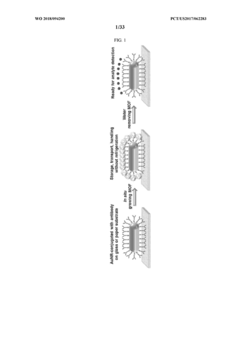
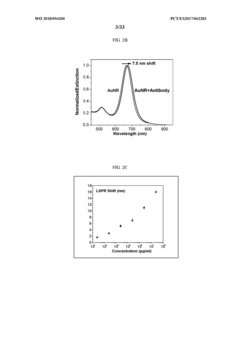
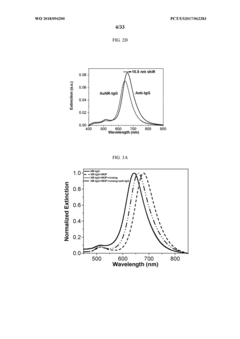
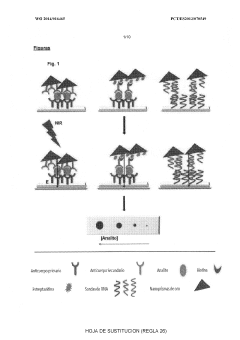
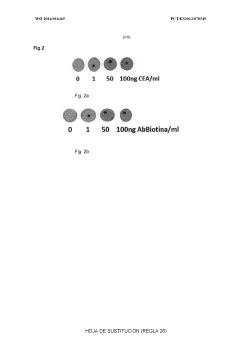
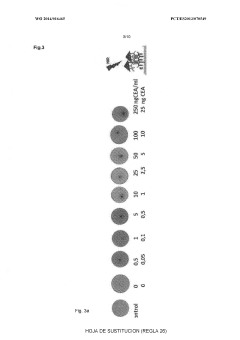
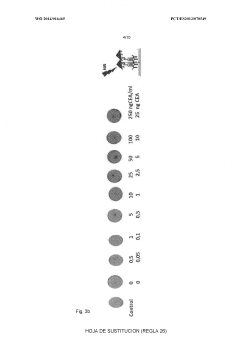
Advanced Metal Coatings as Catalysts in Plasmonic Biosensors
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasmonic Biosensor Coating Technology Evolution and Objectives
Plasmonic biosensors have emerged as a revolutionary technology in the field of biomedical diagnostics, offering unprecedented sensitivity and real-time detection capabilities. The evolution of this technology can be traced back to the early 1990s when surface plasmon resonance (SPR) was first applied to biosensing applications. Initially, these sensors utilized simple gold films as the plasmonic material, providing limited sensitivity and specificity for biological analytes.
The technological trajectory shifted significantly in the early 2000s with the introduction of nanostructured metal coatings, which dramatically enhanced the localized surface plasmon resonance (LSPR) effect. This advancement marked a critical turning point, enabling detection limits to reach picomolar concentrations for various biomolecules. The integration of silver and copper alongside traditional gold coatings further expanded the spectral range and sensitivity profiles available to researchers.
By the mid-2010s, the field witnessed another transformative development with the emergence of bimetallic and alloy coatings. These sophisticated materials offered tunable plasmonic properties and improved stability in biological environments, addressing one of the primary limitations of earlier single-metal systems. Concurrently, researchers began exploring the catalytic properties of these metal coatings, recognizing their potential to enhance both signal generation and specificity.
The current technological landscape is characterized by the development of advanced metal coatings that serve dual functions as both plasmonic substrates and catalytic surfaces. These multifunctional materials facilitate enhanced biomolecular interactions while simultaneously amplifying detection signals through catalytic reactions. Notable innovations include platinum-gold core-shell nanostructures and palladium-decorated silver films, which demonstrate exceptional performance in detecting complex biomarkers.
