Regulatory and analytics needs for plasmid DNA production used as templates in mRNA manufacturing
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasmid DNA Regulatory Framework and Production Goals
Plasmid DNA (pDNA) has emerged as a critical component in the manufacturing of messenger RNA (mRNA) therapeutics and vaccines, serving as the template for in vitro transcription. The regulatory landscape for pDNA production has evolved significantly over the past decade, reflecting the growing importance of this technology in modern medicine, particularly highlighted by the rapid development of mRNA vaccines during the COVID-19 pandemic.
The regulatory framework for pDNA production spans multiple jurisdictions, with the FDA, EMA, and other global regulatory bodies establishing increasingly specific guidelines. These regulations focus on ensuring the safety, purity, and consistency of pDNA as a starting material for mRNA manufacturing. Key regulatory considerations include the absence of antibiotic resistance genes, minimization of bacterial genomic DNA contamination, and elimination of endotoxins that could compromise patient safety.
Current regulatory standards require comprehensive characterization of pDNA, including sequence verification, supercoiling analysis, and assessment of residual host cell proteins. The FDA's guidance on Chemistry, Manufacturing, and Controls (CMC) for plasmid DNA used in gene therapies provides a foundation, though specific guidance for pDNA as mRNA templates continues to evolve as the technology matures.
The production goals for pDNA in mRNA manufacturing center around achieving high yield, purity, and scalability while maintaining regulatory compliance. Industry targets typically include bacterial genomic DNA contamination below 1%, endotoxin levels under 10 EU/mg, and protein contamination below detectable limits. Additionally, production processes aim for consistent supercoiled pDNA content exceeding 80% to ensure optimal transcription efficiency.
Scalability represents a significant production goal, with manufacturers seeking to transition from laboratory-scale production (milligrams) to industrial-scale manufacturing (multiple grams to kilograms) to meet global vaccine and therapeutic demands. This scale-up must maintain product quality while reducing cost-per-dose to ensure accessibility of resulting mRNA products.
Process robustness and reproducibility constitute another critical production goal, as batch-to-batch consistency is essential for regulatory approval and commercial viability. This includes developing standardized fermentation conditions, optimized cell lysis protocols, and purification strategies that can be validated across multiple production runs.
The intersection of regulatory requirements and production goals has driven innovation in analytical methods for pDNA characterization, including next-generation sequencing for comprehensive genetic analysis, improved chromatographic techniques for purity assessment, and advanced spectroscopic methods for structural verification. These analytical capabilities not only ensure regulatory compliance but also facilitate process optimization to meet ambitious production targets.
The regulatory framework for pDNA production spans multiple jurisdictions, with the FDA, EMA, and other global regulatory bodies establishing increasingly specific guidelines. These regulations focus on ensuring the safety, purity, and consistency of pDNA as a starting material for mRNA manufacturing. Key regulatory considerations include the absence of antibiotic resistance genes, minimization of bacterial genomic DNA contamination, and elimination of endotoxins that could compromise patient safety.
Current regulatory standards require comprehensive characterization of pDNA, including sequence verification, supercoiling analysis, and assessment of residual host cell proteins. The FDA's guidance on Chemistry, Manufacturing, and Controls (CMC) for plasmid DNA used in gene therapies provides a foundation, though specific guidance for pDNA as mRNA templates continues to evolve as the technology matures.
The production goals for pDNA in mRNA manufacturing center around achieving high yield, purity, and scalability while maintaining regulatory compliance. Industry targets typically include bacterial genomic DNA contamination below 1%, endotoxin levels under 10 EU/mg, and protein contamination below detectable limits. Additionally, production processes aim for consistent supercoiled pDNA content exceeding 80% to ensure optimal transcription efficiency.
Scalability represents a significant production goal, with manufacturers seeking to transition from laboratory-scale production (milligrams) to industrial-scale manufacturing (multiple grams to kilograms) to meet global vaccine and therapeutic demands. This scale-up must maintain product quality while reducing cost-per-dose to ensure accessibility of resulting mRNA products.
