Purification challenges for in vitro transcribed mRNA: removing dsRNA and residual DNA impurities
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Purification Background and Objectives
Messenger RNA (mRNA) therapeutics have emerged as a revolutionary platform in modern medicine, with applications spanning from vaccines to protein replacement therapies. The field gained unprecedented attention following the successful deployment of mRNA-based COVID-19 vaccines, demonstrating the potential of this technology to address urgent global health challenges. The evolution of mRNA therapeutics traces back to the 1990s, with significant acceleration in the past decade due to innovations in delivery systems and manufacturing processes.
The purification of in vitro transcribed (IVT) mRNA represents a critical bottleneck in the production pipeline. Historically, mRNA purification techniques have evolved from basic precipitation methods to sophisticated chromatographic approaches. The primary objective in this domain is to develop efficient, scalable, and cost-effective purification strategies that can consistently yield mRNA products with pharmaceutical-grade purity.
Double-stranded RNA (dsRNA) contaminants and residual DNA impurities present particular challenges in the purification process. These contaminants can trigger innate immune responses, leading to reduced translation efficiency and potential safety concerns in therapeutic applications. The presence of dsRNA activates pattern recognition receptors such as TLR3 and RIG-I, inducing type I interferon responses that can compromise the efficacy of mRNA therapeutics.
Current technological trends in mRNA purification focus on developing methods that can selectively remove dsRNA and DNA impurities while maintaining the integrity and functionality of the target mRNA. The field is witnessing a shift from traditional methods like LiCl precipitation toward more sophisticated approaches including affinity chromatography, size exclusion techniques, and enzymatic treatments.
The technical objectives for advancing mRNA purification include: developing high-resolution separation methods capable of distinguishing between single-stranded mRNA and structurally similar contaminants; establishing scalable processes suitable for industrial production; reducing purification-related costs to improve the economic viability of mRNA therapeutics; and implementing robust quality control measures to ensure batch-to-batch consistency.
Regulatory considerations further shape the technical landscape, with agencies like the FDA and EMA establishing increasingly stringent requirements for impurity profiles in mRNA products. Meeting these standards necessitates continuous innovation in analytical methods to accurately quantify trace contaminants and validate purification processes.
The ultimate goal of research in this field is to establish a purification platform that can consistently deliver mRNA with impurity levels below immunogenic thresholds, while maintaining high yield and preserving the structural integrity essential for translation efficiency. Achieving this balance represents a fundamental challenge that, when overcome, will significantly advance the clinical and commercial potential of mRNA therapeutics.
The purification of in vitro transcribed (IVT) mRNA represents a critical bottleneck in the production pipeline. Historically, mRNA purification techniques have evolved from basic precipitation methods to sophisticated chromatographic approaches. The primary objective in this domain is to develop efficient, scalable, and cost-effective purification strategies that can consistently yield mRNA products with pharmaceutical-grade purity.
Double-stranded RNA (dsRNA) contaminants and residual DNA impurities present particular challenges in the purification process. These contaminants can trigger innate immune responses, leading to reduced translation efficiency and potential safety concerns in therapeutic applications. The presence of dsRNA activates pattern recognition receptors such as TLR3 and RIG-I, inducing type I interferon responses that can compromise the efficacy of mRNA therapeutics.
Current technological trends in mRNA purification focus on developing methods that can selectively remove dsRNA and DNA impurities while maintaining the integrity and functionality of the target mRNA. The field is witnessing a shift from traditional methods like LiCl precipitation toward more sophisticated approaches including affinity chromatography, size exclusion techniques, and enzymatic treatments.
The technical objectives for advancing mRNA purification include: developing high-resolution separation methods capable of distinguishing between single-stranded mRNA and structurally similar contaminants; establishing scalable processes suitable for industrial production; reducing purification-related costs to improve the economic viability of mRNA therapeutics; and implementing robust quality control measures to ensure batch-to-batch consistency.