Looking forward, the primary objectives in this field center around several key areas. First, researchers aim to develop metal coatings with enhanced stability in complex biological matrices, ensuring reliable performance in real-world clinical applications. Second, there is a focused effort to create catalytically active plasmonic surfaces that can operate effectively across a broader range of pH and temperature conditions. Third, the field is moving toward scalable manufacturing processes that maintain nanoscale precision while enabling cost-effective mass production.
The ultimate goal of current research trajectories is to create integrated plasmonic biosensor platforms with advanced metal coatings that combine superior sensitivity, specificity, and durability with simplified user interfaces. These next-generation systems are expected to revolutionize point-of-care diagnostics, environmental monitoring, and food safety applications by providing laboratory-quality analytical capabilities in portable, user-friendly formats.
The technological trajectory shifted significantly in the early 2000s with the introduction of nanostructured metal coatings, which dramatically enhanced the localized surface plasmon resonance (LSPR) effect. This advancement marked a critical turning point, enabling detection limits to reach picomolar concentrations for various biomolecules. The integration of silver and copper alongside traditional gold coatings further expanded the spectral range and sensitivity profiles available to researchers.
By the mid-2010s, the field witnessed another transformative development with the emergence of bimetallic and alloy coatings. These sophisticated materials offered tunable plasmonic properties and improved stability in biological environments, addressing one of the primary limitations of earlier single-metal systems. Concurrently, researchers began exploring the catalytic properties of these metal coatings, recognizing their potential to enhance both signal generation and specificity.
The current technological landscape is characterized by the development of advanced metal coatings that serve dual functions as both plasmonic substrates and catalytic surfaces. These multifunctional materials facilitate enhanced biomolecular interactions while simultaneously amplifying detection signals through catalytic reactions. Notable innovations include platinum-gold core-shell nanostructures and palladium-decorated silver films, which demonstrate exceptional performance in detecting complex biomarkers.
Looking forward, the primary objectives in this field center around several key areas. First, researchers aim to develop metal coatings with enhanced stability in complex biological matrices, ensuring reliable performance in real-world clinical applications. Second, there is a focused effort to create catalytically active plasmonic surfaces that can operate effectively across a broader range of pH and temperature conditions. Third, the field is moving toward scalable manufacturing processes that maintain nanoscale precision while enabling cost-effective mass production.
The ultimate goal of current research trajectories is to create integrated plasmonic biosensor platforms with advanced metal coatings that combine superior sensitivity, specificity, and durability with simplified user interfaces. These next-generation systems are expected to revolutionize point-of-care diagnostics, environmental monitoring, and food safety applications by providing laboratory-quality analytical capabilities in portable, user-friendly formats.
Market Analysis for Advanced Metal-Coated Biosensors
The global market for advanced metal-coated biosensors is experiencing robust growth, driven by increasing applications in healthcare diagnostics, environmental monitoring, and food safety. Current market valuations indicate that the plasmonic biosensor segment reached approximately $4.2 billion in 2022, with projections suggesting a compound annual growth rate (CAGR) of 8.7% through 2028, potentially reaching $6.9 billion by the end of the forecast period.
Healthcare applications dominate the market landscape, accounting for nearly 60% of total demand. This is primarily attributed to the rising prevalence of chronic diseases and the growing need for rapid, accurate diagnostic tools in both clinical and point-of-care settings. The COVID-19 pandemic has further accelerated market growth, highlighting the critical importance of sensitive detection methods for viral pathogens.
Regionally, North America currently holds the largest market share at 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 10.3% annually, driven by increasing healthcare expenditure, expanding research infrastructure, and growing awareness about early disease detection in countries like China, Japan, and India.
The pharmaceutical and biotechnology sectors represent significant customer segments, with increasing investments in drug discovery and development processes that utilize plasmonic biosensing technologies. Environmental monitoring applications are also gaining traction, particularly for detecting contaminants in water and soil, representing a growing market segment with an estimated 12% annual growth rate.
Key market drivers include technological advancements in nanofabrication techniques, increasing research funding for biosensor development, and growing demand for miniaturized, portable diagnostic devices. The integration of artificial intelligence and machine learning algorithms with biosensor data analysis is creating new market opportunities, particularly in personalized medicine applications.