Process robustness and reproducibility constitute another critical production goal, as batch-to-batch consistency is essential for regulatory approval and commercial viability. This includes developing standardized fermentation conditions, optimized cell lysis protocols, and purification strategies that can be validated across multiple production runs.
The intersection of regulatory requirements and production goals has driven innovation in analytical methods for pDNA characterization, including next-generation sequencing for comprehensive genetic analysis, improved chromatographic techniques for purity assessment, and advanced spectroscopic methods for structural verification. These analytical capabilities not only ensure regulatory compliance but also facilitate process optimization to meet ambitious production targets.
Market Analysis for Plasmid DNA in mRNA Manufacturing
The global market for plasmid DNA in mRNA manufacturing has experienced unprecedented growth following the successful deployment of mRNA vaccines during the COVID-19 pandemic. This market segment is projected to reach $1.2 billion by 2026, growing at a CAGR of 28.7% from 2021. The rapid expansion is primarily driven by the increasing adoption of mRNA therapeutics beyond vaccines, extending into oncology, rare diseases, and autoimmune disorders.
Demand for high-quality plasmid DNA has surged dramatically as pharmaceutical companies scale up mRNA production capabilities. Current market estimates indicate that each commercial-scale mRNA vaccine batch requires approximately 3-5 grams of plasmid DNA as template material, creating substantial recurring demand for GMP-grade plasmids.
Regional analysis reveals North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The remaining 5% is distributed across other regions. This distribution correlates strongly with the concentration of advanced biopharmaceutical manufacturing infrastructure and regulatory frameworks that support novel therapeutic modalities.
Market segmentation shows that pharmaceutical and biotechnology companies constitute the largest customer segment (65%), followed by contract development and manufacturing organizations (CDMOs) (25%) and academic/research institutions (10%). The pharmaceutical segment's dominance reflects the capital-intensive nature of mRNA therapeutic development and commercialization.
Supply chain dynamics present significant market challenges, with current production capacity struggling to meet escalating demand. Lead times for GMP-grade plasmid DNA production have extended to 6-9 months in many cases, creating bottlenecks in mRNA manufacturing pipelines. This supply-demand imbalance has prompted substantial investments in expanding plasmid manufacturing capacity globally.
Pricing trends indicate premium valuations for plasmid DNA meeting stringent regulatory requirements for clinical and commercial applications. Current market rates range from $15,000-$25,000 per gram for research-grade plasmids to $80,000-$150,000 per gram for GMP-grade materials suitable for commercial manufacturing.
Market forecasts suggest continued robust growth as mRNA technology applications expand beyond vaccines into therapeutic areas with larger patient populations. Industry analysts predict that by 2030, plasmid DNA demand for mRNA manufacturing could increase five-fold from current levels, necessitating significant capacity expansion and technological innovation in plasmid production methods.
Demand for high-quality plasmid DNA has surged dramatically as pharmaceutical companies scale up mRNA production capabilities. Current market estimates indicate that each commercial-scale mRNA vaccine batch requires approximately 3-5 grams of plasmid DNA as template material, creating substantial recurring demand for GMP-grade plasmids.
Regional analysis reveals North America dominates the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). The remaining 5% is distributed across other regions. This distribution correlates strongly with the concentration of advanced biopharmaceutical manufacturing infrastructure and regulatory frameworks that support novel therapeutic modalities.
Market segmentation shows that pharmaceutical and biotechnology companies constitute the largest customer segment (65%), followed by contract development and manufacturing organizations (CDMOs) (25%) and academic/research institutions (10%). The pharmaceutical segment's dominance reflects the capital-intensive nature of mRNA therapeutic development and commercialization.
Supply chain dynamics present significant market challenges, with current production capacity struggling to meet escalating demand. Lead times for GMP-grade plasmid DNA production have extended to 6-9 months in many cases, creating bottlenecks in mRNA manufacturing pipelines. This supply-demand imbalance has prompted substantial investments in expanding plasmid manufacturing capacity globally.