Regulatory considerations further shape the technical landscape, with agencies like the FDA and EMA establishing increasingly stringent requirements for impurity profiles in mRNA products. Meeting these standards necessitates continuous innovation in analytical methods to accurately quantify trace contaminants and validate purification processes.
The ultimate goal of research in this field is to establish a purification platform that can consistently deliver mRNA with impurity levels below immunogenic thresholds, while maintaining high yield and preserving the structural integrity essential for translation efficiency. Achieving this balance represents a fundamental challenge that, when overcome, will significantly advance the clinical and commercial potential of mRNA therapeutics.
Market Analysis for High-Purity mRNA Products
The global market for high-purity mRNA products has experienced exponential growth, primarily driven by the success of mRNA-based COVID-19 vaccines. This market segment is projected to reach $15 billion by 2026, with a compound annual growth rate of 28% from 2021. The therapeutic applications extend beyond vaccines to include cancer immunotherapy, protein replacement therapies, and gene editing, creating diverse revenue streams for manufacturers who can deliver consistently pure mRNA products.
Healthcare providers and pharmaceutical companies are increasingly demanding mRNA products with minimal dsRNA and DNA impurities, as these contaminants can trigger unwanted immune responses and reduce translation efficiency. Regulatory bodies worldwide have established stringent purity standards, with the FDA and EMA requiring less than 0.05% residual DNA and virtually undetectable dsRNA levels in clinical-grade mRNA products.
The premium pricing structure for high-purity mRNA reflects this demand, with purified mRNA commanding 30-40% higher prices compared to standard grades. This price differential has created a competitive advantage for companies that have developed proprietary purification technologies, allowing them to capture larger market shares and establish long-term supply agreements with major pharmaceutical partners.
Regional market analysis reveals North America currently dominates with 45% market share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to show the fastest growth rate of 35% annually through 2026, driven by increasing investments in biotechnology infrastructure and government initiatives supporting mRNA technology development in countries like China, Japan, and South Korea.
Customer segmentation shows distinct requirements across different buyer groups. Pharmaceutical companies prioritize scalable purification processes that maintain consistency across batches, while academic and research institutions focus on flexibility and cost-effectiveness. Contract manufacturing organizations seek purification technologies that can be integrated into existing production workflows without significant capital investment.
Market forecasts indicate that companies offering integrated purification solutions that address both dsRNA and DNA contamination simultaneously will capture the highest market value. The trend toward continuous manufacturing processes is expected to further reshape market dynamics, with an estimated 60% of mRNA manufacturers planning to implement continuous purification systems within the next five years to improve efficiency and reduce production costs.
Healthcare providers and pharmaceutical companies are increasingly demanding mRNA products with minimal dsRNA and DNA impurities, as these contaminants can trigger unwanted immune responses and reduce translation efficiency. Regulatory bodies worldwide have established stringent purity standards, with the FDA and EMA requiring less than 0.05% residual DNA and virtually undetectable dsRNA levels in clinical-grade mRNA products.
The premium pricing structure for high-purity mRNA reflects this demand, with purified mRNA commanding 30-40% higher prices compared to standard grades. This price differential has created a competitive advantage for companies that have developed proprietary purification technologies, allowing them to capture larger market shares and establish long-term supply agreements with major pharmaceutical partners.
Regional market analysis reveals North America currently dominates with 45% market share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to show the fastest growth rate of 35% annually through 2026, driven by increasing investments in biotechnology infrastructure and government initiatives supporting mRNA technology development in countries like China, Japan, and South Korea.
Customer segmentation shows distinct requirements across different buyer groups. Pharmaceutical companies prioritize scalable purification processes that maintain consistency across batches, while academic and research institutions focus on flexibility and cost-effectiveness. Contract manufacturing organizations seek purification technologies that can be integrated into existing production workflows without significant capital investment.
