What Drives Patents in Plasmonic Biosensor Technologies
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasmonic Biosensor Technology Background and Objectives
Plasmonic biosensor technology represents a revolutionary approach in the field of biosensing, combining the principles of plasmonics with biological detection mechanisms. The evolution of this technology can be traced back to the early 1990s when researchers first began exploring the potential of surface plasmon resonance (SPR) for biological sensing applications. Over the subsequent decades, the field has witnessed remarkable advancements, transitioning from basic SPR sensors to more sophisticated localized surface plasmon resonance (LSPR) platforms and nanostructured plasmonic materials.
The technological trajectory has been characterized by continuous improvements in sensitivity, selectivity, and miniaturization. Early plasmonic biosensors were primarily laboratory-based instruments requiring specialized expertise, but recent developments have pushed toward portable, user-friendly devices suitable for point-of-care diagnostics. This evolution reflects the broader trend in biosensing technology toward decentralized testing and real-time monitoring capabilities.
Patent activities in plasmonic biosensor technologies have shown exponential growth since the early 2000s, with significant acceleration observed after 2010. This surge coincides with breakthroughs in nanofabrication techniques and increased understanding of plasmon-enhanced spectroscopy. The patent landscape reveals a progressive shift from fundamental sensing principles to application-specific innovations targeting medical diagnostics, environmental monitoring, and food safety.
The primary technical objective in this field is to develop highly sensitive, specific, and reliable biosensing platforms capable of detecting biomarkers at increasingly lower concentrations. Current research aims to achieve detection limits in the femtomolar to attomolar range, representing a thousand-fold improvement over conventional methods. Additionally, there is a strong focus on multiplexed detection capabilities, allowing simultaneous analysis of multiple analytes from a single sample.
Another critical objective driving patent activity is the integration of plasmonic biosensors with complementary technologies such as microfluidics, smartphone-based readout systems, and artificial intelligence for data interpretation. This convergence aims to create comprehensive diagnostic platforms that combine the high sensitivity of plasmonic detection with user-friendly interfaces and automated analysis.
Looking forward, the field is trending toward sustainable and cost-effective manufacturing processes, biodegradable materials, and energy-efficient designs. These developments align with global sustainability goals and address the need for accessible diagnostic technologies in resource-limited settings. The ultimate technological goal remains the creation of reliable, affordable plasmonic biosensing platforms that can democratize advanced diagnostic capabilities across diverse healthcare environments.
The technological trajectory has been characterized by continuous improvements in sensitivity, selectivity, and miniaturization. Early plasmonic biosensors were primarily laboratory-based instruments requiring specialized expertise, but recent developments have pushed toward portable, user-friendly devices suitable for point-of-care diagnostics. This evolution reflects the broader trend in biosensing technology toward decentralized testing and real-time monitoring capabilities.
Patent activities in plasmonic biosensor technologies have shown exponential growth since the early 2000s, with significant acceleration observed after 2010. This surge coincides with breakthroughs in nanofabrication techniques and increased understanding of plasmon-enhanced spectroscopy. The patent landscape reveals a progressive shift from fundamental sensing principles to application-specific innovations targeting medical diagnostics, environmental monitoring, and food safety.
The primary technical objective in this field is to develop highly sensitive, specific, and reliable biosensing platforms capable of detecting biomarkers at increasingly lower concentrations. Current research aims to achieve detection limits in the femtomolar to attomolar range, representing a thousand-fold improvement over conventional methods. Additionally, there is a strong focus on multiplexed detection capabilities, allowing simultaneous analysis of multiple analytes from a single sample.
Another critical objective driving patent activity is the integration of plasmonic biosensors with complementary technologies such as microfluidics, smartphone-based readout systems, and artificial intelligence for data interpretation. This convergence aims to create comprehensive diagnostic platforms that combine the high sensitivity of plasmonic detection with user-friendly interfaces and automated analysis.
Looking forward, the field is trending toward sustainable and cost-effective manufacturing processes, biodegradable materials, and energy-efficient designs. These developments align with global sustainability goals and address the need for accessible diagnostic technologies in resource-limited settings. The ultimate technological goal remains the creation of reliable, affordable plasmonic biosensing platforms that can democratize advanced diagnostic capabilities across diverse healthcare environments.