Pricing trends indicate premium valuations for plasmid DNA meeting stringent regulatory requirements for clinical and commercial applications. Current market rates range from $15,000-$25,000 per gram for research-grade plasmids to $80,000-$150,000 per gram for GMP-grade materials suitable for commercial manufacturing.
Market forecasts suggest continued robust growth as mRNA technology applications expand beyond vaccines into therapeutic areas with larger patient populations. Industry analysts predict that by 2030, plasmid DNA demand for mRNA manufacturing could increase five-fold from current levels, necessitating significant capacity expansion and technological innovation in plasmid production methods.
Technical Challenges in Plasmid DNA Production
The production of plasmid DNA as templates for mRNA manufacturing faces several significant technical challenges that impact quality, scalability, and regulatory compliance. The complexity begins with bacterial cultivation, where maintaining consistent growth conditions is critical yet difficult to achieve at scale. Variations in temperature, pH, oxygen levels, and nutrient availability can significantly affect plasmid yield and quality, creating batch-to-batch inconsistencies that are problematic for pharmaceutical applications.
Downstream processing presents another major hurdle, particularly in the purification stages. The removal of host cell proteins, genomic DNA, endotoxins, and other bacterial contaminants requires sophisticated chromatography techniques and filtration methods. These purification processes must achieve extremely high levels of purity (>95%) while maintaining the structural integrity of the plasmid DNA, a delicate balance that becomes increasingly difficult at commercial scale.
Analytical characterization of plasmid DNA represents a substantial technical challenge due to the complex nature of these large, supercoiled molecules. Current methods for assessing plasmid identity, purity, potency, and stability have limitations in sensitivity, specificity, and throughput. Techniques such as agarose gel electrophoresis, HPLC, and qPCR each provide valuable but incomplete information, necessitating multiple complementary methods for comprehensive characterization.
Stability issues further complicate plasmid DNA production. These molecules are susceptible to degradation through various mechanisms including oxidation, deamination, and mechanical shearing during processing. Developing formulations that maintain plasmid integrity throughout manufacturing, storage, and transportation remains challenging, particularly as regulatory agencies require increasingly stringent stability data.
Scale-up from laboratory to commercial production introduces additional complexities. Traditional fermentation and purification processes that work efficiently at small scale often encounter unforeseen challenges when expanded. Issues such as heat transfer limitations in larger bioreactors, increased processing times leading to degradation, and filter clogging during purification can significantly impact yield and quality at commercial scale.
Regulatory compliance adds another layer of complexity, with agencies requiring extensive characterization, validation, and consistency data. The lack of harmonized global standards specifically for plasmid DNA as starting materials for mRNA vaccines creates uncertainty in manufacturing requirements, potentially necessitating different processes for different markets.
Downstream processing presents another major hurdle, particularly in the purification stages. The removal of host cell proteins, genomic DNA, endotoxins, and other bacterial contaminants requires sophisticated chromatography techniques and filtration methods. These purification processes must achieve extremely high levels of purity (>95%) while maintaining the structural integrity of the plasmid DNA, a delicate balance that becomes increasingly difficult at commercial scale.
Analytical characterization of plasmid DNA represents a substantial technical challenge due to the complex nature of these large, supercoiled molecules. Current methods for assessing plasmid identity, purity, potency, and stability have limitations in sensitivity, specificity, and throughput. Techniques such as agarose gel electrophoresis, HPLC, and qPCR each provide valuable but incomplete information, necessitating multiple complementary methods for comprehensive characterization.
Stability issues further complicate plasmid DNA production. These molecules are susceptible to degradation through various mechanisms including oxidation, deamination, and mechanical shearing during processing. Developing formulations that maintain plasmid integrity throughout manufacturing, storage, and transportation remains challenging, particularly as regulatory agencies require increasingly stringent stability data.
