High-throughput screening of cationic lipids for targeted LNP delivery to specific immune cell subsets
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LNP Delivery Technology Background and Objectives
Lipid nanoparticles (LNPs) have emerged as revolutionary delivery vehicles for nucleic acid therapeutics, with their prominence dramatically highlighted by the successful deployment of mRNA COVID-19 vaccines. The evolution of LNP technology spans several decades, beginning with liposomal drug delivery systems in the 1960s and progressing through significant milestones including the FDA approval of Doxil in 1995 and Onpattro in 2018, the first siRNA therapeutic utilizing LNP delivery.
The current technological landscape of LNPs is characterized by their versatile structure comprising ionizable lipids, helper phospholipids, cholesterol, and PEGylated lipids. This architecture enables efficient encapsulation of nucleic acid cargo and facilitates cellular uptake through endosomal escape mechanisms. Despite these advances, targeted delivery to specific immune cell populations remains a significant challenge, limiting the therapeutic potential of LNP-based treatments for immunological disorders, cancer immunotherapy, and autoimmune diseases.
Cationic lipids represent a critical component of LNPs, influencing their physicochemical properties, biodistribution, and cellular uptake efficiency. Traditional approaches to LNP formulation have relied heavily on empirical testing with limited throughput, resulting in suboptimal targeting capabilities. The field now recognizes the need for systematic, high-throughput screening methodologies to identify cationic lipid structures that confer selective tropism toward specific immune cell subsets.
Recent technological breakthroughs in combinatorial chemistry, microfluidics, and high-content screening have created unprecedented opportunities to explore vast chemical spaces of lipid structures. These advances, coupled with developments in immunological profiling and single-cell analysis techniques, provide the foundation for next-generation targeted LNP delivery systems.
The primary objectives of high-throughput screening for immune cell-targeted LNPs include: (1) establishing robust screening platforms capable of evaluating thousands of cationic lipid candidates; (2) identifying structure-activity relationships that govern immune cell subset targeting; (3) developing predictive models to accelerate rational design of cell-specific LNPs; and (4) validating lead formulations in physiologically relevant models.
Achievement of these objectives would transform the therapeutic landscape by enabling precise modulation of immune responses through cell-specific delivery of nucleic acid therapeutics. This capability would benefit applications ranging from cancer immunotherapy and vaccine development to treatment of autoimmune disorders and inflammatory diseases. Furthermore, targeted delivery would potentially reduce off-target effects, lower required doses, and improve the safety profile of nucleic acid therapeutics.
The convergence of nanotechnology, immunology, and genomics presents a unique opportunity to address these challenges and realize the full potential of LNP technology in precision medicine.
The current technological landscape of LNPs is characterized by their versatile structure comprising ionizable lipids, helper phospholipids, cholesterol, and PEGylated lipids. This architecture enables efficient encapsulation of nucleic acid cargo and facilitates cellular uptake through endosomal escape mechanisms. Despite these advances, targeted delivery to specific immune cell populations remains a significant challenge, limiting the therapeutic potential of LNP-based treatments for immunological disorders, cancer immunotherapy, and autoimmune diseases.
Cationic lipids represent a critical component of LNPs, influencing their physicochemical properties, biodistribution, and cellular uptake efficiency. Traditional approaches to LNP formulation have relied heavily on empirical testing with limited throughput, resulting in suboptimal targeting capabilities. The field now recognizes the need for systematic, high-throughput screening methodologies to identify cationic lipid structures that confer selective tropism toward specific immune cell subsets.
Recent technological breakthroughs in combinatorial chemistry, microfluidics, and high-content screening have created unprecedented opportunities to explore vast chemical spaces of lipid structures. These advances, coupled with developments in immunological profiling and single-cell analysis techniques, provide the foundation for next-generation targeted LNP delivery systems.
The primary objectives of high-throughput screening for immune cell-targeted LNPs include: (1) establishing robust screening platforms capable of evaluating thousands of cationic lipid candidates; (2) identifying structure-activity relationships that govern immune cell subset targeting; (3) developing predictive models to accelerate rational design of cell-specific LNPs; and (4) validating lead formulations in physiologically relevant models.
Achievement of these objectives would transform the therapeutic landscape by enabling precise modulation of immune responses through cell-specific delivery of nucleic acid therapeutics. This capability would benefit applications ranging from cancer immunotherapy and vaccine development to treatment of autoimmune disorders and inflammatory diseases. Furthermore, targeted delivery would potentially reduce off-target effects, lower required doses, and improve the safety profile of nucleic acid therapeutics.