Market forecasts indicate that companies offering integrated purification solutions that address both dsRNA and DNA contamination simultaneously will capture the highest market value. The trend toward continuous manufacturing processes is expected to further reshape market dynamics, with an estimated 60% of mRNA manufacturers planning to implement continuous purification systems within the next five years to improve efficiency and reduce production costs.
Current Challenges in IVT mRNA Purification
In vitro transcribed (IVT) mRNA has emerged as a revolutionary platform for vaccines, therapeutics, and gene therapy applications. However, the purification process remains one of the most significant bottlenecks in mRNA production pipelines. Current purification methods face substantial challenges in efficiently removing two critical contaminants: double-stranded RNA (dsRNA) impurities and residual DNA templates.
The presence of dsRNA in IVT mRNA preparations poses a major challenge as these impurities trigger innate immune responses through pattern recognition receptors like TLR3, RIG-I, and MDA5. This immunostimulatory effect can lead to translation inhibition and degradation of the therapeutic mRNA, significantly reducing efficacy. Conventional purification methods such as HPLC and chromatography techniques struggle to completely separate dsRNA from single-stranded mRNA due to their similar physicochemical properties.
Residual DNA template contamination presents another critical purification hurdle. Despite DNase treatment during manufacturing, trace amounts of DNA templates often persist in final mRNA products. These DNA contaminants can trigger immune responses and potentially cause insertional mutagenesis risks. Regulatory agencies have established strict guidelines limiting DNA impurities in pharmaceutical-grade mRNA products, typically to less than 10 ng DNA per dose.
Current purification technologies demonstrate varying degrees of effectiveness in addressing these challenges. Chromatographic methods like HPLC and fast protein liquid chromatography (FPLC) offer high resolution but suffer from limited scalability and high operational costs. Precipitation-based methods provide better scalability but often result in lower purity and yield losses. Tangential flow filtration (TFF) offers good scalability but struggles with selective removal of similarly sized impurities.
The industry also faces significant analytical challenges in quantifying low levels of dsRNA and DNA impurities. Current detection methods like RT-qPCR and immunological assays have sensitivity limitations that complicate quality control processes. The lack of standardized analytical methods further complicates regulatory compliance and batch-to-batch consistency.
Scale-up challenges represent another significant hurdle in mRNA purification. Many laboratory-scale purification techniques fail to maintain efficiency when scaled to commercial production volumes. This scale-up gap has created bottlenecks in manufacturing capacity, particularly evident during the rapid deployment of mRNA COVID-19 vaccines.
Cost considerations further complicate purification strategies, with high-purity chromatography resins and specialized filtration membranes significantly impacting production economics. The trade-off between purity, yield, and cost remains a central challenge for manufacturers seeking to optimize purification processes while maintaining economic viability.
The presence of dsRNA in IVT mRNA preparations poses a major challenge as these impurities trigger innate immune responses through pattern recognition receptors like TLR3, RIG-I, and MDA5. This immunostimulatory effect can lead to translation inhibition and degradation of the therapeutic mRNA, significantly reducing efficacy. Conventional purification methods such as HPLC and chromatography techniques struggle to completely separate dsRNA from single-stranded mRNA due to their similar physicochemical properties.
Residual DNA template contamination presents another critical purification hurdle. Despite DNase treatment during manufacturing, trace amounts of DNA templates often persist in final mRNA products. These DNA contaminants can trigger immune responses and potentially cause insertional mutagenesis risks. Regulatory agencies have established strict guidelines limiting DNA impurities in pharmaceutical-grade mRNA products, typically to less than 10 ng DNA per dose.
Current purification technologies demonstrate varying degrees of effectiveness in addressing these challenges. Chromatographic methods like HPLC and fast protein liquid chromatography (FPLC) offer high resolution but suffer from limited scalability and high operational costs. Precipitation-based methods provide better scalability but often result in lower purity and yield losses. Tangential flow filtration (TFF) offers good scalability but struggles with selective removal of similarly sized impurities.
