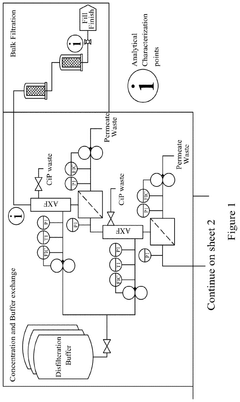
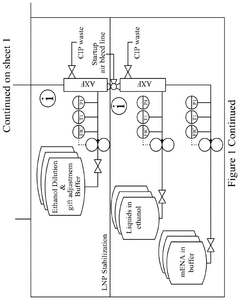
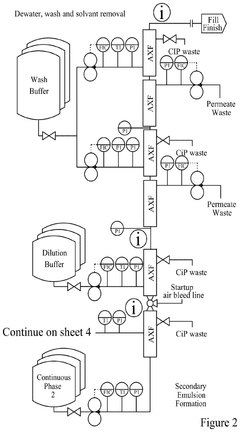
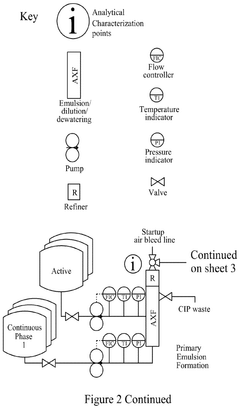
Continuous manufacturing of LNPs: impact of solvent removal and in-line dilution on product quality
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LNP Continuous Manufacturing Background and Objectives
Lipid nanoparticles (LNPs) have emerged as revolutionary delivery vehicles for nucleic acid therapeutics, most notably demonstrated in the rapid development and deployment of mRNA-based COVID-19 vaccines. The traditional batch manufacturing process for LNPs, while effective for clinical trials and initial commercial production, faces significant challenges in scaling to meet global demand and ensuring consistent product quality across batches.
Continuous manufacturing represents a paradigm shift in pharmaceutical production, offering advantages in process control, scalability, and product consistency. For LNPs specifically, continuous manufacturing promises to address critical limitations of batch processes, including variations in particle size distribution, encapsulation efficiency, and lipid composition that can significantly impact therapeutic efficacy and safety profiles.
The evolution of LNP manufacturing technology has progressed from rudimentary lipid film hydration methods to more sophisticated techniques such as microfluidic mixing. Each advancement has aimed to improve control over particle characteristics while increasing production capacity. The current technological frontier focuses on developing fully integrated continuous manufacturing platforms that maintain precise control over critical process parameters throughout the production cycle.
A particularly challenging aspect of LNP continuous manufacturing involves solvent removal and in-line dilution steps. These processes critically influence the final LNP structure, stability, and drug encapsulation efficiency. Traditional approaches often involve post-production batch processing for solvent exchange, creating bottlenecks that undermine the advantages of continuous manufacturing.
The primary objectives of advancing continuous LNP manufacturing technology include: establishing robust process analytical technology (PAT) for real-time quality monitoring; developing integrated solvent removal systems that maintain nanoparticle integrity; optimizing in-line dilution strategies to prevent particle aggregation or drug leakage; and designing scalable systems capable of commercial-level production while maintaining consistent product quality attributes.
Regulatory considerations further shape the technological landscape, with agencies increasingly encouraging continuous manufacturing adoption while requiring comprehensive validation of process control strategies. This regulatory environment drives innovation toward systems with enhanced monitoring capabilities and demonstrable process understanding.
The ultimate goal is to develop a fully continuous, end-to-end manufacturing platform for LNPs that maintains tight control over critical quality attributes while offering the flexibility to produce various LNP formulations for different therapeutic applications. Success in this endeavor would significantly reduce production costs, increase manufacturing capacity, and potentially accelerate the development of novel nucleic acid therapeutics beyond vaccines to include treatments for genetic disorders, cancer, and other conditions with significant unmet medical needs.
Continuous manufacturing represents a paradigm shift in pharmaceutical production, offering advantages in process control, scalability, and product consistency. For LNPs specifically, continuous manufacturing promises to address critical limitations of batch processes, including variations in particle size distribution, encapsulation efficiency, and lipid composition that can significantly impact therapeutic efficacy and safety profiles.