Scale-up from laboratory to commercial production introduces additional complexities. Traditional fermentation and purification processes that work efficiently at small scale often encounter unforeseen challenges when expanded. Issues such as heat transfer limitations in larger bioreactors, increased processing times leading to degradation, and filter clogging during purification can significantly impact yield and quality at commercial scale.
Regulatory compliance adds another layer of complexity, with agencies requiring extensive characterization, validation, and consistency data. The lack of harmonized global standards specifically for plasmid DNA as starting materials for mRNA vaccines creates uncertainty in manufacturing requirements, potentially necessitating different processes for different markets.
Current Analytical Methods for Plasmid DNA Quality Control
01 Regulatory compliance for plasmid DNA production
Regulatory compliance is essential in plasmid DNA production to ensure safety and efficacy standards are met. This involves adhering to guidelines established by regulatory bodies, implementing quality management systems, and maintaining proper documentation throughout the production process. Compliance requirements cover aspects such as facility design, production methods, testing protocols, and validation procedures to ensure the final product meets established standards for therapeutic or research applications.- Regulatory compliance for plasmid DNA production: Regulatory compliance is essential in plasmid DNA production, particularly for pharmaceutical and therapeutic applications. This involves adhering to Good Manufacturing Practices (GMP), quality control standards, and regulatory guidelines established by authorities such as FDA and EMA. Compliance ensures the safety, efficacy, and consistency of plasmid DNA products, which is critical for their approval and use in clinical settings.
- Analytical methods for plasmid DNA quality assessment: Various analytical techniques are employed to assess the quality and purity of plasmid DNA. These include chromatography, electrophoresis, spectroscopy, and PCR-based methods that evaluate parameters such as identity, purity, potency, and stability. Advanced analytics help detect contaminants, confirm sequence integrity, and ensure the plasmid DNA meets specifications required for research, clinical, or commercial applications.
- Production process optimization and scale-up: Optimizing plasmid DNA production processes involves developing efficient bacterial fermentation strategies, improving cell lysis methods, and enhancing purification techniques. Scale-up considerations include maintaining consistent quality across batch sizes, addressing equipment limitations, and implementing process controls. These optimizations aim to increase yield, reduce costs, and maintain product quality during commercial-scale manufacturing.
- Documentation and data management systems: Comprehensive documentation and data management systems are crucial for plasmid DNA production. These systems track production parameters, analytical results, and quality control data throughout the manufacturing process. Electronic records, laboratory information management systems (LIMS), and specialized software solutions help maintain data integrity, facilitate regulatory submissions, and support audit trails required for compliance with industry standards.
- Quality risk management and validation strategies: Quality risk management approaches identify, assess, and mitigate potential risks in plasmid DNA production. Validation strategies ensure that production processes consistently yield products meeting predetermined specifications. This includes equipment qualification, process validation, cleaning validation, and analytical method validation. Implementing these strategies helps manufacturers comply with regulatory requirements while ensuring product quality and patient safety.