Market Analysis of Plasmonic Biosensor Applications
The global market for plasmonic biosensor technologies has experienced significant growth in recent years, driven by increasing demand for rapid, sensitive, and portable diagnostic tools across multiple sectors. The current market size is estimated to reach $1.2 billion by 2025, with a compound annual growth rate of approximately 8.7% from 2020 to 2025, reflecting the expanding applications and technological advancements in this field.
Healthcare and clinical diagnostics represent the largest application segment, accounting for over 40% of the market share. This dominance stems from the critical need for early disease detection, point-of-care testing, and personalized medicine approaches. The COVID-19 pandemic has further accelerated market growth, highlighting the importance of rapid diagnostic capabilities in managing public health crises.
Food safety and environmental monitoring constitute the second-largest application segment. Growing concerns about foodborne illnesses, water contamination, and environmental pollutants have increased demand for sensitive detection methods. Regulatory requirements across different countries have also contributed to market expansion in these sectors.
Pharmaceutical and biotechnology research applications form another significant market segment. The ability of plasmonic biosensors to provide label-free, real-time monitoring of biomolecular interactions makes them valuable tools in drug discovery, development, and quality control processes.
Geographically, North America leads the market with approximately 35% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is projected to witness the highest growth rate due to increasing healthcare expenditure, growing awareness about early disease diagnosis, and expanding research infrastructure in countries like China, Japan, and India.
Key market drivers include technological advancements that improve sensitivity, specificity, and multiplexing capabilities of plasmonic biosensors. Miniaturization trends and integration with smartphone technology are expanding point-of-care applications, particularly in resource-limited settings. The growing prevalence of chronic diseases and aging populations worldwide further fuels market demand for rapid diagnostic solutions.
Market challenges include high development costs, technical complexity in sensor design, and competition from alternative biosensing technologies. Additionally, regulatory hurdles for clinical applications and standardization issues across different platforms may impede market growth in certain segments.
Emerging opportunities exist in wearable biosensor development, integration with artificial intelligence for data analysis, and applications in emerging fields such as precision agriculture and food authentication. These trends suggest continued market expansion and diversification of plasmonic biosensor applications in the coming years.
Healthcare and clinical diagnostics represent the largest application segment, accounting for over 40% of the market share. This dominance stems from the critical need for early disease detection, point-of-care testing, and personalized medicine approaches. The COVID-19 pandemic has further accelerated market growth, highlighting the importance of rapid diagnostic capabilities in managing public health crises.
Food safety and environmental monitoring constitute the second-largest application segment. Growing concerns about foodborne illnesses, water contamination, and environmental pollutants have increased demand for sensitive detection methods. Regulatory requirements across different countries have also contributed to market expansion in these sectors.
Pharmaceutical and biotechnology research applications form another significant market segment. The ability of plasmonic biosensors to provide label-free, real-time monitoring of biomolecular interactions makes them valuable tools in drug discovery, development, and quality control processes.
Geographically, North America leads the market with approximately 35% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is projected to witness the highest growth rate due to increasing healthcare expenditure, growing awareness about early disease diagnosis, and expanding research infrastructure in countries like China, Japan, and India.
Key market drivers include technological advancements that improve sensitivity, specificity, and multiplexing capabilities of plasmonic biosensors. Miniaturization trends and integration with smartphone technology are expanding point-of-care applications, particularly in resource-limited settings. The growing prevalence of chronic diseases and aging populations worldwide further fuels market demand for rapid diagnostic solutions.
Market challenges include high development costs, technical complexity in sensor design, and competition from alternative biosensing technologies. Additionally, regulatory hurdles for clinical applications and standardization issues across different platforms may impede market growth in certain segments.
Emerging opportunities exist in wearable biosensor development, integration with artificial intelligence for data analysis, and applications in emerging fields such as precision agriculture and food authentication. These trends suggest continued market expansion and diversification of plasmonic biosensor applications in the coming years.
Global Landscape and Technical Barriers in Plasmonic Biosensing
The global landscape of plasmonic biosensing technologies reveals significant geographical concentration in research and development activities. North America, particularly the United States, maintains leadership with approximately 40% of global patents and publications, driven by substantial funding from agencies like NIH and DARPA, alongside strong university-industry collaborations. Europe follows closely with 30% market share, demonstrating particular strength in theoretical research and novel material applications, with countries like Germany, UK, and France leading innovation efforts through programs such as Horizon Europe.