The evolution of LNP manufacturing technology has progressed from rudimentary lipid film hydration methods to more sophisticated techniques such as microfluidic mixing. Each advancement has aimed to improve control over particle characteristics while increasing production capacity. The current technological frontier focuses on developing fully integrated continuous manufacturing platforms that maintain precise control over critical process parameters throughout the production cycle.
A particularly challenging aspect of LNP continuous manufacturing involves solvent removal and in-line dilution steps. These processes critically influence the final LNP structure, stability, and drug encapsulation efficiency. Traditional approaches often involve post-production batch processing for solvent exchange, creating bottlenecks that undermine the advantages of continuous manufacturing.
The primary objectives of advancing continuous LNP manufacturing technology include: establishing robust process analytical technology (PAT) for real-time quality monitoring; developing integrated solvent removal systems that maintain nanoparticle integrity; optimizing in-line dilution strategies to prevent particle aggregation or drug leakage; and designing scalable systems capable of commercial-level production while maintaining consistent product quality attributes.
Regulatory considerations further shape the technological landscape, with agencies increasingly encouraging continuous manufacturing adoption while requiring comprehensive validation of process control strategies. This regulatory environment drives innovation toward systems with enhanced monitoring capabilities and demonstrable process understanding.
The ultimate goal is to develop a fully continuous, end-to-end manufacturing platform for LNPs that maintains tight control over critical quality attributes while offering the flexibility to produce various LNP formulations for different therapeutic applications. Success in this endeavor would significantly reduce production costs, increase manufacturing capacity, and potentially accelerate the development of novel nucleic acid therapeutics beyond vaccines to include treatments for genetic disorders, cancer, and other conditions with significant unmet medical needs.
Market Demand Analysis for Continuous LNP Production
The global market for lipid nanoparticle (LNP) delivery systems has experienced unprecedented growth following the successful deployment of mRNA-based COVID-19 vaccines. This surge has created substantial demand for continuous manufacturing processes that can ensure consistent LNP quality while meeting large-scale production requirements.
Current market analysis indicates that the LNP drug delivery market is projected to grow at a compound annual growth rate of 15.8% through 2028, driven primarily by the expanding pipeline of RNA therapeutics and vaccines. The successful commercialization of Pfizer-BioNTech and Moderna COVID-19 vaccines has validated LNP technology at an industrial scale, creating significant pull for optimized manufacturing solutions.
Pharmaceutical companies are increasingly seeking continuous manufacturing platforms for LNP production to address several critical market needs. First, batch-to-batch variability remains a significant challenge in traditional manufacturing methods, affecting product quality and regulatory compliance. Continuous processes offer better control over critical parameters such as mixing conditions, dilution rates, and solvent removal, resulting in more consistent particle size distribution and encapsulation efficiency.
Cost reduction represents another major market driver. Traditional batch manufacturing of LNPs involves significant capital investment and operational expenses. Industry reports suggest that continuous manufacturing can potentially reduce production costs by 20-30% through improved resource utilization, reduced labor requirements, and minimized waste generation.
Scalability concerns are particularly acute as RNA therapeutics move beyond vaccines into chronic disease treatments requiring larger and more consistent supply chains. Continuous manufacturing platforms that can seamlessly scale from clinical to commercial production without compromising product quality are highly sought after by pharmaceutical developers and contract manufacturing organizations.
Regulatory agencies, including the FDA and EMA, have expressed support for continuous manufacturing technologies, recognizing their potential to enhance product quality and manufacturing robustness. This regulatory encouragement has further stimulated market interest in continuous LNP production systems with integrated quality control capabilities.
The impact of solvent removal and in-line dilution on product quality has emerged as a critical focus area, as these processes directly affect LNP size, polydispersity, and drug encapsulation efficiency. Market research indicates that technologies enabling precise control of these parameters could command premium pricing, with pharmaceutical companies willing to invest in systems that demonstrably improve product consistency and reduce development timelines.
Regional analysis shows North America leading the demand for continuous LNP manufacturing technologies, followed by Europe and rapidly growing interest from Asia-Pacific markets, particularly China and South Korea, where significant investments in RNA therapeutics are underway.