The convergence of nanotechnology, immunology, and genomics presents a unique opportunity to address these challenges and realize the full potential of LNP technology in precision medicine.
Market Analysis for Targeted Immune Cell Delivery Systems
The targeted delivery of therapeutics to specific immune cell subsets represents a significant market opportunity within the broader lipid nanoparticle (LNP) and drug delivery sectors. The global market for targeted drug delivery systems is currently valued at approximately $26 billion and is projected to grow at a CAGR of 8.7% through 2028, with immune cell-specific delivery systems emerging as a high-growth segment.
Demand for targeted immune cell delivery technologies is primarily driven by the expanding field of immunotherapy, which has revolutionized treatment approaches for cancer, autoimmune disorders, and infectious diseases. The oncology segment currently dominates market demand, accounting for roughly 40% of applications, followed by autoimmune diseases (25%) and vaccines (20%).
Pharmaceutical and biotechnology companies are increasingly seeking delivery technologies that can selectively target specific immune cell populations such as dendritic cells, T cells, B cells, and macrophages. This precision approach offers significant advantages over conventional delivery methods, including reduced off-target effects, lower dosage requirements, and improved therapeutic efficacy.
Regional analysis indicates North America holds the largest market share (approximately 45%) due to robust R&D infrastructure and significant investment in advanced therapeutic development. Europe follows at 30%, while Asia-Pacific represents the fastest-growing region with increasing investment in biotechnology and pharmaceutical research.
Key market segments for targeted LNP delivery systems include cancer immunotherapy, autoimmune disease treatment, vaccine development, and cell-based therapies. The cancer immunotherapy segment is experiencing particularly strong growth, driven by the clinical success of mRNA-based therapeutics and CAR-T cell therapies.
Customer analysis reveals pharmaceutical giants are actively seeking licensing opportunities for novel delivery technologies, while mid-sized biotechnology companies are forming strategic partnerships to access specialized delivery platforms. Academic research institutions represent another significant customer segment, particularly for early-stage technology development.
Market barriers include regulatory hurdles associated with novel delivery systems, manufacturing scalability challenges, and competition from alternative delivery technologies such as viral vectors and polymer-based systems. However, the superior safety profile and versatility of lipid-based delivery systems position them favorably against these alternatives.
Pricing models for targeted delivery technologies typically involve upfront licensing fees ranging from $5-20 million, milestone payments tied to clinical development stages, and royalties on commercial sales (typically 5-10%). This creates substantial revenue potential for developers of novel cationic lipid screening platforms and targeted LNP formulations.
Demand for targeted immune cell delivery technologies is primarily driven by the expanding field of immunotherapy, which has revolutionized treatment approaches for cancer, autoimmune disorders, and infectious diseases. The oncology segment currently dominates market demand, accounting for roughly 40% of applications, followed by autoimmune diseases (25%) and vaccines (20%).
Pharmaceutical and biotechnology companies are increasingly seeking delivery technologies that can selectively target specific immune cell populations such as dendritic cells, T cells, B cells, and macrophages. This precision approach offers significant advantages over conventional delivery methods, including reduced off-target effects, lower dosage requirements, and improved therapeutic efficacy.
Regional analysis indicates North America holds the largest market share (approximately 45%) due to robust R&D infrastructure and significant investment in advanced therapeutic development. Europe follows at 30%, while Asia-Pacific represents the fastest-growing region with increasing investment in biotechnology and pharmaceutical research.
Key market segments for targeted LNP delivery systems include cancer immunotherapy, autoimmune disease treatment, vaccine development, and cell-based therapies. The cancer immunotherapy segment is experiencing particularly strong growth, driven by the clinical success of mRNA-based therapeutics and CAR-T cell therapies.
Customer analysis reveals pharmaceutical giants are actively seeking licensing opportunities for novel delivery technologies, while mid-sized biotechnology companies are forming strategic partnerships to access specialized delivery platforms. Academic research institutions represent another significant customer segment, particularly for early-stage technology development.
Market barriers include regulatory hurdles associated with novel delivery systems, manufacturing scalability challenges, and competition from alternative delivery technologies such as viral vectors and polymer-based systems. However, the superior safety profile and versatility of lipid-based delivery systems position them favorably against these alternatives.