The industry also faces significant analytical challenges in quantifying low levels of dsRNA and DNA impurities. Current detection methods like RT-qPCR and immunological assays have sensitivity limitations that complicate quality control processes. The lack of standardized analytical methods further complicates regulatory compliance and batch-to-batch consistency.
Scale-up challenges represent another significant hurdle in mRNA purification. Many laboratory-scale purification techniques fail to maintain efficiency when scaled to commercial production volumes. This scale-up gap has created bottlenecks in manufacturing capacity, particularly evident during the rapid deployment of mRNA COVID-19 vaccines.
Cost considerations further complicate purification strategies, with high-purity chromatography resins and specialized filtration membranes significantly impacting production economics. The trade-off between purity, yield, and cost remains a central challenge for manufacturers seeking to optimize purification processes while maintaining economic viability.
Established Methods for dsRNA and DNA Removal
01 Chromatography-based purification methods
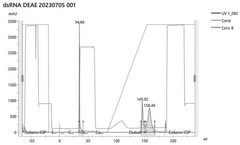
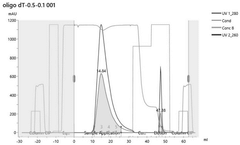
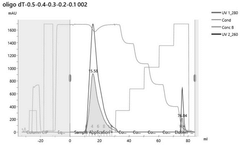
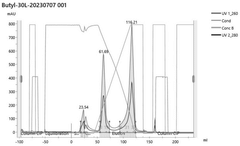
Various chromatography techniques are employed for purifying in vitro transcribed mRNA from contaminants such as dsRNA and residual DNA. These methods include affinity chromatography, ion-exchange chromatography, and size-exclusion chromatography. These techniques separate mRNA from impurities based on differences in molecular properties such as charge, size, or specific binding interactions, resulting in highly purified mRNA suitable for therapeutic applications.- Chromatography-based purification methods: Various chromatography techniques are employed for purifying in vitro transcribed mRNA from contaminants including dsRNA and residual DNA. These methods include affinity chromatography, ion-exchange chromatography, and size-exclusion chromatography. These techniques separate mRNA from impurities based on differences in molecular properties such as charge, size, and binding affinity, allowing for the removal of dsRNA byproducts and DNA templates that remain after transcription.
- Enzymatic treatment for impurity removal: Enzymatic approaches are utilized to specifically degrade unwanted nucleic acid impurities in mRNA preparations. DNase treatment is commonly employed to eliminate template DNA, while specific RNases can selectively degrade dsRNA without affecting the desired single-stranded mRNA product. These enzymatic methods can be applied either during or after the transcription process to enhance the purity of the final mRNA product.
- Precipitation and extraction techniques: Various precipitation and extraction methods are used to separate mRNA from impurities. These include lithium chloride precipitation, which selectively precipitates RNA while leaving DNA in solution, alcohol precipitation techniques, and phase separation using organic solvents. These approaches exploit differences in solubility between mRNA, dsRNA, and DNA under various chemical conditions to achieve purification.
- Advanced filtration and membrane-based purification: Membrane-based separation techniques including ultrafiltration, tangential flow filtration, and specialized membrane adsorbers are employed for mRNA purification. These methods can effectively separate mRNA from smaller impurities and contaminants based on molecular size and charge differences. The techniques allow for scalable purification processes that can handle large volumes while maintaining high recovery of the target mRNA.
- Quality control and analytical methods: Various analytical techniques are employed to assess the purity of mRNA preparations and detect residual dsRNA and DNA impurities. These include quantitative PCR for DNA detection, bioanalyzer-based methods for RNA integrity analysis, and specialized assays for dsRNA detection. These quality control methods are essential for ensuring that the purified mRNA meets the required specifications for downstream applications such as vaccine production or gene therapy.