02 Analytical methods for plasmid DNA quality control
Various analytical techniques are employed to assess the quality and purity of plasmid DNA during production. These methods include chromatography, electrophoresis, spectroscopy, and PCR-based assays to evaluate parameters such as DNA concentration, purity, identity, and structural integrity. Advanced analytical tools help detect contaminants, impurities, and structural variations that could affect the functionality and safety of the plasmid DNA product.Expand Specific Solutions03 Production process optimization and scale-up
Optimizing plasmid DNA production processes involves developing efficient methods for bacterial fermentation, cell lysis, and purification to maximize yield while maintaining quality. Scale-up strategies address challenges in transitioning from laboratory to industrial production scales, including bioreactor design, process parameters control, and downstream processing adjustments. These optimizations aim to increase productivity while ensuring consistent product quality across different production batches.Expand Specific Solutions04 Data management and documentation systems
Comprehensive data management systems are crucial for plasmid DNA production to ensure traceability, facilitate regulatory compliance, and support quality assurance. These systems include electronic documentation of production parameters, analytical results, batch records, and deviation reports. Advanced software solutions enable efficient data collection, analysis, storage, and retrieval, supporting both operational efficiency and regulatory requirements for data integrity throughout the product lifecycle.Expand Specific Solutions05 Novel technologies for plasmid DNA production and analysis
Emerging technologies are transforming plasmid DNA production and analysis, including automated systems, continuous manufacturing processes, and advanced analytical platforms. These innovations enhance production efficiency, improve product quality, and enable more precise characterization of plasmid DNA. Novel approaches such as cell-free production systems, microfluidic devices, and real-time monitoring technologies offer potential advantages in terms of speed, scalability, and analytical capabilities for next-generation plasmid DNA manufacturing.Expand Specific Solutions
Key Industry Players in Plasmid DNA Production
The plasmid DNA production market for mRNA manufacturing is currently in a growth phase, with increasing regulatory scrutiny and analytical demands driving innovation. The global market is expanding rapidly, projected to reach significant scale as mRNA therapeutics gain traction beyond COVID-19 vaccines. Companies like Aldevron, GenScript, and Cytiva (Global Life Sciences Solutions) have established strong positions in plasmid manufacturing, while pharmaceutical giants including Pfizer, Moderna, and Novartis are vertically integrating capabilities. CureVac and Applied DNA Sciences are advancing technological innovations in plasmid design and production. The technology is maturing but still evolving, with challenges in scalability, quality control, and regulatory compliance driving continuous improvement in manufacturing processes and analytics.
CureVac SE
Technical Solution: CureVac has engineered a specialized plasmid DNA production platform tailored for their proprietary RNActive® mRNA technology. Their system employs a high-yield fermentation process with optimized growth media formulations that enhance plasmid stability while minimizing bacterial genomic DNA contamination. CureVac's regulatory approach incorporates a comprehensive quality-by-design methodology, with critical process parameters identified and controlled throughout manufacturing[2]. The company has developed specialized analytical methods for plasmid characterization, including advanced sequencing techniques that verify nucleotide composition with single-base resolution and identify potential sequence variants. Their platform includes automated in-process testing systems that monitor plasmid yield and quality attributes during production, allowing for real-time adjustments to maximize consistency. CureVac's regulatory documentation framework is designed to meet both EMA and FDA requirements, with particular attention to ICH Q5D guidelines for cell substrates used in biologics production[5]. Their analytics suite includes specialized assays for supercoiling analysis, residual protein quantification, and endotoxin testing optimized for downstream mRNA applications.
Strengths: Purpose-built system specifically designed for mRNA vaccine applications; extensive experience with regulatory submissions in multiple markets; integrated quality systems that facilitate compliance. Weaknesses: Technology platform may be optimized primarily for CureVac's specific mRNA constructs; potentially higher production costs compared to standard plasmid manufacturing; proprietary nature may limit technology transfer options.
Novartis AG
Technical Solution: Novartis has developed a comprehensive plasmid DNA manufacturing platform that integrates advanced analytics with robust regulatory compliance systems. Their approach utilizes a high-throughput fermentation process with proprietary E. coli strains engineered for enhanced plasmid stability and reduced genomic DNA contamination. The company employs a multi-tiered purification strategy that combines traditional methods with novel chromatographic techniques to achieve exceptional purity profiles that meet stringent regulatory requirements for mRNA template applications[2]. Novartis has implemented an advanced analytical framework that includes next-generation sequencing for complete sequence verification, circular dichroism spectroscopy for structural analysis, and specialized chromatographic methods for supercoiling assessment. Their regulatory strategy incorporates ICH Q8-Q11 principles, with particular emphasis on quality-by-design approaches that establish design spaces for critical process parameters[4]. The company has developed specialized software systems for tracking regulatory compliance across global jurisdictions, facilitating streamlined submission processes for both clinical and commercial applications. Novartis also employs advanced stability monitoring protocols that assess plasmid integrity under various storage conditions, ensuring consistent performance in downstream mRNA manufacturing.