Pricing models for targeted delivery technologies typically involve upfront licensing fees ranging from $5-20 million, milestone payments tied to clinical development stages, and royalties on commercial sales (typically 5-10%). This creates substantial revenue potential for developers of novel cationic lipid screening platforms and targeted LNP formulations.
Current Challenges in Cationic Lipid Screening
Despite significant advancements in lipid nanoparticle (LNP) technology for RNA delivery, the screening of cationic lipids for targeted delivery to specific immune cell subsets faces numerous technical challenges. The current high-throughput screening methodologies remain inadequate for efficiently identifying optimal lipid formulations with cell-type specificity, particularly for immune cells that present unique biological barriers.
The sheer chemical diversity of potential cationic lipid structures creates an astronomically large design space. With variations in head groups, linker regions, hydrophobic tails, and ionizable domains, researchers face a combinatorial challenge that conventional screening approaches cannot adequately address. Current libraries typically contain only hundreds to a few thousand candidates, representing a minute fraction of the theoretical possibility space.
Physiochemical characterization bottlenecks significantly impede progress. Traditional methods for assessing critical parameters such as size distribution, surface charge, encapsulation efficiency, and stability are often low-throughput and require substantial sample volumes. This creates a fundamental mismatch between the capacity to synthesize lipid variants and the ability to thoroughly characterize their properties.
Immune cell targeting adds another layer of complexity. Different immune cell subsets (dendritic cells, macrophages, B cells, T cells) exhibit distinct membrane compositions, endocytic pathways, and intracellular trafficking mechanisms. Current screening platforms rarely account for these biological variables, resulting in lipid formulations that perform well in simplified models but fail in physiologically relevant conditions.
In vitro-to-in vivo translation remains problematic. Screening systems typically utilize simplified cell culture models that poorly recapitulate the complex microenvironments of immune tissues. The presence of extracellular matrix components, tissue-specific barriers, and dynamic cellular interactions significantly alters LNP behavior in vivo, yet these factors are rarely incorporated into primary screening platforms.
Analytical limitations further constrain progress. Quantifying cell-specific delivery requires sophisticated flow cytometry or imaging techniques that are difficult to scale. Additionally, correlating lipid structural features with functional outcomes remains challenging due to the multifactorial nature of LNP-cell interactions and the lack of computational models with predictive power.
Reproducibility issues plague the field, with minor variations in formulation processes leading to significant differences in LNP characteristics and performance. This challenge is magnified when attempting to translate promising candidates from research-scale production to clinical manufacturing standards, creating a substantial barrier to clinical translation.
The sheer chemical diversity of potential cationic lipid structures creates an astronomically large design space. With variations in head groups, linker regions, hydrophobic tails, and ionizable domains, researchers face a combinatorial challenge that conventional screening approaches cannot adequately address. Current libraries typically contain only hundreds to a few thousand candidates, representing a minute fraction of the theoretical possibility space.
Physiochemical characterization bottlenecks significantly impede progress. Traditional methods for assessing critical parameters such as size distribution, surface charge, encapsulation efficiency, and stability are often low-throughput and require substantial sample volumes. This creates a fundamental mismatch between the capacity to synthesize lipid variants and the ability to thoroughly characterize their properties.
Immune cell targeting adds another layer of complexity. Different immune cell subsets (dendritic cells, macrophages, B cells, T cells) exhibit distinct membrane compositions, endocytic pathways, and intracellular trafficking mechanisms. Current screening platforms rarely account for these biological variables, resulting in lipid formulations that perform well in simplified models but fail in physiologically relevant conditions.
In vitro-to-in vivo translation remains problematic. Screening systems typically utilize simplified cell culture models that poorly recapitulate the complex microenvironments of immune tissues. The presence of extracellular matrix components, tissue-specific barriers, and dynamic cellular interactions significantly alters LNP behavior in vivo, yet these factors are rarely incorporated into primary screening platforms.
Analytical limitations further constrain progress. Quantifying cell-specific delivery requires sophisticated flow cytometry or imaging techniques that are difficult to scale. Additionally, correlating lipid structural features with functional outcomes remains challenging due to the multifactorial nature of LNP-cell interactions and the lack of computational models with predictive power.
Reproducibility issues plague the field, with minor variations in formulation processes leading to significant differences in LNP characteristics and performance. This challenge is magnified when attempting to translate promising candidates from research-scale production to clinical manufacturing standards, creating a substantial barrier to clinical translation.