Asia has emerged as the fastest-growing region, with China's rapid advancement particularly noteworthy. Chinese institutions have increased their patent filings by over 300% in the past decade, focusing on mass-production techniques and cost reduction strategies. Japan and South Korea maintain specialized niches in miniaturization and integration technologies for plasmonic biosensors.
Despite this global progress, several technical barriers persist across regions. Signal-to-noise ratio limitations remain a fundamental challenge, with current technologies struggling to achieve consistent detection below picomolar concentrations in complex biological samples. This challenge is particularly pronounced in point-of-care applications where environmental variables cannot be tightly controlled.
Fabrication scalability presents another significant hurdle. While laboratory-scale production of plasmonic nanostructures demonstrates excellent performance, transitioning to mass production while maintaining nanoscale precision and reproducibility remains problematic. Current fabrication techniques often suffer from batch-to-batch variations exceeding 15%, limiting commercial viability.
Biocompatibility and surface functionalization issues continue to impede clinical applications. The long-term stability of biomolecule-nanoparticle interfaces in physiological conditions rarely exceeds several days, insufficient for many monitoring applications. Additionally, non-specific binding in complex biological matrices like whole blood or tissue extracts can generate false positive rates as high as 20-30% in some systems.
Integration challenges with existing diagnostic platforms represent another barrier. Most plasmonic biosensing technologies require specialized equipment and expertise, limiting their adoption in standard clinical settings. The lack of standardized protocols for performance evaluation further complicates comparative assessments across different technological approaches.
Regulatory pathways remain unclear in many jurisdictions, with FDA and EMA guidelines still evolving for these novel diagnostic technologies. This regulatory uncertainty has slowed investment in late-stage development and commercialization efforts, particularly for in vivo applications where safety concerns are paramount.
Asia has emerged as the fastest-growing region, with China's rapid advancement particularly noteworthy. Chinese institutions have increased their patent filings by over 300% in the past decade, focusing on mass-production techniques and cost reduction strategies. Japan and South Korea maintain specialized niches in miniaturization and integration technologies for plasmonic biosensors.
Despite this global progress, several technical barriers persist across regions. Signal-to-noise ratio limitations remain a fundamental challenge, with current technologies struggling to achieve consistent detection below picomolar concentrations in complex biological samples. This challenge is particularly pronounced in point-of-care applications where environmental variables cannot be tightly controlled.
Fabrication scalability presents another significant hurdle. While laboratory-scale production of plasmonic nanostructures demonstrates excellent performance, transitioning to mass production while maintaining nanoscale precision and reproducibility remains problematic. Current fabrication techniques often suffer from batch-to-batch variations exceeding 15%, limiting commercial viability.
Biocompatibility and surface functionalization issues continue to impede clinical applications. The long-term stability of biomolecule-nanoparticle interfaces in physiological conditions rarely exceeds several days, insufficient for many monitoring applications. Additionally, non-specific binding in complex biological matrices like whole blood or tissue extracts can generate false positive rates as high as 20-30% in some systems.
Integration challenges with existing diagnostic platforms represent another barrier. Most plasmonic biosensing technologies require specialized equipment and expertise, limiting their adoption in standard clinical settings. The lack of standardized protocols for performance evaluation further complicates comparative assessments across different technological approaches.
Regulatory pathways remain unclear in many jurisdictions, with FDA and EMA guidelines still evolving for these novel diagnostic technologies. This regulatory uncertainty has slowed investment in late-stage development and commercialization efforts, particularly for in vivo applications where safety concerns are paramount.
Current Technical Solutions in Plasmonic Biosensor Design
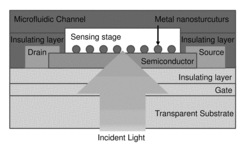
01 Surface plasmon resonance (SPR) biosensor technologies
Surface plasmon resonance biosensors utilize the optical phenomenon that occurs at the interface between a metal and a dielectric medium to detect biomolecular interactions. These biosensors offer label-free, real-time detection with high sensitivity for various analytes. The technology involves measuring changes in the refractive index near the sensor surface when target molecules bind to immobilized receptors, causing a shift in the resonance angle or wavelength that can be quantified to determine concentration or binding affinity.- Surface plasmon resonance (SPR) biosensor technologies: Surface plasmon resonance biosensors utilize the optical phenomenon occurring at metal-dielectric interfaces to detect biomolecular interactions without labels. These biosensors measure changes in refractive index near the sensor surface when target analytes bind to immobilized receptors. The technology offers real-time, label-free detection with high sensitivity for applications in medical diagnostics, environmental monitoring, and pharmaceutical research.