Strengths: Extensive regulatory expertise across multiple markets; sophisticated analytical capabilities for comprehensive characterization; robust quality systems with established compliance history. Weaknesses: Large organizational structure may result in longer development timelines; system may be less flexible for novel constructs requiring rapid adaptation; potentially higher costs associated with comprehensive quality systems.
Critical Patents and Innovations in Plasmid DNA Analytics
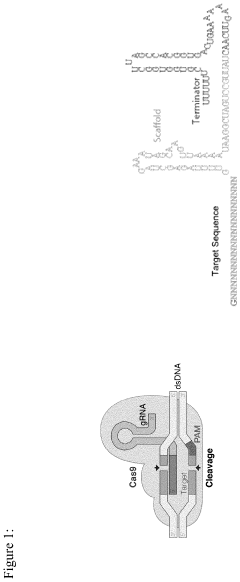
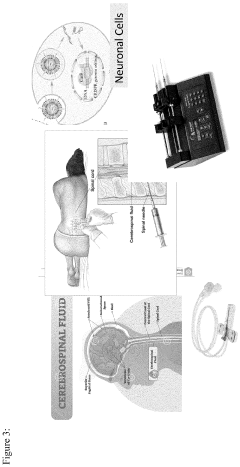
Method of early diagnosis and composition of gRNA for treating mental health diseases using genome editing and cell and gene therapy
PatentPendingUS20240011053A1
Innovation
- A nonviral LNP-mediated CRISPR-Cas9 mRNA-based strategy for in vivo brain-specific genome editing is employed, where mRNA encoding Cas9 endonuclease and guide RNA (gRNA) are delivered directly to the brain or cerebrospinal fluid, minimizing off-target effects by designing gRNA with high specificity and using lipid nanoparticles to enhance delivery efficacy.
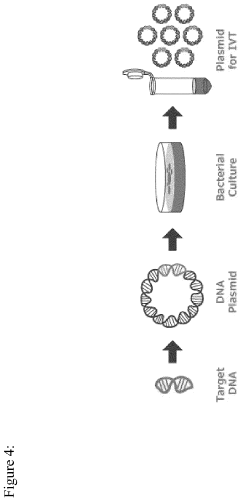
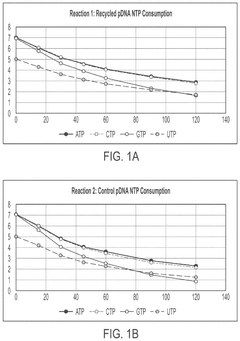
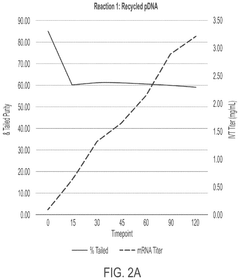
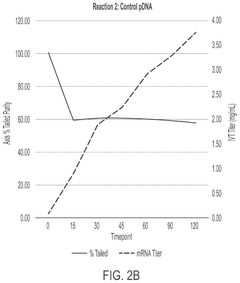
In vitro transcription DNA purification and recycling
PatentPendingUS20250115897A1
Innovation
- A method is developed to recycle the DNA template from IVT reactions by removing mRNA and other reaction components and impurities, resulting in a purified recycled template DNA solution that can be reused in subsequent IVT reactions.
Regulatory Compliance Strategies for GMP Plasmid Production
Regulatory Compliance Strategies for GMP Plasmid Production
The production of plasmid DNA as templates for mRNA manufacturing requires adherence to stringent regulatory frameworks to ensure safety, efficacy, and quality. Regulatory bodies worldwide, including the FDA, EMA, and PMDA, have established specific guidelines for plasmid production under Good Manufacturing Practice (GMP) conditions. These guidelines focus on quality control, process validation, and documentation requirements throughout the production lifecycle.
A comprehensive regulatory compliance strategy begins with understanding the classification of plasmid DNA within the regulatory landscape. Depending on its intended use, plasmid DNA may be classified as an active pharmaceutical ingredient (API), an intermediate, or a critical raw material. This classification determines the applicable regulatory requirements and the level of GMP compliance necessary for production facilities.