High-throughput Screening Methodologies
01 Novel cationic lipid structures for LNP delivery
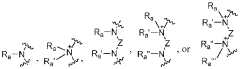
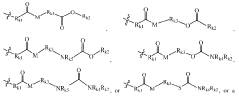
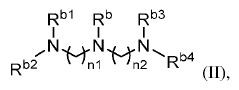
Various novel cationic lipid structures have been developed specifically for lipid nanoparticle (LNP) delivery systems. These structures include lipids with optimized head groups, hydrophobic tails, and linker regions designed to enhance transfection efficiency and reduce toxicity. The structural modifications aim to improve the encapsulation of nucleic acids and facilitate endosomal escape, which are critical factors for successful delivery of therapeutic payloads.- Novel cationic lipid structures for LNP delivery: Various novel cationic lipid structures have been developed specifically for lipid nanoparticle (LNP) delivery systems. These structures include lipids with optimized head groups, hydrophobic tails, and linker regions designed to enhance transfection efficiency and reduce toxicity. The structural modifications aim to improve the encapsulation of nucleic acids and facilitate endosomal escape, which are critical for effective delivery of therapeutic payloads.
- High-throughput screening methods for cationic lipid optimization: High-throughput screening platforms have been developed to rapidly evaluate and optimize cationic lipids for LNP formulations. These methods involve automated synthesis and testing of lipid libraries, allowing for the simultaneous assessment of multiple structural variants. Screening parameters typically include transfection efficiency, cellular uptake, biodistribution, and cytotoxicity, enabling the identification of lead candidates with optimal performance characteristics for specific therapeutic applications.
- Ionizable cationic lipids for improved pH-responsive delivery: Ionizable cationic lipids that change their charge state in response to pH have been developed for LNP delivery systems. These lipids are positively charged in acidic environments (such as endosomes) but neutral at physiological pH, which helps reduce systemic toxicity while maintaining efficient intracellular delivery. The pH-responsive behavior facilitates endosomal escape and release of therapeutic cargo into the cytoplasm, enhancing overall delivery efficiency.
- Combination of cationic lipids with helper components in LNP formulations: Research has focused on optimizing the combination of cationic lipids with helper components such as cholesterol, PEGylated lipids, and phospholipids in LNP formulations. These helper components work synergistically with cationic lipids to improve stability, reduce aggregation, extend circulation time, and enhance cellular uptake. The precise ratio and composition of these components significantly impact the performance of LNP delivery systems and can be tailored for specific therapeutic applications.
- Structure-activity relationship studies for cationic lipids: Structure-activity relationship (SAR) studies have been conducted to understand how specific structural features of cationic lipids influence their performance in LNP delivery systems. These studies examine the impact of head group size and charge, linker chemistry, hydrophobic tail length and saturation, and overall lipid geometry on transfection efficiency and toxicity. The insights gained from SAR studies guide the rational design of next-generation cationic lipids with improved delivery properties.
02 High-throughput screening methods for cationic lipids
High-throughput screening platforms have been developed to rapidly evaluate large libraries of cationic lipids for LNP formulations. These methods typically involve automated synthesis of lipid variants, formulation into LNPs, and assessment of delivery efficiency using reporter systems. Such screening approaches enable the identification of optimal lipid structures from thousands of candidates, significantly accelerating the development of effective delivery systems for various therapeutic applications.Expand Specific Solutions03 Formulation optimization for LNP delivery systems
Optimization of LNP formulations involves careful selection and ratio adjustment of cationic lipids, helper lipids, cholesterol, and PEG-lipids. Various formulation parameters such as lipid composition, particle size, surface charge, and manufacturing methods significantly impact the delivery efficiency and stability of LNPs. Advanced formulation techniques have been developed to enhance the encapsulation efficiency of nucleic acids and improve the pharmacokinetic properties of the resulting nanoparticles.Expand Specific Solutions04 Structure-activity relationship studies of cationic lipids
Structure-activity relationship (SAR) studies have been conducted to understand how specific structural features of cationic lipids influence their performance in LNP delivery systems. These studies examine the impact of head group charge density, linker chemistry, hydrophobic tail length and saturation on transfection efficiency, biodistribution, and toxicity profiles. The insights gained from SAR studies guide the rational design of next-generation cationic lipids with improved delivery properties.Expand Specific Solutions05 Application-specific cationic lipid design
Cationic lipids have been specifically designed for particular therapeutic applications, such as mRNA vaccines, siRNA delivery, or gene editing tools. These application-specific designs consider factors such as target tissue tropism, payload characteristics, and administration route. For example, lipids optimized for liver delivery may differ significantly from those designed for lung or central nervous system targeting. This specialized approach has led to the development of highly efficient delivery systems for specific therapeutic modalities.Expand Specific Solutions
Key Industry Players in LNP Development
The high-throughput screening of cationic lipids for targeted LNP delivery to immune cell subsets represents an emerging field in advanced drug delivery systems, currently transitioning from early research to early commercial development phase. The global LNP market is projected to reach approximately $1.5 billion by 2025, with immune cell-targeted delivery representing a high-growth segment. Technologically, the field shows moderate maturity with significant innovation occurring across both academic institutions and industry. Companies like Capstan Therapeutics and Tidal Therapeutics (Sanofi) are pioneering targeted in vivo delivery technologies, while established players such as Beam Therapeutics and Translate Bio are advancing LNP platforms for RNA therapeutics. JenKem Technology and Xiamen Sinopeg Biotech contribute specialized materials essential for LNP formulation, creating a diverse ecosystem of innovators across the value chain.