02 Enzymatic treatment for impurity removal
Enzymatic treatments are utilized to specifically degrade dsRNA and DNA impurities in mRNA preparations. DNase enzymes are commonly used to digest residual DNA templates, while specific RNases can selectively degrade dsRNA without affecting the desired single-stranded mRNA. These enzymatic approaches can be combined with other purification methods to achieve higher purity levels of the final mRNA product.Expand Specific Solutions03 Precipitation and extraction techniques
Various precipitation and extraction methods are employed to separate mRNA from impurities. These include lithium chloride precipitation, which selectively precipitates RNA while leaving DNA in solution, and phenol-chloroform extraction to remove proteins and separate nucleic acids. Additional techniques like alcohol precipitation with ethanol or isopropanol help concentrate the purified mRNA while removing salts and small molecules.Expand Specific Solutions04 Modified nucleotides and transcription conditions
The incorporation of modified nucleotides during in vitro transcription can reduce the formation of dsRNA impurities. By optimizing transcription conditions such as temperature, buffer composition, and enzyme concentrations, the yield of correctly folded mRNA can be increased while minimizing the production of dsRNA byproducts. These modifications to the transcription process can significantly improve the purity of the resulting mRNA before additional purification steps.Expand Specific Solutions05 Advanced filtration and membrane-based purification
Tangential flow filtration, ultrafiltration, and other membrane-based techniques are used to purify mRNA based on molecular size differences. These methods can effectively separate mRNA from smaller impurities like nucleotides and salts, as well as larger contaminants like DNA fragments and dsRNA. Specialized membranes with specific molecular weight cut-offs allow for selective retention of the desired mRNA while allowing impurities to pass through or be retained based on their size.Expand Specific Solutions
Key Industry Players in mRNA Manufacturing
The mRNA purification landscape is evolving rapidly, with the market currently in a growth phase driven by COVID-19 vaccine development. The global mRNA therapeutics market is projected to reach $5.3 billion by 2026, expanding at a CAGR of 13.2%. Key players addressing dsRNA and DNA impurity challenges include BioNTech and Moderna as market leaders, with CureVac, Translate Bio, and Arcturus Therapeutics making significant technological advances. TriLink BioTechnologies and Acuitas Therapeutics provide critical purification technologies. The field is transitioning from early-stage development to commercial maturity, with companies like Sanofi and GlaxoSmithKline leveraging partnerships to enhance purification capabilities, while academic institutions like University of Pennsylvania contribute fundamental research to overcome these technical barriers.
CureVac SE
Technical Solution: CureVac has developed a distinctive purification platform for mRNA therapeutics called RNAoptimizer that specifically addresses dsRNA and DNA contamination challenges. Their approach begins with a proprietary in vitro transcription process using optimized reaction conditions to minimize dsRNA formation from the start. For purification, CureVac employs a sequential enzymatic treatment strategy, first using DNase I to eliminate template DNA followed by a phosphatase treatment to remove 5'-triphosphates that can trigger innate immune responses. The company has pioneered a specialized chromatography technique using modified oligo(dT) matrices that selectively capture poly(A)-tailed mRNA while allowing impurities to wash through. This is complemented by a tangential flow filtration (TFF) step that removes small molecular contaminants while concentrating the purified mRNA. CureVac's platform also incorporates a novel dsRNA-specific binding protein technology that selectively removes remaining dsRNA contaminants. Their quality control process includes advanced analytical methods such as next-generation sequencing to verify the absence of DNA fragments and specialized immunological assays to confirm minimal dsRNA-mediated immune activation.
Strengths: Proprietary RNAoptimizer platform with integrated purification strategy; specialized oligo(dT) chromatography for high selectivity; innovative dsRNA-binding protein technology for contaminant removal. Weaknesses: Complex multi-component purification system requiring precise optimization; potential challenges in large-scale manufacturing consistency; relatively higher production costs compared to simpler purification methods.
ModernaTX, Inc.