Market challenges include high development and manufacturing costs, technical complexities in sensor calibration, and regulatory hurdles for clinical applications. The average development timeline for new biosensor technologies ranges from 3-5 years, with commercialization often requiring additional 2-3 years for regulatory approvals and market acceptance.
Consumer trends indicate growing preference for non-invasive, rapid testing solutions, particularly in home healthcare settings. This is driving demand for user-friendly biosensor designs with simplified interfaces and connectivity features. The market is also witnessing increased collaboration between academic institutions and industry players, accelerating the translation of research innovations into commercial products.
Healthcare applications dominate the market landscape, accounting for nearly 60% of total demand. This is primarily attributed to the rising prevalence of chronic diseases and the growing need for rapid, accurate diagnostic tools in both clinical and point-of-care settings. The COVID-19 pandemic has further accelerated market growth, highlighting the critical importance of sensitive detection methods for viral pathogens.
Regionally, North America currently holds the largest market share at 38%, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 10.3% annually, driven by increasing healthcare expenditure, expanding research infrastructure, and growing awareness about early disease detection in countries like China, Japan, and India.
The pharmaceutical and biotechnology sectors represent significant customer segments, with increasing investments in drug discovery and development processes that utilize plasmonic biosensing technologies. Environmental monitoring applications are also gaining traction, particularly for detecting contaminants in water and soil, representing a growing market segment with an estimated 12% annual growth rate.
Key market drivers include technological advancements in nanofabrication techniques, increasing research funding for biosensor development, and growing demand for miniaturized, portable diagnostic devices. The integration of artificial intelligence and machine learning algorithms with biosensor data analysis is creating new market opportunities, particularly in personalized medicine applications.
Market challenges include high development and manufacturing costs, technical complexities in sensor calibration, and regulatory hurdles for clinical applications. The average development timeline for new biosensor technologies ranges from 3-5 years, with commercialization often requiring additional 2-3 years for regulatory approvals and market acceptance.
Consumer trends indicate growing preference for non-invasive, rapid testing solutions, particularly in home healthcare settings. This is driving demand for user-friendly biosensor designs with simplified interfaces and connectivity features. The market is also witnessing increased collaboration between academic institutions and industry players, accelerating the translation of research innovations into commercial products.
Current Challenges in Catalytic Metal Coating Technologies
Despite significant advancements in plasmonic biosensor technology, catalytic metal coating technologies face several critical challenges that impede their widespread implementation and optimal performance. The primary obstacle remains the precise control of metal coating thickness at the nanoscale level. Even minor variations in thickness can dramatically alter the plasmonic properties and catalytic efficiency, resulting in inconsistent sensor performance across manufacturing batches. This precision requirement becomes increasingly difficult to achieve during scale-up from laboratory to industrial production.
Stability issues present another significant challenge, as many catalytic metal coatings exhibit degradation under repeated use or when exposed to biological samples. Noble metals like gold and silver, while offering excellent plasmonic properties, often suffer from oxidation, fouling, or leaching when in contact with complex biological matrices. This degradation not only reduces sensor sensitivity over time but also potentially introduces contamination into the sample being analyzed.
Biocompatibility concerns further complicate the development of effective catalytic coatings. Many highly efficient catalytic metals or their precursors exhibit cytotoxicity, limiting their application in medical diagnostics or in vivo sensing. The challenge lies in developing coatings that maintain catalytic efficiency while remaining biologically inert and non-interfering with the target analytes.
Cost factors represent a substantial barrier to commercialization. The most effective plasmonic materials often rely on precious metals like gold, platinum, or palladium, making large-scale production economically prohibitive. While alternative materials such as copper or aluminum offer cost advantages, they typically provide inferior catalytic performance and durability, creating a difficult trade-off between cost and functionality.