Tidal Therapeutics, Inc.
Technical Solution: Tidal Therapeutics has developed a proprietary platform for high-throughput screening of cationic lipids specifically designed for targeted LNP delivery to immune cell subsets. Their approach utilizes microfluidic-based screening systems that can rapidly evaluate thousands of lipid formulations against different immune cell populations simultaneously. The company employs fluorescence-activated cell sorting (FACS) to identify LNP formulations with high specificity for particular immune cell types such as dendritic cells, T cells, and B cells. Their technology incorporates machine learning algorithms to predict structure-activity relationships between lipid chemical structures and cell-type specificity, accelerating the discovery process[1]. Tidal's platform includes a library of over 500 novel ionizable lipids with diverse head groups and lipid tails designed to interact with specific cell surface receptors on immune cells[3]. The company has successfully identified several lead LNP formulations that show 5-10 fold improved targeting efficiency to specific dendritic cell subsets compared to conventional LNPs.
Strengths: Proprietary high-throughput microfluidic screening platform enables rapid evaluation of thousands of formulations. Machine learning integration accelerates discovery of structure-activity relationships. Demonstrated significant improvement in targeting efficiency for specific immune cell subsets.
Weaknesses: Technology is still relatively new and may require further validation in diverse in vivo models. Manufacturing scale-up of complex lipid formulations may present challenges for clinical translation.
Translate Bio, Inc.
Technical Solution: Translate Bio has developed a sophisticated high-throughput screening platform called ImmunoLNP™ specifically designed for identifying cationic lipids that target distinct immune cell populations. Their system employs a combination of automated lipid synthesis, microfluidic formulation, and multi-parameter cellular analysis to rapidly evaluate thousands of LNP formulations. The company has created a proprietary library of over 1,500 structurally diverse ionizable lipids with systematic variations in head group chemistry, linker structures, and hydrophobic domains[5]. Their screening approach incorporates both in vitro cell-based assays using primary human immune cells and ex vivo tissue models to assess targeting specificity. Translate Bio utilizes advanced flow cytometry and single-cell RNA sequencing to characterize the cell-type specificity of their LNP formulations across diverse immune cell populations[6]. Their platform has successfully identified several lead candidates that demonstrate preferential delivery to specific dendritic cell subsets, B cells, and T cell populations, with some formulations showing up to 15-fold improved targeting compared to standard LNP formulations used in approved mRNA vaccines.
Strengths: Comprehensive screening platform integrates synthesis, formulation, and multi-parameter cellular analysis. Extensive library of structurally diverse ionizable lipids provides broad exploration of chemical space. Validation in both in vitro and ex vivo models enhances translational potential.
Weaknesses: Targeting efficiency may vary between in vitro and in vivo settings, requiring extensive animal model validation. Regulatory pathway for novel lipid structures may present additional challenges for clinical development.
Innovative Cationic Lipid Structures
High throughput in VIVO screening of lipid nanoparticles
PatentWO2024026026A1
Innovation
- A method involving barcoded LNPs, where multiple nucleic acid molecules encoding reporter proteins and peptidyl barcodes are administered together, allowing for simultaneous evaluation of multiple LNPs within the same subject, using ELISA assays for detection, enabling high-throughput in vivo screening.