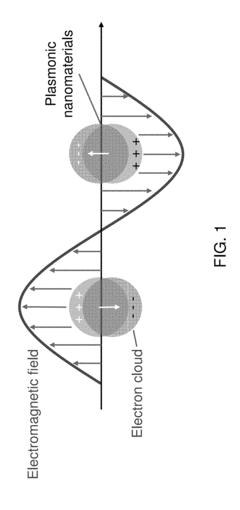
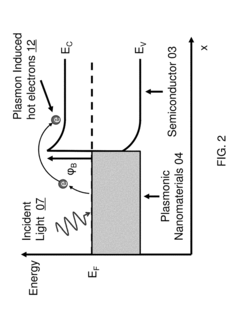
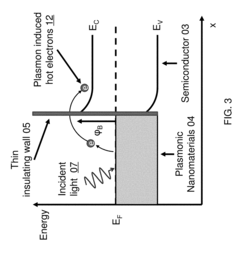
- Nanostructured plasmonic biosensors: Nanostructured plasmonic biosensors incorporate engineered metallic nanostructures such as nanoparticles, nanorods, and nanoholes to enhance sensing capabilities. These nanostructures create localized surface plasmon resonance effects that significantly improve detection sensitivity and enable miniaturization. The technology allows for detection of biomolecules at extremely low concentrations and can be integrated into portable diagnostic devices for point-of-care applications.
- Integrated plasmonic-photonic biosensing systems: Integrated plasmonic-photonic biosensing systems combine plasmonic elements with photonic components such as waveguides, resonators, and interferometers to create hybrid sensing platforms. These integrated systems leverage the advantages of both technologies to achieve enhanced sensitivity, multiplexing capabilities, and improved signal-to-noise ratios. The integration enables compact, chip-scale devices suitable for high-throughput screening and complex bioanalytical applications.
- Plasmonic biosensors with advanced surface functionalization: Plasmonic biosensors with advanced surface functionalization techniques employ specialized chemical modifications to enhance selectivity and reduce non-specific binding. These approaches include self-assembled monolayers, polymer brushes, and biomimetic interfaces that optimize the immobilization of capture molecules while maintaining their biological activity. The functionalized surfaces improve sensor performance in complex biological samples such as blood, serum, or environmental matrices.
- Multiplexed plasmonic biosensor arrays: Multiplexed plasmonic biosensor arrays enable simultaneous detection of multiple analytes on a single sensing platform. These arrays incorporate spatially separated sensing elements, each functionalized with different recognition molecules, allowing parallel analysis of numerous biomarkers. The technology utilizes advanced imaging techniques, microfluidics, and data processing algorithms to extract and analyze signals from multiple sensing spots, providing comprehensive diagnostic information from a single test.