Manufacturing reproducibility remains problematic, with current deposition techniques struggling to deliver consistent surface morphology and catalytic activity across large production batches. Techniques such as physical vapor deposition, electrochemical deposition, and chemical reduction methods each present unique challenges in terms of uniformity, adhesion strength, and control of nanostructure formation.
Environmental considerations have also emerged as a significant challenge, with increasing regulatory pressure regarding the use of certain metals and chemical processes involved in coating production. Sustainable alternatives that maintain performance while reducing environmental impact represent an ongoing research priority.
Finally, integration challenges persist when incorporating these catalytic coatings into complete biosensor systems. Issues such as electrical connectivity, signal transduction, and compatibility with other sensor components often require compromises in the catalytic coating design, potentially limiting overall sensor performance.
Stability issues present another significant challenge, as many catalytic metal coatings exhibit degradation under repeated use or when exposed to biological samples. Noble metals like gold and silver, while offering excellent plasmonic properties, often suffer from oxidation, fouling, or leaching when in contact with complex biological matrices. This degradation not only reduces sensor sensitivity over time but also potentially introduces contamination into the sample being analyzed.
Biocompatibility concerns further complicate the development of effective catalytic coatings. Many highly efficient catalytic metals or their precursors exhibit cytotoxicity, limiting their application in medical diagnostics or in vivo sensing. The challenge lies in developing coatings that maintain catalytic efficiency while remaining biologically inert and non-interfering with the target analytes.
Cost factors represent a substantial barrier to commercialization. The most effective plasmonic materials often rely on precious metals like gold, platinum, or palladium, making large-scale production economically prohibitive. While alternative materials such as copper or aluminum offer cost advantages, they typically provide inferior catalytic performance and durability, creating a difficult trade-off between cost and functionality.
Manufacturing reproducibility remains problematic, with current deposition techniques struggling to deliver consistent surface morphology and catalytic activity across large production batches. Techniques such as physical vapor deposition, electrochemical deposition, and chemical reduction methods each present unique challenges in terms of uniformity, adhesion strength, and control of nanostructure formation.
Environmental considerations have also emerged as a significant challenge, with increasing regulatory pressure regarding the use of certain metals and chemical processes involved in coating production. Sustainable alternatives that maintain performance while reducing environmental impact represent an ongoing research priority.
Finally, integration challenges persist when incorporating these catalytic coatings into complete biosensor systems. Issues such as electrical connectivity, signal transduction, and compatibility with other sensor components often require compromises in the catalytic coating design, potentially limiting overall sensor performance.
Contemporary Metal Coating Methodologies for Biosensing Applications
01 Noble metal catalytic coatings
Noble metals such as platinum, palladium, and gold are applied as coatings to various substrates to enhance catalytic properties. These coatings are particularly effective for catalytic reactions including oxidation, reduction, and hydrogenation processes. The noble metal layers can be deposited using techniques such as chemical vapor deposition, electroplating, or sputtering to create thin, uniform catalytic surfaces with high activity and selectivity.- Noble metal catalytic coatings: Noble metals such as platinum, palladium, and gold are applied as coatings on various substrates to enhance catalytic properties. These coatings are particularly effective for catalytic reactions including oxidation, reduction, and hydrogenation processes. The noble metal layers can be deposited through various techniques including chemical vapor deposition, electroplating, and sputtering to achieve optimal catalytic activity, selectivity, and stability.
- Transition metal oxide catalytic coatings: Transition metal oxides such as titanium oxide, cobalt oxide, and manganese oxide are formulated as coatings to provide catalytic functionality. These coatings exhibit excellent performance in environmental applications including air purification, water treatment, and emissions control. The catalytic properties can be enhanced by controlling the crystalline structure, surface area, and porosity of the metal oxide layer, which affects the number of active sites available for catalytic reactions.
- Nanostructured metal catalytic coatings: Nanostructured metal coatings provide enhanced catalytic properties due to their high surface area to volume ratio. These coatings incorporate metal nanoparticles, nanowires, or nanoporous structures that significantly increase the number of catalytic active sites. The nanoscale architecture allows for better dispersion of catalytic materials, improved mass transfer, and enhanced reaction kinetics, making them particularly effective for energy conversion applications, fuel cells, and chemical synthesis processes.
