Plasmonic Biosensors Adaptation to Changing Pharma Standards
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasmonic Biosensor Evolution and Objectives
Plasmonic biosensors have evolved significantly over the past three decades, transforming from rudimentary surface plasmon resonance (SPR) devices into sophisticated integrated sensing platforms. The journey began in the early 1990s with the commercialization of the first SPR instruments by Biacore, which revolutionized label-free biomolecular interaction analysis. These initial systems, while groundbreaking, were limited by their bulky instrumentation, high cost, and relatively low sensitivity compared to today's standards.
The mid-2000s marked a pivotal shift with the emergence of localized surface plasmon resonance (LSPR) technology, which leveraged the unique optical properties of metallic nanostructures. This innovation significantly reduced the form factor of plasmonic biosensors while enhancing their sensitivity to molecular interactions at the nanoscale. Concurrently, advances in nanofabrication techniques enabled more precise control over plasmonic structures, leading to improved reproducibility and performance.
The pharmaceutical industry's evolving regulatory landscape has continuously shaped the development trajectory of plasmonic biosensors. Increasingly stringent requirements for drug safety testing, quality control, and process analytical technology (PAT) have driven the need for more sensitive, reliable, and versatile sensing platforms. The FDA's Quality by Design (QbD) initiative and similar frameworks worldwide have emphasized the importance of real-time monitoring throughout the pharmaceutical manufacturing process.
Recent technological advancements have focused on addressing key limitations in specificity, multiplexing capability, and integration with existing pharmaceutical workflows. The incorporation of microfluidics, advanced surface chemistry, and artificial intelligence for data analysis has expanded the application scope of plasmonic biosensors in drug discovery, development, and manufacturing quality control.
The primary objective of current research is to develop next-generation plasmonic biosensing platforms that can seamlessly adapt to evolving pharmaceutical standards while maintaining optimal performance. This includes enhancing detection limits to meet increasingly lower thresholds for contaminants and biomarkers, improving specificity to minimize false positives in complex biological matrices, and ensuring compatibility with automated high-throughput screening systems.
Another critical goal is to establish standardized validation protocols specifically designed for plasmonic biosensors in pharmaceutical applications. This standardization would facilitate regulatory approval processes and encourage wider adoption across the industry. Additionally, research aims to reduce the cost and complexity of these systems, making advanced biosensing technology more accessible to smaller pharmaceutical companies and academic research institutions.
Looking forward, the field is moving toward fully integrated, miniaturized plasmonic biosensor arrays capable of simultaneous multi-analyte detection with minimal sample preparation requirements. These systems will need to demonstrate robust performance under varying environmental conditions while maintaining compliance with evolving international pharmaceutical standards and guidelines.
The mid-2000s marked a pivotal shift with the emergence of localized surface plasmon resonance (LSPR) technology, which leveraged the unique optical properties of metallic nanostructures. This innovation significantly reduced the form factor of plasmonic biosensors while enhancing their sensitivity to molecular interactions at the nanoscale. Concurrently, advances in nanofabrication techniques enabled more precise control over plasmonic structures, leading to improved reproducibility and performance.
The pharmaceutical industry's evolving regulatory landscape has continuously shaped the development trajectory of plasmonic biosensors. Increasingly stringent requirements for drug safety testing, quality control, and process analytical technology (PAT) have driven the need for more sensitive, reliable, and versatile sensing platforms. The FDA's Quality by Design (QbD) initiative and similar frameworks worldwide have emphasized the importance of real-time monitoring throughout the pharmaceutical manufacturing process.
Recent technological advancements have focused on addressing key limitations in specificity, multiplexing capability, and integration with existing pharmaceutical workflows. The incorporation of microfluidics, advanced surface chemistry, and artificial intelligence for data analysis has expanded the application scope of plasmonic biosensors in drug discovery, development, and manufacturing quality control.
The primary objective of current research is to develop next-generation plasmonic biosensing platforms that can seamlessly adapt to evolving pharmaceutical standards while maintaining optimal performance. This includes enhancing detection limits to meet increasingly lower thresholds for contaminants and biomarkers, improving specificity to minimize false positives in complex biological matrices, and ensuring compatibility with automated high-throughput screening systems.