02 Nanostructured plasmonic biosensors
Nanostructured plasmonic biosensors incorporate engineered metallic nanostructures such as nanoparticles, nanorods, or nanoholes to enhance sensing capabilities. These nanostructures create localized surface plasmon resonance (LSPR) effects that concentrate electromagnetic fields, significantly improving detection sensitivity and enabling the detection of smaller analytes or lower concentrations. The nanostructured approach allows for miniaturization of sensing platforms while maintaining or improving performance metrics compared to conventional plasmonic sensors.Expand Specific Solutions03 Integrated plasmonic biosensor systems
Integrated plasmonic biosensor systems combine plasmonic sensing elements with microfluidics, electronics, and data processing components to create complete analytical platforms. These integrated systems automate sample handling, signal acquisition, and data analysis, making them suitable for point-of-care diagnostics and field applications. The integration enables multiplexed detection of multiple analytes simultaneously and improves reproducibility by controlling environmental factors that might affect measurement accuracy.Expand Specific Solutions04 Plasmonic biosensors for specific biomedical applications
Plasmonic biosensors designed for specific biomedical applications focus on detecting disease biomarkers, pathogens, or therapeutic drug monitoring. These specialized biosensors incorporate recognition elements such as antibodies, aptamers, or molecularly imprinted polymers that provide high specificity for target analytes relevant to clinical diagnostics. The sensing platforms are optimized for biological samples like blood, urine, or saliva, with features to minimize interference from non-specific binding and matrix effects while maintaining sensitivity in complex biological environments.Expand Specific Solutions05 Signal enhancement techniques for plasmonic biosensors
Signal enhancement techniques for plasmonic biosensors employ various strategies to improve detection limits and sensitivity. These include secondary amplification methods using enzymatic reactions, sandwich assay configurations, and plasmonic coupling effects between nanostructures. Advanced signal processing algorithms and machine learning approaches are also implemented to extract meaningful data from complex sensor responses. These enhancement techniques enable the detection of ultra-low concentrations of analytes, extending the application range of plasmonic biosensors to areas requiring exceptional sensitivity.Expand Specific Solutions
Leading Companies and Research Institutions in Plasmonic Biosensors
The plasmonic biosensor technology patent landscape is evolving within a rapidly growing market, currently in its growth phase with increasing commercial applications. The field is characterized by strong academic leadership from institutions like MIT, Washington University, and EPFL, alongside corporate innovation from established players such as Corning, Texas Instruments, and Philips. While academic institutions dominate fundamental research, companies like Precision Biosensor and BOE Technology are commercializing applications. The technology shows varying maturity levels across applications, with point-of-care diagnostics reaching commercial readiness while more advanced applications remain in development. This competitive landscape reflects the technology's transition from research to commercial implementation, with increasing cross-sector collaborations accelerating market adoption.
École Polytechnique Fédérale de Lausanne
Technical Solution: 洛桑联邦理工学院(EPFL)在等离子体生物传感器领域的专利技术主要围绕纳米光子学和集成光学系统展开。其核心技术方案包括开发基于亚波长金属纳米结构的等离子体传感平台,通过精确设计纳米天线和光子晶体结构,实现对生物分子的超灵敏检测。EPFL研究人员开发了创新的纳米光学结构,如金属-绝缘体-金属(MIM)波导和等离子体纳米聚焦器,能够将光场局限在极小体积内,显著增强生物分子与光场的相互作用。该校还开发了集成光子芯片上的等离子体传感阵列,结合微流控技术和实时数据处理算法,实现多参数并行检测。EPFL的专利技术特别强调传感器的微型化和集成度,已应用于单细胞分析、蛋白质组学研究和环境监测领域,检测灵敏度达到纳摩尔(10^-9)级别。
优势:纳米光子学和微纳加工技术领先,跨学科研究能力强,产学研合作紧密,欧洲研发网络资源丰富。劣势:技术商业化进程相对缓慢,专利保护地域性限制,与产业界的技术转移机制有待完善。
Massachusetts Institute of Technology
Technical Solution: MIT在等离子体生物传感器领域开发了基于表面等离子体共振(SPR)的高灵敏度传感平台,结合纳米结构金属薄膜和专有信号处理算法。其技术方案包括使用纳米孔阵列增强局部表面等离子体共振效应,实现单分子检测能力。MIT研究人员开发的等离子体生物传感器可同时检测多种生物标志物,灵敏度达到飞摩尔(10^-15)级别,比传统ELISA方法提高约100倍。该技术还整合了微流控系统,实现样品自动化处理和实时数据分析,特别适用于即时检测(POC)应用场景。MIT的专利组合涵盖了从基础纳米结构设计到完整检测系统的全产业链技术保护。
优势:拥有领先的纳米制造技术和信号处理算法,检测灵敏度极高,多重检测能力强,学术影响力大。缺点:技术转化为商业产品的周期较长,制造成本较高,部分高端应用场景市场规模有限。
Key Patent Analysis and Technological Breakthroughs
Portable plasmonic system for disease detection
PatentActiveUS20180217138A1
Innovation
- A portable biosensing system utilizing a plasmonic field effect transistor or photoconductor platform with a microfluidic control unit, back illumination, and a lock-in amplifier to directly convert plasmonic energy into an electrical signal, enabling real-time measurement and multiplexing capabilities.