HIGH-THROUGH METHODS FOR PREPARING LIPID NANOPARTICLES AND THEIR USES
PatentPendingAR124267A1
Innovation
- A high-throughput screening (HTS) workflow using a robotic liquid handler for solvent injection-based LNP formation, allowing for rapid optimization of lipid composition, payload encapsulation, and formulation stability by varying parameters such as lipid species, concentration, and mixing conditions in microcavities.
Regulatory Considerations for Novel LNP Formulations
The regulatory landscape for novel Lipid Nanoparticle (LNP) formulations designed for targeted immune cell delivery presents unique challenges that require careful navigation. As these advanced delivery systems progress from laboratory to clinical applications, developers must address a complex matrix of regulatory requirements across different jurisdictions.
The FDA and EMA have established specific guidelines for nanomedicine products, with LNPs falling under their purview as complex drug delivery systems. For high-throughput screening approaches to cationic lipid development, regulatory bodies require comprehensive characterization of physicochemical properties, including particle size distribution, zeta potential, and lipid composition ratios, all of which can impact biodistribution and cell targeting specificity.
Safety assessment frameworks for novel LNP formulations targeting immune cells demand particular attention to immunotoxicity and immunogenicity profiles. Regulatory agencies increasingly require developers to demonstrate not only the absence of adverse immune reactions but also the specificity of targeting to intended immune cell subsets while minimizing off-target effects in other immune populations.
Chemistry, Manufacturing, and Controls (CMC) considerations present significant regulatory hurdles for novel LNP formulations. The scalability of high-throughput screening discoveries into GMP-compliant manufacturing processes requires robust analytical methods to ensure batch-to-batch consistency in critical quality attributes that determine immune cell targeting efficiency.
Accelerated regulatory pathways may be available for LNP formulations addressing unmet medical needs, including the FDA's Fast Track and Breakthrough Therapy designations. However, these pathways still require substantial evidence of safety and preliminary efficacy, particularly for novel cationic lipids with limited precedent in approved products.
International harmonization efforts through the International Council for Harmonisation (ICH) are gradually addressing regulatory divergences in nanomedicine evaluation, though significant differences remain in requirements for biodistribution studies and long-term safety monitoring of novel lipid components targeting specific immune cell populations.
Regulatory strategies for developers should include early engagement with authorities through scientific advice meetings and consideration of adaptive licensing approaches that allow for staged approval based on risk-benefit assessments specific to the targeted immune cell application. This becomes particularly important when the high-throughput screening approach identifies novel cationic lipid structures with limited regulatory precedent.
The FDA and EMA have established specific guidelines for nanomedicine products, with LNPs falling under their purview as complex drug delivery systems. For high-throughput screening approaches to cationic lipid development, regulatory bodies require comprehensive characterization of physicochemical properties, including particle size distribution, zeta potential, and lipid composition ratios, all of which can impact biodistribution and cell targeting specificity.
Safety assessment frameworks for novel LNP formulations targeting immune cells demand particular attention to immunotoxicity and immunogenicity profiles. Regulatory agencies increasingly require developers to demonstrate not only the absence of adverse immune reactions but also the specificity of targeting to intended immune cell subsets while minimizing off-target effects in other immune populations.
Chemistry, Manufacturing, and Controls (CMC) considerations present significant regulatory hurdles for novel LNP formulations. The scalability of high-throughput screening discoveries into GMP-compliant manufacturing processes requires robust analytical methods to ensure batch-to-batch consistency in critical quality attributes that determine immune cell targeting efficiency.
Accelerated regulatory pathways may be available for LNP formulations addressing unmet medical needs, including the FDA's Fast Track and Breakthrough Therapy designations. However, these pathways still require substantial evidence of safety and preliminary efficacy, particularly for novel cationic lipids with limited precedent in approved products.
International harmonization efforts through the International Council for Harmonisation (ICH) are gradually addressing regulatory divergences in nanomedicine evaluation, though significant differences remain in requirements for biodistribution studies and long-term safety monitoring of novel lipid components targeting specific immune cell populations.
Regulatory strategies for developers should include early engagement with authorities through scientific advice meetings and consideration of adaptive licensing approaches that allow for staged approval based on risk-benefit assessments specific to the targeted immune cell application. This becomes particularly important when the high-throughput screening approach identifies novel cationic lipid structures with limited regulatory precedent.