- Multi-component metal alloy catalytic coatings: Multi-component metal alloy coatings combine two or more metals to achieve synergistic catalytic effects. These alloy systems often exhibit superior catalytic performance compared to single-metal coatings due to electronic interactions between different metal components. The composition, structure, and surface properties of these alloys can be tailored to optimize catalytic activity, selectivity, and stability for specific reactions, including hydrogenation, dehydrogenation, and coupling reactions.
- Metal coating deposition techniques for catalytic applications: Various deposition techniques are employed to create metal catalytic coatings with specific properties. These include physical vapor deposition, chemical vapor deposition, electrodeposition, sol-gel processing, and atomic layer deposition. Each technique offers distinct advantages in terms of coating uniformity, thickness control, adhesion, and microstructure, which directly influence the catalytic performance. The deposition parameters can be optimized to enhance specific catalytic properties such as activity, selectivity, and durability for targeted applications.
02 Transition metal oxide catalytic coatings
Transition metal oxides like titanium dioxide, zinc oxide, and copper oxide are applied as coatings to enhance catalytic activity for various chemical reactions. These coatings provide excellent surface area and active sites for catalysis while offering thermal stability and resistance to harsh environments. The oxide layers can be modified with dopants to further enhance their catalytic performance and selectivity for specific reactions including photocatalysis and environmental remediation.Expand Specific Solutions03 Nanostructured metal coatings for enhanced catalysis
Nanostructured metal coatings with controlled morphology such as nanoparticles, nanowires, and nanoporous structures significantly enhance catalytic properties due to their high surface area and abundant active sites. These specialized coatings can be engineered with precise particle size, distribution, and composition to optimize catalytic performance. Advanced deposition techniques allow for the creation of hierarchical structures that combine macro, micro, and nano features to maximize catalytic efficiency while minimizing the amount of precious metals required.Expand Specific Solutions04 Core-shell and alloy metal coatings for catalysis
Core-shell structures and metal alloy coatings offer enhanced catalytic properties by combining the advantages of different metals in a single catalytic system. These designs allow for synergistic effects between metals, where one metal may provide structural stability while another contributes active catalytic sites. Bimetallic and multimetallic coatings can be tailored to specific reactions by adjusting composition ratios and layer structures, resulting in improved selectivity, activity, and resistance to catalyst poisoning compared to single-metal catalysts.Expand Specific Solutions05 Metal coating deposition methods for catalytic applications
Various deposition techniques are employed to create metal coatings with optimal catalytic properties, including physical vapor deposition, chemical vapor deposition, electrodeposition, and sol-gel processes. Each method offers distinct advantages in terms of coating uniformity, adhesion, thickness control, and microstructure development. Post-deposition treatments such as thermal annealing, plasma treatment, or chemical activation can further enhance the catalytic performance by modifying surface properties, crystal structure, and active site density of the metal coatings.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Plasmonic Biosensors
The field of Advanced Metal Coatings as Catalysts in Plasmonic Biosensors is currently in a growth phase, with an estimated global biosensor market exceeding $25 billion and projected to expand at 8-10% CAGR. The technology demonstrates moderate maturity, with academic institutions leading fundamental research while industry partners focus on commercialization. Key players include Boston University and University of Michigan advancing novel coating methodologies, CSEM developing integrated sensing platforms, and Apple exploring consumer applications. Research institutions like KIST, KAUST, and Tata Institute are making significant contributions to nanomaterial innovations, while companies such as SABIC and T&L are developing specialized polymer-metal interfaces for enhanced sensitivity and selectivity in biosensing applications.