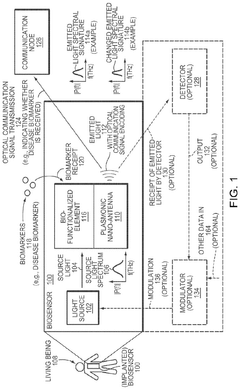
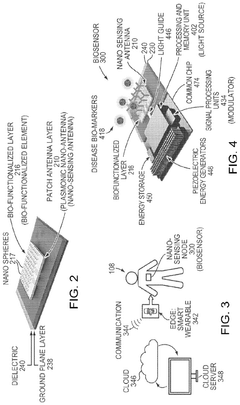
Implantable Biosensor and Communication Node With Plasmonic Nano-Antenna
PatentPendingUS20240350045A1
Innovation
- The development of biosensors and communication nodes equipped with plasmonic nano-antennas that can sense biomarkers and transmit optical communication signals, leveraging a chirp-spread spectrum excitation and detection method for simultaneous communication and sensing, and utilizing edge computing and networking for data processing and transmission.
Intellectual Property Strategy and Patent Landscape
The patent landscape for plasmonic biosensor technologies reveals strategic patterns that drive innovation and market positioning in this rapidly evolving field. Analysis of patent filings shows concentrated activity in three primary jurisdictions: the United States, Europe, and China, with the US maintaining leadership in terms of both quantity and citation impact. This geographic distribution reflects the significant investment in biosensor research infrastructure and commercial applications in these regions.
Patent filing trends indicate a substantial increase in plasmonic biosensor patents over the past decade, with a compound annual growth rate of approximately 18%. This acceleration correlates with breakthroughs in nanofabrication techniques and increased demand for point-of-care diagnostic solutions. The patent landscape is characterized by a mix of broad foundational patents covering core plasmonic principles and more specialized applications targeting specific biomarkers or detection methodologies.
Key patent holders include both established multinational corporations and specialized biotechnology firms. Companies like Roche, Abbott, and Philips maintain extensive patent portfolios focused on clinical applications, while academic institutions such as MIT, Stanford, and the Chinese Academy of Sciences lead in fundamental research patents. This dichotomy creates a complex licensing environment where commercial development often requires cross-licensing agreements between multiple stakeholders.
Patent analysis reveals four primary innovation drivers: enhanced sensitivity through novel nanostructures, multiplexed detection capabilities, miniaturization for portable applications, and integration with complementary technologies such as microfluidics and artificial intelligence. The most valuable patents typically combine innovations across multiple categories, creating robust intellectual property positions that are difficult to circumvent.
Strategic patent filing patterns demonstrate a clear evolution from broad conceptual claims toward more specific implementation details and system integration approaches. Early-stage patents focused on fundamental plasmonic principles have given way to application-specific innovations targeting particular disease biomarkers or environmental contaminants. This progression reflects the technology's maturation and increasing commercial relevance.
For emerging players in this space, strategic options include: developing improvements to existing technologies while navigating around established patents, focusing on underserved application areas with less patent density, or pursuing collaborative development through licensing agreements with key patent holders. The fragmented nature of the patent landscape creates opportunities for innovative approaches that combine elements from multiple technical domains while establishing new intellectual property positions.
Patent filing trends indicate a substantial increase in plasmonic biosensor patents over the past decade, with a compound annual growth rate of approximately 18%. This acceleration correlates with breakthroughs in nanofabrication techniques and increased demand for point-of-care diagnostic solutions. The patent landscape is characterized by a mix of broad foundational patents covering core plasmonic principles and more specialized applications targeting specific biomarkers or detection methodologies.
Key patent holders include both established multinational corporations and specialized biotechnology firms. Companies like Roche, Abbott, and Philips maintain extensive patent portfolios focused on clinical applications, while academic institutions such as MIT, Stanford, and the Chinese Academy of Sciences lead in fundamental research patents. This dichotomy creates a complex licensing environment where commercial development often requires cross-licensing agreements between multiple stakeholders.
Patent analysis reveals four primary innovation drivers: enhanced sensitivity through novel nanostructures, multiplexed detection capabilities, miniaturization for portable applications, and integration with complementary technologies such as microfluidics and artificial intelligence. The most valuable patents typically combine innovations across multiple categories, creating robust intellectual property positions that are difficult to circumvent.