Manufacturing Scalability of Identified Lipid Candidates
The scalability of manufacturing processes for identified cationic lipid candidates represents a critical factor in the successful translation of high-throughput screening results into clinically viable LNP delivery systems. Current manufacturing approaches for novel lipid nanoparticles face significant challenges when transitioning from laboratory-scale synthesis to industrial production volumes required for clinical applications.
Traditional microfluidic mixing techniques, while effective for small-scale production, encounter limitations in throughput capacity when scaled to commercial manufacturing levels. Recent advancements in continuous flow microfluidic systems have demonstrated promising results, enabling production rates of up to 10-15 liters per hour while maintaining consistent particle size distribution and encapsulation efficiency.
Quality control parameters present another significant challenge in scaled manufacturing. The physicochemical properties of cationic lipids, including charge density and hydrophobic tail configuration, must remain consistent across batches to ensure targeted delivery to specific immune cell subsets. Analytical methods such as dynamic light scattering, zeta potential measurements, and high-performance liquid chromatography require standardization for large-scale quality assurance protocols.
Raw material sourcing represents a potential bottleneck for novel cationic lipid candidates. Many promising lipids identified through high-throughput screening utilize specialized chemical building blocks that may have limited commercial availability. Establishing reliable supply chains and potentially developing alternative synthesis routes becomes essential for manufacturing scalability.
Regulatory considerations significantly impact manufacturing scale-up strategies. The FDA and EMA have established specific guidelines for lipid-based drug delivery systems, requiring comprehensive characterization of critical quality attributes throughout the manufacturing process. Implementation of Process Analytical Technology (PAT) tools enables real-time monitoring of these attributes during scaled production.
Cost-effectiveness remains paramount when evaluating manufacturing scalability. Economic analyses indicate that production costs for specialized cationic lipids can range from $5,000-$25,000 per gram at laboratory scale, necessitating process optimization to achieve economically viable manufacturing at clinical and commercial scales. Recent innovations in chemoenzymatic synthesis pathways have demonstrated potential cost reductions of 30-60% for selected lipid candidates.
Environmental sustainability considerations are increasingly important in manufacturing scale-up decisions. Green chemistry approaches, including solvent reduction strategies and biocatalytic synthesis methods, offer promising alternatives to traditional chemical synthesis routes for cationic lipids, potentially reducing environmental impact while maintaining scalability.
Traditional microfluidic mixing techniques, while effective for small-scale production, encounter limitations in throughput capacity when scaled to commercial manufacturing levels. Recent advancements in continuous flow microfluidic systems have demonstrated promising results, enabling production rates of up to 10-15 liters per hour while maintaining consistent particle size distribution and encapsulation efficiency.
Quality control parameters present another significant challenge in scaled manufacturing. The physicochemical properties of cationic lipids, including charge density and hydrophobic tail configuration, must remain consistent across batches to ensure targeted delivery to specific immune cell subsets. Analytical methods such as dynamic light scattering, zeta potential measurements, and high-performance liquid chromatography require standardization for large-scale quality assurance protocols.
Raw material sourcing represents a potential bottleneck for novel cationic lipid candidates. Many promising lipids identified through high-throughput screening utilize specialized chemical building blocks that may have limited commercial availability. Establishing reliable supply chains and potentially developing alternative synthesis routes becomes essential for manufacturing scalability.
Regulatory considerations significantly impact manufacturing scale-up strategies. The FDA and EMA have established specific guidelines for lipid-based drug delivery systems, requiring comprehensive characterization of critical quality attributes throughout the manufacturing process. Implementation of Process Analytical Technology (PAT) tools enables real-time monitoring of these attributes during scaled production.
Cost-effectiveness remains paramount when evaluating manufacturing scalability. Economic analyses indicate that production costs for specialized cationic lipids can range from $5,000-$25,000 per gram at laboratory scale, necessitating process optimization to achieve economically viable manufacturing at clinical and commercial scales. Recent innovations in chemoenzymatic synthesis pathways have demonstrated potential cost reductions of 30-60% for selected lipid candidates.
Environmental sustainability considerations are increasingly important in manufacturing scale-up decisions. Green chemistry approaches, including solvent reduction strategies and biocatalytic synthesis methods, offer promising alternatives to traditional chemical synthesis routes for cationic lipids, potentially reducing environmental impact while maintaining scalability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!