Current market analysis indicates that the LNP drug delivery market is projected to grow at a compound annual growth rate of 15.8% through 2028, driven primarily by the expanding pipeline of RNA therapeutics and vaccines. The successful commercialization of Pfizer-BioNTech and Moderna COVID-19 vaccines has validated LNP technology at an industrial scale, creating significant pull for optimized manufacturing solutions.
Pharmaceutical companies are increasingly seeking continuous manufacturing platforms for LNP production to address several critical market needs. First, batch-to-batch variability remains a significant challenge in traditional manufacturing methods, affecting product quality and regulatory compliance. Continuous processes offer better control over critical parameters such as mixing conditions, dilution rates, and solvent removal, resulting in more consistent particle size distribution and encapsulation efficiency.
Cost reduction represents another major market driver. Traditional batch manufacturing of LNPs involves significant capital investment and operational expenses. Industry reports suggest that continuous manufacturing can potentially reduce production costs by 20-30% through improved resource utilization, reduced labor requirements, and minimized waste generation.
Scalability concerns are particularly acute as RNA therapeutics move beyond vaccines into chronic disease treatments requiring larger and more consistent supply chains. Continuous manufacturing platforms that can seamlessly scale from clinical to commercial production without compromising product quality are highly sought after by pharmaceutical developers and contract manufacturing organizations.
Regulatory agencies, including the FDA and EMA, have expressed support for continuous manufacturing technologies, recognizing their potential to enhance product quality and manufacturing robustness. This regulatory encouragement has further stimulated market interest in continuous LNP production systems with integrated quality control capabilities.
The impact of solvent removal and in-line dilution on product quality has emerged as a critical focus area, as these processes directly affect LNP size, polydispersity, and drug encapsulation efficiency. Market research indicates that technologies enabling precise control of these parameters could command premium pricing, with pharmaceutical companies willing to invest in systems that demonstrably improve product consistency and reduce development timelines.
Regional analysis shows North America leading the demand for continuous LNP manufacturing technologies, followed by Europe and rapidly growing interest from Asia-Pacific markets, particularly China and South Korea, where significant investments in RNA therapeutics are underway.
Technical Challenges in Solvent Removal for LNP Manufacturing
Solvent removal represents one of the most critical and challenging steps in the continuous manufacturing of Lipid Nanoparticles (LNPs). The process typically involves the rapid mixing of lipids dissolved in organic solvents with aqueous solutions containing nucleic acids, followed by the essential step of removing these organic solvents. This removal process directly impacts the final LNP structure, stability, encapsulation efficiency, and ultimately, therapeutic efficacy.
Current solvent removal techniques in LNP manufacturing face several significant technical hurdles. Conventional methods such as tangential flow filtration (TFF), dialysis, and hollow fiber filtration struggle to maintain consistent product quality when scaled up for continuous manufacturing. The primary challenge lies in achieving uniform solvent removal rates across the entire LNP suspension without causing particle aggregation or destabilization.
Temperature control during solvent removal presents another substantial challenge. Even minor temperature fluctuations can significantly alter lipid phase transitions, potentially leading to inconsistent particle size distribution and reduced encapsulation efficiency. Continuous manufacturing systems must incorporate precise temperature control mechanisms that can respond rapidly to process variations.
The kinetics of solvent removal also critically affects LNP formation. Too rapid removal can cause structural deformities and aggregation, while too slow removal may lead to drug leakage and reduced loading efficiency. Finding the optimal removal rate that balances these factors remains technically challenging, particularly in continuous flow systems where residence time must be carefully controlled.
Residual solvent levels present regulatory concerns, as organic solvents like ethanol are classified as Class 2 or 3 solvents by regulatory agencies. Continuous manufacturing systems must reliably reduce solvent concentrations below established safety thresholds while maintaining process efficiency. Current analytical methods for real-time monitoring of residual solvent levels lack the sensitivity and speed required for effective process control in continuous manufacturing settings.
Scale-up considerations further complicate solvent removal. As production volumes increase, maintaining uniform mixing conditions and consistent solvent removal rates becomes increasingly difficult. Heat transfer limitations, pressure drops, and flow pattern changes can all contribute to product variability at larger scales.