Strategic patent filing patterns demonstrate a clear evolution from broad conceptual claims toward more specific implementation details and system integration approaches. Early-stage patents focused on fundamental plasmonic principles have given way to application-specific innovations targeting particular disease biomarkers or environmental contaminants. This progression reflects the technology's maturation and increasing commercial relevance.
For emerging players in this space, strategic options include: developing improvements to existing technologies while navigating around established patents, focusing on underserved application areas with less patent density, or pursuing collaborative development through licensing agreements with key patent holders. The fragmented nature of the patent landscape creates opportunities for innovative approaches that combine elements from multiple technical domains while establishing new intellectual property positions.
Regulatory Framework and Standardization Challenges
The regulatory landscape for plasmonic biosensor technologies presents significant challenges for patent development and commercialization. Currently, there exists a fragmented regulatory framework across different regions, with the FDA in the United States, the EMA in Europe, and the NMPA in China each maintaining distinct requirements for biosensor approval. These disparities create substantial hurdles for companies seeking global market access, often necessitating multiple parallel development pathways to satisfy various regulatory bodies.
Standardization remains particularly underdeveloped in the plasmonic biosensor field, with no universally accepted protocols for performance validation, sensitivity thresholds, or reproducibility metrics. This lack of standardization directly impacts patent quality and defensibility, as claims regarding sensor performance cannot be evaluated against established benchmarks. The International Organization for Standardization (ISO) has begun addressing this gap through technical committees focused on nanotechnologies and biocompatibility, but comprehensive standards specific to plasmonic biosensors remain elusive.
Regulatory considerations significantly influence patent strategies in this domain. Companies must navigate complex requirements for clinical validation, particularly for diagnostic applications where regulatory bodies demand extensive clinical trials. This regulatory burden creates a patent paradox: while early filing is essential to secure intellectual property rights, premature filing before regulatory pathway clarity may result in patents that fail to adequately protect commercially viable implementations.
The intersection of multiple regulatory domains further complicates the patent landscape. Plasmonic biosensors often fall under medical device regulations, laboratory developed test frameworks, and in some cases, pharmaceutical companion diagnostic rules. This regulatory overlap creates uncertainty regarding which standards apply, potentially leaving patent holders vulnerable to post-market challenges if their technology fails to meet evolving regulatory expectations.
Recent regulatory developments show promising trends toward harmonization. The International Medical Device Regulators Forum (IMDRF) has initiated efforts to create globally recognized standards for innovative diagnostic technologies, including certain biosensor platforms. Similarly, the Clinical Laboratory Standards Institute (CLSI) has published guidelines for analytical validation that increasingly incorporate novel sensing technologies. These harmonization efforts may eventually streamline the path from patent to market, reducing the current fragmentation that hampers innovation.
Standardization remains particularly underdeveloped in the plasmonic biosensor field, with no universally accepted protocols for performance validation, sensitivity thresholds, or reproducibility metrics. This lack of standardization directly impacts patent quality and defensibility, as claims regarding sensor performance cannot be evaluated against established benchmarks. The International Organization for Standardization (ISO) has begun addressing this gap through technical committees focused on nanotechnologies and biocompatibility, but comprehensive standards specific to plasmonic biosensors remain elusive.
Regulatory considerations significantly influence patent strategies in this domain. Companies must navigate complex requirements for clinical validation, particularly for diagnostic applications where regulatory bodies demand extensive clinical trials. This regulatory burden creates a patent paradox: while early filing is essential to secure intellectual property rights, premature filing before regulatory pathway clarity may result in patents that fail to adequately protect commercially viable implementations.
The intersection of multiple regulatory domains further complicates the patent landscape. Plasmonic biosensors often fall under medical device regulations, laboratory developed test frameworks, and in some cases, pharmaceutical companion diagnostic rules. This regulatory overlap creates uncertainty regarding which standards apply, potentially leaving patent holders vulnerable to post-market challenges if their technology fails to meet evolving regulatory expectations.
Recent regulatory developments show promising trends toward harmonization. The International Medical Device Regulators Forum (IMDRF) has initiated efforts to create globally recognized standards for innovative diagnostic technologies, including certain biosensor platforms. Similarly, the Clinical Laboratory Standards Institute (CLSI) has published guidelines for analytical validation that increasingly incorporate novel sensing technologies. These harmonization efforts may eventually streamline the path from patent to market, reducing the current fragmentation that hampers innovation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!