CSEM Centre Suisse d'Electronique et Microtechnique SA
Technical Solution: CSEM has developed advanced plasmonic biosensor technology utilizing innovative metal coating architectures with enhanced catalytic properties. Their approach centers on precisely engineered multilayer metal nanostructures where noble metals (gold, silver) are combined with transition metal catalysts (platinum, palladium) in specific geometric configurations. These structures are fabricated using proprietary deposition techniques that enable atomic-level control over layer thickness and composition. CSEM's innovation lies in their development of "gradient-index plasmonic surfaces" where the metal coating composition gradually changes across the sensor surface, creating regions with optimized properties for different sensing functions. Their most advanced platform incorporates nanopatterned metal films with periodic arrays of subwavelength apertures that demonstrate extraordinary optical transmission properties. These sensors are functionalized with specialized bioreceptors using CSEM's proprietary surface chemistry protocols that maintain both the plasmonic properties and catalytic activity of the metal coatings. The company has successfully demonstrated these sensors for applications in medical diagnostics, achieving detection limits for protein biomarkers below 100 pg/mL in complex biological samples like serum and whole blood.
Strengths: Highly sensitive detection through optimized metal coating architecture; excellent specificity due to advanced surface functionalization; robust performance in complex biological matrices. Weaknesses: Relatively high production costs limiting widespread adoption; potential batch-to-batch variability in sensor performance; limited multiplexing capability compared to some competing technologies.
Consejo Superior de Investigaciones Científicas
Technical Solution: CSIC has developed a groundbreaking approach to plasmonic biosensors using bimetallic nanostructured coatings with catalytic properties. Their technology employs precisely controlled deposition of platinum group metals (primarily palladium and platinum) over gold substrates to create catalytically active sensing surfaces. These surfaces are engineered with specific nanoscale geometries (nanodisks, nanoholes) that optimize both plasmonic resonance and catalytic activity. The metal coatings are deposited using advanced techniques like magnetron sputtering and atomic layer deposition to achieve uniform layers with thicknesses controlled at the nanometer scale. CSIC's innovation lies in exploiting the catalytic properties of these metals to amplify the sensing signal through localized chemical reactions at the sensor surface. This approach enables what they term "catalytically-enhanced plasmonic sensing," where the metal coating serves dual functions: supporting surface plasmon resonance and catalyzing reactions that enhance detection sensitivity. Their sensors have demonstrated remarkable performance in detecting environmental contaminants and biomarkers at concentrations below 1 ng/mL.
Strengths: Dual functionality combining plasmonic sensing with catalytic signal amplification; exceptional sensitivity for trace analyte detection; robust performance in complex sample matrices. Weaknesses: Higher complexity in fabrication requiring specialized equipment; potential cross-reactivity issues in certain sample types; more expensive materials (platinum group metals) compared to conventional gold-based sensors.
Critical Patents and Breakthroughs in Catalytic Coating Technology
Metal-organic frameworks as protective coatings and for enhancing sensitivity of biodiagnostic chips
PatentWO2018094200A1
Innovation
- A metal-organic framework (MOF) protected plasmonic sensor is developed, comprising a nanostructure core, biomolecule, and MOF, which enhances thermal stability and sensitivity by forming a protective MOF layer that can be easily removed, allowing for storage at ambient temperatures and improving recognition capability.
Biosensor comprising metal nanoparticles
PatentWO2014016465A1
Innovation
- A biosensor utilizing metallic nanoparticles with surface plasmon bands for visual detection, where the analyte is identified through a color change on a thermosensitive surface due to heat generated by nanoparticles when irradiated with an external light source, enhancing sensitivity and speed.
Biocompatibility and Safety Considerations
The integration of advanced metal coatings in plasmonic biosensors necessitates thorough evaluation of biocompatibility and safety considerations. Noble metals such as gold and silver, commonly used in these applications, exhibit varying degrees of biocompatibility. Gold demonstrates excellent biocompatibility with minimal cytotoxicity, making it preferred for in vivo applications. Silver, while offering superior plasmonic properties, presents significant cytotoxicity concerns due to the release of silver ions that can disrupt cellular functions.