Technical Solution: Moderna has developed a proprietary purification platform for mRNA therapeutics that addresses dsRNA and DNA impurities. Their approach combines chromatography techniques with enzymatic treatments to achieve high-purity mRNA. The process includes a multi-stage purification workflow starting with DNase treatment to degrade template DNA, followed by HPLC purification using reverse-phase and anion-exchange chromatography to separate full-length mRNA from truncated species and dsRNA contaminants. Moderna employs a cellulose-based chromatography method that selectively binds dsRNA impurities while allowing purified ssRNA to flow through. Their platform also incorporates a proprietary lipid nanoparticle (LNP) delivery system that encapsulates the purified mRNA, protecting it from degradation and facilitating cellular uptake. This comprehensive approach has enabled Moderna to achieve >95% purity in their clinical-grade mRNA products, with dsRNA content reduced to <0.05% and residual DNA below detectable limits.
Strengths: Industry-leading purification technology with proven clinical success in COVID-19 vaccines; integrated manufacturing platform allowing consistent quality control; proprietary LNP delivery system enhancing stability. Weaknesses: Complex multi-step purification process increases production costs; scalability challenges for global distribution; potential intellectual property constraints limiting technology sharing.
Critical Purification Technologies and Patents
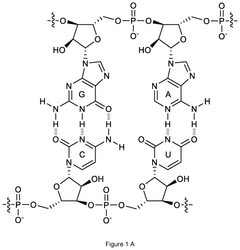
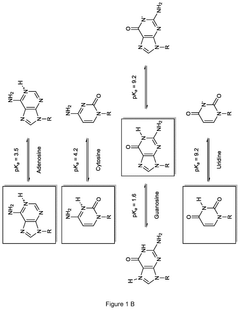
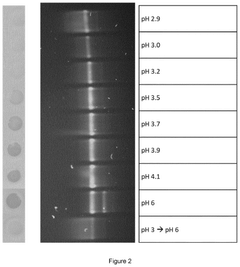
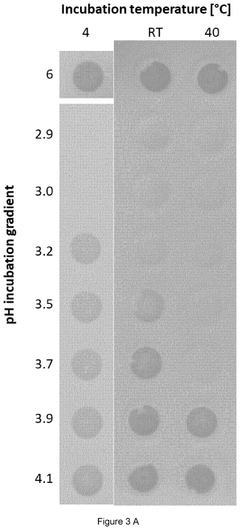
METHODS FOR THE REMOVAL OF DOUBLE-AND/OR MULTI-STRANDED NUCLEIC ACID IMPURITIES FROM RNA PREPARATIONS BY LOW pH TREATMENT
PatentPendingUS20250207121A1
Innovation
- A method involving incubation of RNA preparations at a pH range of 1 to 5 to dissociate double-and/or multi-stranded nucleic acid impurities, followed by purification to remove the dissociated fragments using chromatographic techniques at similar or adjusted pH levels.
A method for purifying in vitro transcribed mRNA and its application
PatentActiveCN117603958B
Innovation
- A combined method of lithium chloride precipitation, Oligo dT affinity chromatography, and DEAE ion exchange chromatography is used to gradually remove free nucleotides and small nucleotide fragments in the mRNA through NaCl gradient elution and pH gradient elution. , protein and double-stranded RNA impurities to achieve the preparation of high-purity mRNA.
Regulatory Requirements for mRNA-Based Therapeutics
The regulatory landscape for mRNA-based therapeutics is complex and evolving rapidly, particularly regarding purification standards for in vitro transcribed (IVT) mRNA products. Regulatory bodies worldwide, including the FDA, EMA, and PMDA, have established stringent requirements for the removal of double-stranded RNA (dsRNA) and residual DNA impurities, recognizing their potential to trigger immunogenicity and compromise therapeutic efficacy.
Current regulatory guidelines mandate that mRNA therapeutics must demonstrate consistently low levels of dsRNA contaminants, typically below 0.1% of total RNA content. These requirements stem from evidence that dsRNA can activate pattern recognition receptors like TLR3 and RIG-I, potentially leading to undesired immune responses and reduced translation efficiency of the therapeutic mRNA.