The integration of solvent removal with subsequent processing steps, particularly in-line dilution, introduces additional complexity. The transition between these steps must be seamlessly coordinated to prevent intermediate product degradation or quality issues. Current technologies often struggle to provide the necessary level of process integration and control required for consistent LNP quality in continuous manufacturing environments.
Current solvent removal techniques in LNP manufacturing face several significant technical hurdles. Conventional methods such as tangential flow filtration (TFF), dialysis, and hollow fiber filtration struggle to maintain consistent product quality when scaled up for continuous manufacturing. The primary challenge lies in achieving uniform solvent removal rates across the entire LNP suspension without causing particle aggregation or destabilization.
Temperature control during solvent removal presents another substantial challenge. Even minor temperature fluctuations can significantly alter lipid phase transitions, potentially leading to inconsistent particle size distribution and reduced encapsulation efficiency. Continuous manufacturing systems must incorporate precise temperature control mechanisms that can respond rapidly to process variations.
The kinetics of solvent removal also critically affects LNP formation. Too rapid removal can cause structural deformities and aggregation, while too slow removal may lead to drug leakage and reduced loading efficiency. Finding the optimal removal rate that balances these factors remains technically challenging, particularly in continuous flow systems where residence time must be carefully controlled.
Residual solvent levels present regulatory concerns, as organic solvents like ethanol are classified as Class 2 or 3 solvents by regulatory agencies. Continuous manufacturing systems must reliably reduce solvent concentrations below established safety thresholds while maintaining process efficiency. Current analytical methods for real-time monitoring of residual solvent levels lack the sensitivity and speed required for effective process control in continuous manufacturing settings.
Scale-up considerations further complicate solvent removal. As production volumes increase, maintaining uniform mixing conditions and consistent solvent removal rates becomes increasingly difficult. Heat transfer limitations, pressure drops, and flow pattern changes can all contribute to product variability at larger scales.
The integration of solvent removal with subsequent processing steps, particularly in-line dilution, introduces additional complexity. The transition between these steps must be seamlessly coordinated to prevent intermediate product degradation or quality issues. Current technologies often struggle to provide the necessary level of process integration and control required for consistent LNP quality in continuous manufacturing environments.
Current Solvent Removal and In-line Dilution Methodologies
01 Quality control methods for LNP characterization
Various analytical techniques are employed to characterize lipid nanoparticles and ensure product quality. These methods include dynamic light scattering for particle size determination, zeta potential measurements for surface charge analysis, high-performance liquid chromatography for lipid composition verification, and electron microscopy for morphological assessment. These characterization methods are critical for establishing consistent quality parameters and ensuring batch-to-batch reproducibility of LNP formulations.- Quality control methods for LNP characterization: Various analytical methods are employed to characterize and ensure the quality of lipid nanoparticles. These include dynamic light scattering for particle size determination, zeta potential measurements for surface charge analysis, high-performance liquid chromatography for lipid composition verification, and electron microscopy for morphological assessment. These techniques help maintain consistent quality standards and ensure batch-to-batch reproducibility of LNP formulations.
- Stability enhancement strategies for LNPs: Improving the stability of lipid nanoparticles is crucial for maintaining product quality during storage and administration. This involves optimizing lipid compositions, incorporating stabilizing agents such as antioxidants or cryoprotectants, controlling the pH and ionic strength of the formulation, and developing appropriate lyophilization processes. These strategies help prevent particle aggregation, lipid oxidation, and payload degradation, thereby extending shelf-life and preserving therapeutic efficacy.
- Manufacturing process optimization for LNP quality: The manufacturing process significantly impacts LNP quality attributes. Optimization strategies include precise control of mixing parameters during nanoprecipitation, standardization of extrusion conditions, implementation of microfluidic techniques for consistent particle formation, and development of scalable production methods. Process analytical technology (PAT) tools are integrated to monitor critical quality attributes in real-time, enabling adjustments during manufacturing to ensure consistent product quality.
- Encapsulation efficiency and payload integrity: Maintaining high encapsulation efficiency and protecting the integrity of therapeutic payloads are essential aspects of LNP product quality. This involves optimizing the lipid composition and N/P ratio for nucleic acid payloads, developing methods to prevent payload degradation during formulation, implementing purification techniques to remove unencapsulated material, and establishing analytical methods to accurately quantify encapsulation efficiency and payload stability over time.