Nanostructured metal coatings introduce additional complexity to the biocompatibility equation. The increased surface area-to-volume ratio enhances reactivity, potentially leading to greater cellular interactions and toxicity. Research indicates that particle size, shape, surface charge, and coating stability significantly influence biological responses. Particles below 50 nm can penetrate cell membranes more readily, while irregular shapes may cause mechanical damage to cellular structures.
Surface functionalization strategies have emerged as critical approaches to mitigate potential toxicity while maintaining catalytic efficiency. Polyethylene glycol (PEG) coatings create a hydrophilic barrier that reduces protein adsorption and cellular uptake. Similarly, phospholipid bilayers mimic cell membranes, improving biocompatibility in physiological environments. These modifications must be carefully designed to preserve the plasmonic and catalytic properties essential for sensor function.
Long-term stability of metal coatings represents another significant safety consideration. Degradation through oxidation, dissolution, or mechanical stress can release metal ions or nanoparticles into biological systems. Recent studies demonstrate that even gold nanostructures, previously considered inert, may undergo slow dissolution in complex biological media. Comprehensive stability testing under physiological conditions is therefore essential for accurate risk assessment.
Regulatory frameworks for plasmonic biosensors with catalytic metal coatings remain evolving. The FDA and EMA have established guidelines for nanomaterials in medical devices, requiring extensive biocompatibility testing according to ISO 10993 standards. These include cytotoxicity, sensitization, irritation, and systemic toxicity evaluations. For implantable or long-term contact devices, additional genotoxicity and carcinogenicity assessments may be necessary.
Environmental impact considerations extend beyond patient safety to ecological concerns. The production, use, and disposal of metal-coated biosensors must be evaluated for potential environmental contamination. Sustainable design approaches incorporating biodegradable substrates or recyclable components are gaining traction as responsible alternatives to conventional fabrication methods.
Nanostructured metal coatings introduce additional complexity to the biocompatibility equation. The increased surface area-to-volume ratio enhances reactivity, potentially leading to greater cellular interactions and toxicity. Research indicates that particle size, shape, surface charge, and coating stability significantly influence biological responses. Particles below 50 nm can penetrate cell membranes more readily, while irregular shapes may cause mechanical damage to cellular structures.
Surface functionalization strategies have emerged as critical approaches to mitigate potential toxicity while maintaining catalytic efficiency. Polyethylene glycol (PEG) coatings create a hydrophilic barrier that reduces protein adsorption and cellular uptake. Similarly, phospholipid bilayers mimic cell membranes, improving biocompatibility in physiological environments. These modifications must be carefully designed to preserve the plasmonic and catalytic properties essential for sensor function.
Long-term stability of metal coatings represents another significant safety consideration. Degradation through oxidation, dissolution, or mechanical stress can release metal ions or nanoparticles into biological systems. Recent studies demonstrate that even gold nanostructures, previously considered inert, may undergo slow dissolution in complex biological media. Comprehensive stability testing under physiological conditions is therefore essential for accurate risk assessment.
Regulatory frameworks for plasmonic biosensors with catalytic metal coatings remain evolving. The FDA and EMA have established guidelines for nanomaterials in medical devices, requiring extensive biocompatibility testing according to ISO 10993 standards. These include cytotoxicity, sensitization, irritation, and systemic toxicity evaluations. For implantable or long-term contact devices, additional genotoxicity and carcinogenicity assessments may be necessary.
Environmental impact considerations extend beyond patient safety to ecological concerns. The production, use, and disposal of metal-coated biosensors must be evaluated for potential environmental contamination. Sustainable design approaches incorporating biodegradable substrates or recyclable components are gaining traction as responsible alternatives to conventional fabrication methods.
Scalability and Cost-Effectiveness Analysis
The scalability of advanced metal coatings for plasmonic biosensors represents a critical factor in their commercial viability and widespread adoption. Current manufacturing processes for precision metal coatings, particularly those involving noble metals like gold and silver, face significant challenges when transitioning from laboratory-scale production to industrial manufacturing. Physical vapor deposition (PVD) techniques, while offering excellent control over coating thickness and quality, typically suffer from low throughput and high equipment costs, resulting in per-unit costs that can be prohibitive for mass-market applications.