For residual DNA impurities, regulatory thresholds are even more stringent, with most authorities requiring levels below 10 ng per dose. This reflects concerns about potential integration into the host genome or unwanted biological activity. Manufacturers must implement validated analytical methods capable of detecting DNA contaminants at concentrations as low as 1-5 pg/μL to satisfy regulatory scrutiny.
Quality control documentation for mRNA therapeutics must include comprehensive data on purification processes, with detailed validation of removal efficiency for both dsRNA and DNA impurities. Regulatory submissions typically require multiple orthogonal analytical methods to characterize these impurities, including HPLC, capillary electrophoresis, and specialized immunological assays for dsRNA detection.
Batch-to-batch consistency represents another critical regulatory focus, with authorities requiring manufacturers to demonstrate reproducible purification outcomes across production scales. This necessitates robust process controls and in-process testing protocols specifically targeting dsRNA and DNA contaminant levels throughout manufacturing.
The regulatory framework also addresses stability considerations, requiring evidence that purification-related quality attributes remain within specification throughout the product's shelf life. Manufacturers must demonstrate that dsRNA and DNA impurity profiles do not change significantly under recommended storage conditions.
Recent regulatory trends indicate increasing harmonization of requirements across major markets, though notable differences persist in specific analytical method preferences and acceptance criteria. Companies developing mRNA therapeutics must navigate these nuances while maintaining compliance with core safety standards focused on minimizing immunogenic potential related to purification impurities.
Current regulatory guidelines mandate that mRNA therapeutics must demonstrate consistently low levels of dsRNA contaminants, typically below 0.1% of total RNA content. These requirements stem from evidence that dsRNA can activate pattern recognition receptors like TLR3 and RIG-I, potentially leading to undesired immune responses and reduced translation efficiency of the therapeutic mRNA.
For residual DNA impurities, regulatory thresholds are even more stringent, with most authorities requiring levels below 10 ng per dose. This reflects concerns about potential integration into the host genome or unwanted biological activity. Manufacturers must implement validated analytical methods capable of detecting DNA contaminants at concentrations as low as 1-5 pg/μL to satisfy regulatory scrutiny.
Quality control documentation for mRNA therapeutics must include comprehensive data on purification processes, with detailed validation of removal efficiency for both dsRNA and DNA impurities. Regulatory submissions typically require multiple orthogonal analytical methods to characterize these impurities, including HPLC, capillary electrophoresis, and specialized immunological assays for dsRNA detection.
Batch-to-batch consistency represents another critical regulatory focus, with authorities requiring manufacturers to demonstrate reproducible purification outcomes across production scales. This necessitates robust process controls and in-process testing protocols specifically targeting dsRNA and DNA contaminant levels throughout manufacturing.
The regulatory framework also addresses stability considerations, requiring evidence that purification-related quality attributes remain within specification throughout the product's shelf life. Manufacturers must demonstrate that dsRNA and DNA impurity profiles do not change significantly under recommended storage conditions.
Recent regulatory trends indicate increasing harmonization of requirements across major markets, though notable differences persist in specific analytical method preferences and acceptance criteria. Companies developing mRNA therapeutics must navigate these nuances while maintaining compliance with core safety standards focused on minimizing immunogenic potential related to purification impurities.
Quality Control Strategies for mRNA Purity Assessment
Quality control strategies for mRNA purity assessment have evolved significantly in response to the challenges associated with in vitro transcribed (IVT) mRNA purification. These strategies encompass a comprehensive suite of analytical methods designed to detect, quantify, and characterize impurities that may compromise the safety and efficacy of mRNA therapeutics.
Chromatographic techniques represent the cornerstone of mRNA purity assessment, with high-performance liquid chromatography (HPLC) and its variants being widely employed. Anion exchange chromatography (AEX) effectively separates mRNA from DNA contaminants based on charge differences, while reversed-phase HPLC distinguishes between capped and uncapped mRNA species. Size exclusion chromatography (SEC) provides valuable insights into the size distribution of mRNA products and associated impurities.