- Regulatory considerations and quality standards for LNPs: Meeting regulatory requirements is crucial for LNP product development and commercialization. This includes establishing appropriate specifications for critical quality attributes, developing validated analytical methods for quality control testing, implementing good manufacturing practices (GMP) for production, conducting comprehensive stability studies, and preparing thorough documentation for regulatory submissions. International harmonization of quality standards helps ensure consistent evaluation of LNP products across different regulatory jurisdictions.
02 Stability enhancement strategies for LNPs
Maintaining the stability of lipid nanoparticles during storage and administration is crucial for product quality. Various approaches include optimizing lipid composition with stabilizing agents, lyophilization techniques with appropriate cryoprotectants, controlled storage conditions with specific temperature requirements, and packaging innovations that minimize exposure to degradation factors. These strategies help prevent particle aggregation, lipid oxidation, and payload degradation, thereby extending shelf-life and maintaining therapeutic efficacy.Expand Specific Solutions03 Manufacturing process optimization for LNP quality
The manufacturing process significantly impacts LNP quality attributes. Key considerations include microfluidic mixing parameters for consistent particle formation, purification techniques to remove process impurities, sterile filtration methods to ensure product safety, and scalable production processes that maintain quality during commercial manufacturing. Process analytical technology implementation enables real-time monitoring and control of critical quality attributes throughout the manufacturing process.Expand Specific Solutions04 Encapsulation efficiency and payload protection
The quality of LNP products is heavily dependent on efficient encapsulation and protection of therapeutic payloads such as mRNA or siRNA. Formulation strategies focus on optimizing lipid compositions to enhance nucleic acid complexation, preventing payload degradation during manufacturing and storage, maintaining appropriate pH conditions for payload stability, and ensuring consistent release kinetics at the target site. These factors directly impact the therapeutic efficacy and safety profile of LNP-based products.Expand Specific Solutions05 Regulatory considerations and quality standards for LNPs
Regulatory frameworks guide the quality requirements for lipid nanoparticle products. These include compliance with good manufacturing practices (GMP), implementation of quality-by-design principles during development, establishment of appropriate specifications and acceptance criteria for critical quality attributes, and validation of analytical methods for quality control testing. Comprehensive characterization data packages are required for regulatory submissions, addressing aspects such as impurity profiles, sterility assurance, and stability under various conditions.Expand Specific Solutions
Key Industry Players in Continuous LNP Manufacturing
The continuous manufacturing of Lipid Nanoparticles (LNPs) is currently in a transitional phase from early commercial adoption to broader implementation, with the market expected to grow significantly due to increased demand for mRNA therapeutics. The technology maturity varies across players, with pharmaceutical companies like BioNTech SE and Becton, Dickinson & Co. leading innovation following COVID-19 vaccine development. Academic institutions such as Zhejiang University of Technology and chemical manufacturers including BASF Corp. and Covestro Deutschland AG are contributing to solvent removal and in-line dilution advancements. Equipment providers like Malvern Panalytical Ltd. and Elemental Scientific, Inc. are developing analytical tools for quality control. The competitive landscape is characterized by cross-sector collaboration between pharmaceutical, chemical, and instrumentation companies to overcome technical challenges in continuous LNP manufacturing.
BASF Corp.
Technical Solution: BASF has developed an innovative continuous manufacturing platform for LNPs that leverages their extensive expertise in chemical processing and materials science. Their system utilizes a proprietary microreactor technology that enables precise control over mixing conditions during LNP formation, followed by a continuous solvent exchange process that gradually replaces organic solvents with aqueous buffer. This approach maintains consistent supersaturation conditions throughout the process, resulting in highly uniform particle size distributions (typically PDI < 0.15). BASF's platform incorporates advanced in-line dilution systems that use precision flow controllers to create carefully controlled concentration gradients, minimizing osmotic stress on forming LNPs. Their technology includes specialized surface-modified flow channels that reduce lipid adsorption during processing, addressing a common cause of yield loss in LNP manufacturing. The company has demonstrated that their continuous approach reduces solvent usage by approximately 40% compared to batch processes while maintaining equivalent or superior encapsulation efficiency. BASF has also developed customized computational fluid dynamics models that predict the effects of process parameters on LNP formation, enabling rapid process optimization for new formulations.