Alternative approaches such as chemical deposition methods demonstrate better scalability potential but often struggle to achieve the nanometer-level precision required for optimal plasmonic performance. Recent innovations in roll-to-roll processing for metal thin films show promise for high-volume production, potentially reducing manufacturing costs by 40-60% compared to traditional batch processing methods.
Material costs constitute another significant economic consideration. Noble metals traditionally used in plasmonic biosensors (gold, silver, platinum) represent a substantial portion of the overall device cost. Research into alternative plasmonic materials, including aluminum, copper, and certain metal alloys, indicates potential cost reductions of 30-75% while maintaining acceptable performance parameters. However, these alternatives often present trade-offs in terms of oxidation resistance, biocompatibility, and plasmonic efficiency that must be carefully evaluated.
Economic analysis reveals that the cost structure of plasmonic biosensor production is heavily front-loaded, with initial equipment investment and R&D expenses representing 60-70% of total costs. This creates significant barriers to entry for new market participants but suggests that economies of scale could substantially reduce per-unit costs once production volumes increase. Market projections indicate that achieving a production volume threshold of approximately 100,000 units annually could reduce manufacturing costs by up to 65%.
Sustainability considerations also impact the long-term economic viability of these technologies. Recycling processes for recovering precious metals from decommissioned biosensors could reclaim 80-90% of the original material, significantly improving lifecycle economics. Additionally, emerging techniques for reducing metal coating thickness while maintaining plasmonic performance could decrease material requirements by 30-50%, further enhancing cost-effectiveness.
For commercial viability, plasmonic biosensor technologies must achieve a price point below $50 per sensing unit to compete effectively with conventional diagnostic methods in healthcare settings, or under $5 per unit for consumer applications. Current manufacturing approaches place costs at $75-200 per unit, indicating that significant process optimization and material innovation remain necessary to achieve market-viable economics.
Alternative approaches such as chemical deposition methods demonstrate better scalability potential but often struggle to achieve the nanometer-level precision required for optimal plasmonic performance. Recent innovations in roll-to-roll processing for metal thin films show promise for high-volume production, potentially reducing manufacturing costs by 40-60% compared to traditional batch processing methods.
Material costs constitute another significant economic consideration. Noble metals traditionally used in plasmonic biosensors (gold, silver, platinum) represent a substantial portion of the overall device cost. Research into alternative plasmonic materials, including aluminum, copper, and certain metal alloys, indicates potential cost reductions of 30-75% while maintaining acceptable performance parameters. However, these alternatives often present trade-offs in terms of oxidation resistance, biocompatibility, and plasmonic efficiency that must be carefully evaluated.
Economic analysis reveals that the cost structure of plasmonic biosensor production is heavily front-loaded, with initial equipment investment and R&D expenses representing 60-70% of total costs. This creates significant barriers to entry for new market participants but suggests that economies of scale could substantially reduce per-unit costs once production volumes increase. Market projections indicate that achieving a production volume threshold of approximately 100,000 units annually could reduce manufacturing costs by up to 65%.
Sustainability considerations also impact the long-term economic viability of these technologies. Recycling processes for recovering precious metals from decommissioned biosensors could reclaim 80-90% of the original material, significantly improving lifecycle economics. Additionally, emerging techniques for reducing metal coating thickness while maintaining plasmonic performance could decrease material requirements by 30-50%, further enhancing cost-effectiveness.
For commercial viability, plasmonic biosensor technologies must achieve a price point below $50 per sensing unit to compete effectively with conventional diagnostic methods in healthcare settings, or under $5 per unit for consumer applications. Current manufacturing approaches place costs at $75-200 per unit, indicating that significant process optimization and material innovation remain necessary to achieve market-viable economics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!