Risk-based approaches have become increasingly important in regulatory compliance for plasmid production. This involves systematic identification and mitigation of potential risks associated with the manufacturing process, from bacterial fermentation to downstream purification. Implementing Quality by Design (QbD) principles allows manufacturers to build quality into the process rather than relying solely on final product testing.
Documentation systems form the backbone of regulatory compliance strategies. A robust documentation framework should include standard operating procedures (SOPs), batch records, validation protocols, and change control systems. These documents must demonstrate consistent adherence to established manufacturing processes and provide traceability throughout the production chain.
Analytical method validation represents another critical component of regulatory compliance. Methods used for identity, purity, potency, and safety testing must be validated according to ICH guidelines. This includes demonstrating specificity, accuracy, precision, linearity, and robustness of analytical procedures used to characterize plasmid DNA products.
Supplier qualification programs are essential when sourcing materials for plasmid production. Regulatory agencies expect manufacturers to implement thorough vendor qualification processes, including audits, quality agreements, and ongoing monitoring to ensure the quality and consistency of raw materials.
Environmental monitoring programs must be established to prevent contamination during plasmid production. This includes monitoring for microbial contamination, particulates, and potential cross-contamination, particularly in shared manufacturing facilities. Regulatory bodies increasingly scrutinize these controls to ensure product integrity.
Regulatory filing strategies should be developed early in the product development lifecycle. This includes determining the appropriate regulatory pathways, understanding data requirements for submissions, and planning for regulatory interactions. Early engagement with regulatory authorities through scientific advice meetings can provide valuable guidance on compliance expectations for novel plasmid production processes.
The production of plasmid DNA as templates for mRNA manufacturing requires adherence to stringent regulatory frameworks to ensure safety, efficacy, and quality. Regulatory bodies worldwide, including the FDA, EMA, and PMDA, have established specific guidelines for plasmid production under Good Manufacturing Practice (GMP) conditions. These guidelines focus on quality control, process validation, and documentation requirements throughout the production lifecycle.
A comprehensive regulatory compliance strategy begins with understanding the classification of plasmid DNA within the regulatory landscape. Depending on its intended use, plasmid DNA may be classified as an active pharmaceutical ingredient (API), an intermediate, or a critical raw material. This classification determines the applicable regulatory requirements and the level of GMP compliance necessary for production facilities.
Risk-based approaches have become increasingly important in regulatory compliance for plasmid production. This involves systematic identification and mitigation of potential risks associated with the manufacturing process, from bacterial fermentation to downstream purification. Implementing Quality by Design (QbD) principles allows manufacturers to build quality into the process rather than relying solely on final product testing.
Documentation systems form the backbone of regulatory compliance strategies. A robust documentation framework should include standard operating procedures (SOPs), batch records, validation protocols, and change control systems. These documents must demonstrate consistent adherence to established manufacturing processes and provide traceability throughout the production chain.
Analytical method validation represents another critical component of regulatory compliance. Methods used for identity, purity, potency, and safety testing must be validated according to ICH guidelines. This includes demonstrating specificity, accuracy, precision, linearity, and robustness of analytical procedures used to characterize plasmid DNA products.
Supplier qualification programs are essential when sourcing materials for plasmid production. Regulatory agencies expect manufacturers to implement thorough vendor qualification processes, including audits, quality agreements, and ongoing monitoring to ensure the quality and consistency of raw materials.
Environmental monitoring programs must be established to prevent contamination during plasmid production. This includes monitoring for microbial contamination, particulates, and potential cross-contamination, particularly in shared manufacturing facilities. Regulatory bodies increasingly scrutinize these controls to ensure product integrity.
Regulatory filing strategies should be developed early in the product development lifecycle. This includes determining the appropriate regulatory pathways, understanding data requirements for submissions, and planning for regulatory interactions. Early engagement with regulatory authorities through scientific advice meetings can provide valuable guidance on compliance expectations for novel plasmid production processes.