Strengths: Exceptional particle size uniformity, reduced solvent consumption, and sophisticated modeling capabilities for process optimization. The system's modular design allows for flexible configuration based on specific product requirements. Weaknesses: The specialized microreactor technology may have throughput limitations for very high-volume applications, and the system requires precise control of multiple interacting process variables.
BioNTech SE
Technical Solution: BioNTech SE has developed a proprietary microfluidic-based continuous manufacturing platform for lipid nanoparticles (LNPs) that addresses critical solvent removal challenges. Their system employs a controlled tangential flow filtration (TFF) process that gradually removes organic solvents while maintaining precise control over dilution rates. This approach enables real-time monitoring of particle size distribution and zeta potential during manufacturing. BioNTech's platform incorporates in-line dilution systems that automatically adjust buffer composition to maintain optimal ionic strength during the transition from organic to aqueous phase, which has been shown to significantly improve mRNA encapsulation efficiency (>90%) and reduce batch-to-batch variability to less than 5%. Their continuous manufacturing system includes integrated PAT (Process Analytical Technology) tools that provide feedback control loops to adjust critical process parameters in response to measured product attributes, ensuring consistent LNP quality even with variations in input materials.
Strengths: Superior encapsulation efficiency for mRNA therapeutics, reduced batch-to-batch variability, and integrated real-time monitoring capabilities. The system's automation reduces human error and contamination risks. Weaknesses: The complex control systems require specialized expertise to operate and maintain, and the platform may have limited flexibility for rapid formulation changes compared to batch processes.
Critical Process Parameters Affecting LNP Quality
Continuous process
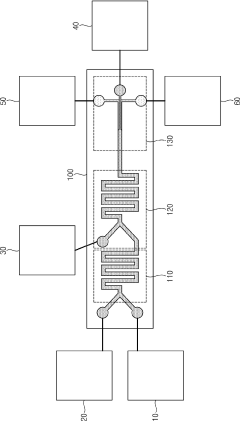
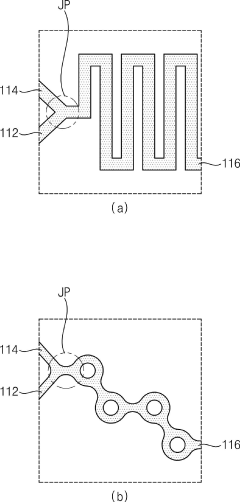
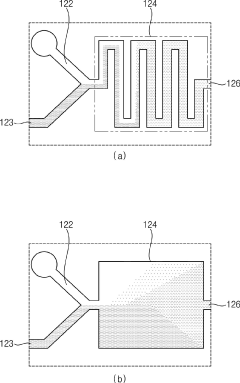
PatentPendingUS20250213480A1
Innovation
- A continuous process using membrane emulsification techniques, involving controlled provision of liquid phases through membranes with apertures, pH buffer adjustments, and optional concentration and microfiltration steps to produce uniform microparticles and nanoparticles.
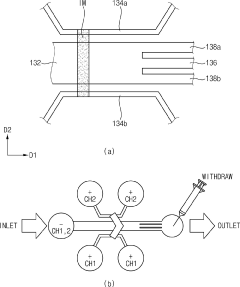
Lipid nanoparticles manufacturing Chip, Lipid nanoparticles manufacturing System having the same, and Lipid nanoparticles manufacturing method
PatentActiveKR1020220154039A
Innovation
- A chip and system for producing lipid nanoparticles that integrate mixing, dilution, and concentration units, utilizing ion exchange channels and voltage application to create ion-rich and ion-depleted zones for precise control over nanoparticle formation and separation, ensuring uniformity and stability.
Regulatory Considerations for Continuous LNP Manufacturing
The regulatory landscape for continuous manufacturing of Lipid Nanoparticles (LNPs) presents unique challenges and opportunities for pharmaceutical companies and regulatory bodies alike. The FDA and EMA have demonstrated increasing support for continuous manufacturing technologies through various guidance documents, recognizing their potential benefits in product consistency and quality assurance. However, specific regulatory frameworks for continuous LNP production remain in development.