Electrophoretic methods, including capillary gel electrophoresis (CGE) and microfluidic-based platforms like the Agilent Bioanalyzer, offer high-resolution separation of nucleic acids based on size. These techniques are particularly valuable for detecting truncated mRNA species and residual DNA fragments, with detection limits approaching 1-5 ng/μL.
Polymerase chain reaction (PCR)-based assays, especially quantitative PCR (qPCR), have emerged as gold standards for the detection of residual DNA impurities. These methods can achieve remarkable sensitivity, detecting DNA contamination at levels as low as 10 pg per dose, which is critical for meeting regulatory requirements.
Immunological assays targeting double-stranded RNA (dsRNA) impurities have gained prominence due to the immunostimulatory potential of these contaminants. Enzyme-linked immunosorbent assays (ELISA) utilizing dsRNA-specific antibodies, such as J2 or 9D5, enable quantification of dsRNA with detection limits in the picogram range.
Next-generation sequencing (NGS) approaches provide comprehensive characterization of mRNA products and their impurities at the nucleotide level. These methods can identify sequence variants, truncations, and modifications that may affect the functionality of the final product.
Spectroscopic techniques, including UV-visible spectroscopy and circular dichroism, offer rapid assessment of overall nucleic acid content and structural characteristics. The A260/A280 ratio serves as a preliminary indicator of protein contamination, while circular dichroism provides insights into the secondary structure of mRNA molecules.
Implementation of these quality control strategies requires careful consideration of method validation parameters, including specificity, sensitivity, accuracy, precision, and robustness. Regulatory agencies increasingly emphasize the importance of orthogonal testing approaches, combining multiple analytical methods to ensure comprehensive assessment of mRNA purity.
Chromatographic techniques represent the cornerstone of mRNA purity assessment, with high-performance liquid chromatography (HPLC) and its variants being widely employed. Anion exchange chromatography (AEX) effectively separates mRNA from DNA contaminants based on charge differences, while reversed-phase HPLC distinguishes between capped and uncapped mRNA species. Size exclusion chromatography (SEC) provides valuable insights into the size distribution of mRNA products and associated impurities.
Electrophoretic methods, including capillary gel electrophoresis (CGE) and microfluidic-based platforms like the Agilent Bioanalyzer, offer high-resolution separation of nucleic acids based on size. These techniques are particularly valuable for detecting truncated mRNA species and residual DNA fragments, with detection limits approaching 1-5 ng/μL.
Polymerase chain reaction (PCR)-based assays, especially quantitative PCR (qPCR), have emerged as gold standards for the detection of residual DNA impurities. These methods can achieve remarkable sensitivity, detecting DNA contamination at levels as low as 10 pg per dose, which is critical for meeting regulatory requirements.
Immunological assays targeting double-stranded RNA (dsRNA) impurities have gained prominence due to the immunostimulatory potential of these contaminants. Enzyme-linked immunosorbent assays (ELISA) utilizing dsRNA-specific antibodies, such as J2 or 9D5, enable quantification of dsRNA with detection limits in the picogram range.
Next-generation sequencing (NGS) approaches provide comprehensive characterization of mRNA products and their impurities at the nucleotide level. These methods can identify sequence variants, truncations, and modifications that may affect the functionality of the final product.
Spectroscopic techniques, including UV-visible spectroscopy and circular dichroism, offer rapid assessment of overall nucleic acid content and structural characteristics. The A260/A280 ratio serves as a preliminary indicator of protein contamination, while circular dichroism provides insights into the secondary structure of mRNA molecules.
Implementation of these quality control strategies requires careful consideration of method validation parameters, including specificity, sensitivity, accuracy, precision, and robustness. Regulatory agencies increasingly emphasize the importance of orthogonal testing approaches, combining multiple analytical methods to ensure comprehensive assessment of mRNA purity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!