent product quality.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!