Quality by Design (QbD) principles are particularly relevant for continuous LNP manufacturing processes. Regulatory agencies expect manufacturers to establish a thorough understanding of critical process parameters (CPPs) that affect critical quality attributes (CQAs). For LNP production, these include parameters related to solvent removal efficiency and in-line dilution rates, which directly impact particle size distribution, encapsulation efficiency, and stability.
Process Analytical Technology (PAT) implementation is a key regulatory expectation for continuous manufacturing. Real-time monitoring of solvent concentration during removal processes and dilution ratios must be validated to ensure consistent product quality. Regulatory submissions should demonstrate robust control strategies with appropriate feedback mechanisms to maintain process parameters within established design spaces.
Validation approaches for continuous LNP manufacturing differ significantly from traditional batch processes. Regulatory agencies require demonstration of state of control throughout extended production runs, with particular attention to start-up and shut-down transitions. The impact of process interruptions on product quality must be thoroughly characterized, especially regarding solvent removal efficiency and dilution homogeneity.
Scale-up considerations present unique regulatory challenges for continuous LNP manufacturing. Unlike traditional batch processes where scale-up follows established paradigms, continuous processes must demonstrate consistent product quality across different throughput rates. Regulatory submissions should include comprehensive comparability studies showing equivalent product quality attributes across various production scales and rates.
Global regulatory harmonization remains an evolving area for continuous LNP manufacturing. While ICH guidelines provide general frameworks for pharmaceutical development and manufacturing, specific guidance for continuous LNP production varies between regions. Companies pursuing global markets must develop regulatory strategies that address the nuanced expectations of different authorities regarding process validation, control strategies, and stability data requirements.
Regulatory risk assessment for continuous LNP manufacturing should focus on potential failure modes related to solvent removal and in-line dilution processes. Manufacturers must demonstrate robust risk mitigation strategies, including redundant monitoring systems and predetermined corrective actions for process deviations that could impact product quality or patient safety.
Quality by Design (QbD) principles are particularly relevant for continuous LNP manufacturing processes. Regulatory agencies expect manufacturers to establish a thorough understanding of critical process parameters (CPPs) that affect critical quality attributes (CQAs). For LNP production, these include parameters related to solvent removal efficiency and in-line dilution rates, which directly impact particle size distribution, encapsulation efficiency, and stability.
Process Analytical Technology (PAT) implementation is a key regulatory expectation for continuous manufacturing. Real-time monitoring of solvent concentration during removal processes and dilution ratios must be validated to ensure consistent product quality. Regulatory submissions should demonstrate robust control strategies with appropriate feedback mechanisms to maintain process parameters within established design spaces.
Validation approaches for continuous LNP manufacturing differ significantly from traditional batch processes. Regulatory agencies require demonstration of state of control throughout extended production runs, with particular attention to start-up and shut-down transitions. The impact of process interruptions on product quality must be thoroughly characterized, especially regarding solvent removal efficiency and dilution homogeneity.
Scale-up considerations present unique regulatory challenges for continuous LNP manufacturing. Unlike traditional batch processes where scale-up follows established paradigms, continuous processes must demonstrate consistent product quality across different throughput rates. Regulatory submissions should include comprehensive comparability studies showing equivalent product quality attributes across various production scales and rates.
Global regulatory harmonization remains an evolving area for continuous LNP manufacturing. While ICH guidelines provide general frameworks for pharmaceutical development and manufacturing, specific guidance for continuous LNP production varies between regions. Companies pursuing global markets must develop regulatory strategies that address the nuanced expectations of different authorities regarding process validation, control strategies, and stability data requirements.
Regulatory risk assessment for continuous LNP manufacturing should focus on potential failure modes related to solvent removal and in-line dilution processes. Manufacturers must demonstrate robust risk mitigation strategies, including redundant monitoring systems and predetermined corrective actions for process deviations that could impact product quality or patient safety.