Risk Assessment and Mitigation in Plasmid DNA Supply Chain
The plasmid DNA supply chain for mRNA vaccine manufacturing presents multiple critical risk points that require systematic assessment and strategic mitigation approaches. Supply chain disruptions can significantly impact production timelines, regulatory compliance, and ultimately patient access to vital therapeutics. Primary risks include raw material shortages, particularly specialized enzymes and nucleotides required for plasmid amplification, which often have limited suppliers globally. These materials frequently face competition from other biotechnology sectors, creating vulnerability during high-demand periods.
Quality inconsistency represents another substantial risk, as batch-to-batch variability in plasmid DNA can compromise downstream mRNA production efficiency and product safety profiles. This variability may stem from production process fluctuations or inconsistent starting materials, potentially triggering regulatory concerns and manufacturing delays.
Contamination risks, particularly from adventitious agents or host cell impurities, pose significant threats to product safety and regulatory acceptance. Even minor contamination events can necessitate batch rejection, causing substantial financial losses and production setbacks. The specialized nature of plasmid DNA production creates additional vulnerability through limited manufacturing capacity, with few contract development and manufacturing organizations (CDMOs) possessing the necessary expertise and infrastructure.
Regulatory compliance risks are particularly acute in this sector, as evolving guidelines for plasmid DNA quality attributes create uncertainty in production parameters. Different regulatory authorities may impose varying requirements, complicating global supply strategies and necessitating market-specific production approaches.
Effective mitigation strategies must include supplier diversification to reduce single-source dependencies, implementing robust quality agreements with detailed specifications and testing protocols. Development of strategic inventory management systems with appropriate safety stocks can buffer against short-term supply disruptions. Investment in analytical technology advancement enables more sensitive detection of impurities and contaminants, supporting both quality control and regulatory compliance efforts.
Continuous process monitoring utilizing real-time analytics and implementing track-and-trace systems throughout the supply chain enhances transparency and enables rapid response to emerging quality issues. Establishing collaborative relationships with regulatory authorities facilitates alignment on evolving requirements and supports proactive compliance strategies. These combined approaches create a resilient supply chain ecosystem capable of maintaining consistent plasmid DNA quality while adapting to production challenges and regulatory developments.
Quality inconsistency represents another substantial risk, as batch-to-batch variability in plasmid DNA can compromise downstream mRNA production efficiency and product safety profiles. This variability may stem from production process fluctuations or inconsistent starting materials, potentially triggering regulatory concerns and manufacturing delays.
Contamination risks, particularly from adventitious agents or host cell impurities, pose significant threats to product safety and regulatory acceptance. Even minor contamination events can necessitate batch rejection, causing substantial financial losses and production setbacks. The specialized nature of plasmid DNA production creates additional vulnerability through limited manufacturing capacity, with few contract development and manufacturing organizations (CDMOs) possessing the necessary expertise and infrastructure.
Regulatory compliance risks are particularly acute in this sector, as evolving guidelines for plasmid DNA quality attributes create uncertainty in production parameters. Different regulatory authorities may impose varying requirements, complicating global supply strategies and necessitating market-specific production approaches.
Effective mitigation strategies must include supplier diversification to reduce single-source dependencies, implementing robust quality agreements with detailed specifications and testing protocols. Development of strategic inventory management systems with appropriate safety stocks can buffer against short-term supply disruptions. Investment in analytical technology advancement enables more sensitive detection of impurities and contaminants, supporting both quality control and regulatory compliance efforts.
Continuous process monitoring utilizing real-time analytics and implementing track-and-trace systems throughout the supply chain enhances transparency and enables rapid response to emerging quality issues. Establishing collaborative relationships with regulatory authorities facilitates alignment on evolving requirements and supports proactive compliance strategies. These combined approaches create a resilient supply chain ecosystem capable of maintaining consistent plasmid DNA quality while adapting to production challenges and regulatory developments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!