Scale-up Strategies and Process Economics
Scaling up the continuous manufacturing of lipid nanoparticles (LNPs) from laboratory to commercial production presents significant challenges that require careful strategic planning and economic considerations. The transition from small-scale to large-scale production necessitates robust process design that maintains product quality while achieving cost efficiency. Current industry approaches typically involve either vertical scaling, where equipment dimensions are increased proportionally, or horizontal scaling through parallel processing units.
For LNP production, microfluidic mixing technology has demonstrated excellent scalability through numbering-up strategies. This approach maintains the critical quality attributes of LNPs by preserving the mixing conditions across multiple parallel mixers. Companies like Precision NanoSystems and Dolomite Microfluidics have successfully implemented such strategies, achieving throughput increases from milliliters to liters per hour without compromising nanoparticle size distribution or encapsulation efficiency.
Economic analysis reveals that solvent removal and in-line dilution processes significantly impact the overall manufacturing costs. Traditional batch-based solvent removal methods like tangential flow filtration (TFF) represent approximately 15-20% of total production costs due to membrane replacement, buffer consumption, and extended processing times. Continuous solvent removal technologies, such as counter-current dialysis or integrated membrane systems, can reduce these costs by 30-40% while improving product consistency.
The capital expenditure for establishing continuous LNP manufacturing facilities ranges from $5-15 million for clinical-scale production to $30-50 million for commercial manufacturing. However, the return on investment typically occurs within 3-5 years due to reduced labor costs, decreased batch failures, and improved facility utilization. Continuous processing can reduce the manufacturing footprint by 40-60% compared to batch operations, further enhancing economic benefits.
Energy consumption represents another critical economic factor. Continuous solvent removal systems typically consume 25-35% less energy than batch processes due to more efficient heat transfer and reduced heating/cooling cycles. This efficiency translates to approximately $0.5-1.5 per gram of LNP production cost savings, which becomes significant at commercial scales exceeding 100 kg annually.
Risk assessment models indicate that implementing continuous manufacturing with integrated solvent removal and in-line dilution reduces product quality variability by 40-60%, thereby decreasing the likelihood of batch rejections. Each rejected batch at commercial scale can cost $500,000-1,000,000, making process reliability a major economic driver. Companies adopting continuous manufacturing report payback periods shortening by 1-2 years when accounting for reduced quality-related losses.
For LNP production, microfluidic mixing technology has demonstrated excellent scalability through numbering-up strategies. This approach maintains the critical quality attributes of LNPs by preserving the mixing conditions across multiple parallel mixers. Companies like Precision NanoSystems and Dolomite Microfluidics have successfully implemented such strategies, achieving throughput increases from milliliters to liters per hour without compromising nanoparticle size distribution or encapsulation efficiency.
Economic analysis reveals that solvent removal and in-line dilution processes significantly impact the overall manufacturing costs. Traditional batch-based solvent removal methods like tangential flow filtration (TFF) represent approximately 15-20% of total production costs due to membrane replacement, buffer consumption, and extended processing times. Continuous solvent removal technologies, such as counter-current dialysis or integrated membrane systems, can reduce these costs by 30-40% while improving product consistency.
The capital expenditure for establishing continuous LNP manufacturing facilities ranges from $5-15 million for clinical-scale production to $30-50 million for commercial manufacturing. However, the return on investment typically occurs within 3-5 years due to reduced labor costs, decreased batch failures, and improved facility utilization. Continuous processing can reduce the manufacturing footprint by 40-60% compared to batch operations, further enhancing economic benefits.
Energy consumption represents another critical economic factor. Continuous solvent removal systems typically consume 25-35% less energy than batch processes due to more efficient heat transfer and reduced heating/cooling cycles. This efficiency translates to approximately $0.5-1.5 per gram of LNP production cost savings, which becomes significant at commercial scales exceeding 100 kg annually.
Risk assessment models indicate that implementing continuous manufacturing with integrated solvent removal and in-line dilution reduces product quality variability by 40-60%, thereby decreasing the likelihood of batch rejections. Each rejected batch at commercial scale can cost $500,000-1,000,000, making process reliability a major economic driver. Companies adopting continuous manufacturing report payback periods shortening by 1-2 years when accounting for reduced quality-related losses.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!