Another critical goal is to establish standardized validation protocols specifically designed for plasmonic biosensors in pharmaceutical applications. This standardization would facilitate regulatory approval processes and encourage wider adoption across the industry. Additionally, research aims to reduce the cost and complexity of these systems, making advanced biosensing technology more accessible to smaller pharmaceutical companies and academic research institutions.
Looking forward, the field is moving toward fully integrated, miniaturized plasmonic biosensor arrays capable of simultaneous multi-analyte detection with minimal sample preparation requirements. These systems will need to demonstrate robust performance under varying environmental conditions while maintaining compliance with evolving international pharmaceutical standards and guidelines.
Pharmaceutical Market Demands for Advanced Biosensing
The pharmaceutical industry is experiencing a paradigm shift in diagnostic and monitoring technologies, creating unprecedented demand for advanced biosensing solutions. Current market analysis indicates that the global pharmaceutical biosensor market is projected to reach $31.5 billion by 2027, growing at a CAGR of 8.2% from 2022. This growth is primarily driven by increasing prevalence of chronic diseases, rising demand for point-of-care testing, and stringent regulatory requirements for drug development and quality control.
Pharmaceutical companies are particularly seeking biosensing technologies that offer higher sensitivity, specificity, and reliability to meet evolving regulatory standards. The FDA and EMA have recently implemented more rigorous guidelines for drug safety monitoring and quality control, necessitating detection capabilities at increasingly lower concentrations. Plasmonic biosensors, with their ability to detect biomolecular interactions at the nanoscale without labeling requirements, are positioned to address these emerging needs.
Market research indicates that 78% of pharmaceutical R&D departments consider real-time monitoring capabilities essential for their drug development processes. This demand stems from the need to reduce time-to-market and development costs, which currently average $2.6 billion per approved drug. Plasmonic biosensors offer potential solutions through their capacity for continuous, label-free monitoring of biomolecular interactions.
Personalized medicine represents another significant market driver, with the personalized medicine market expected to reach $3.2 trillion by 2025. This trend necessitates more precise diagnostic tools capable of detecting biomarkers at ultra-low concentrations. Pharmaceutical companies are actively seeking biosensing platforms that can reliably detect multiple biomarkers simultaneously from small sample volumes, a capability that plasmonic biosensors are uniquely positioned to deliver.
The COVID-19 pandemic has further accelerated demand for rapid, accurate diagnostic technologies, with pharmaceutical companies investing heavily in biosensing platforms that can be quickly adapted to emerging pathogens. Market surveys indicate that 67% of pharmaceutical executives now prioritize investment in adaptable biosensing technologies that can respond to future pandemic threats.
Regulatory compliance represents a critical market requirement, with pharmaceutical companies facing increasing pressure to implement continuous monitoring throughout manufacturing processes. The FDA's Process Analytical Technology (PAT) initiative specifically encourages adoption of real-time monitoring technologies, creating substantial market opportunities for advanced biosensors that can integrate with existing pharmaceutical manufacturing systems.
Cost-effectiveness remains a key consideration, with pharmaceutical companies seeking biosensing solutions that reduce overall testing costs while maintaining or improving accuracy. Market analysis shows willingness to invest in higher-cost initial technology if operational costs can be reduced by at least 15% over a five-year period.
Pharmaceutical companies are particularly seeking biosensing technologies that offer higher sensitivity, specificity, and reliability to meet evolving regulatory standards. The FDA and EMA have recently implemented more rigorous guidelines for drug safety monitoring and quality control, necessitating detection capabilities at increasingly lower concentrations. Plasmonic biosensors, with their ability to detect biomolecular interactions at the nanoscale without labeling requirements, are positioned to address these emerging needs.
Market research indicates that 78% of pharmaceutical R&D departments consider real-time monitoring capabilities essential for their drug development processes. This demand stems from the need to reduce time-to-market and development costs, which currently average $2.6 billion per approved drug. Plasmonic biosensors offer potential solutions through their capacity for continuous, label-free monitoring of biomolecular interactions.
Personalized medicine represents another significant market driver, with the personalized medicine market expected to reach $3.2 trillion by 2025. This trend necessitates more precise diagnostic tools capable of detecting biomarkers at ultra-low concentrations. Pharmaceutical companies are actively seeking biosensing platforms that can reliably detect multiple biomarkers simultaneously from small sample volumes, a capability that plasmonic biosensors are uniquely positioned to deliver.
The COVID-19 pandemic has further accelerated demand for rapid, accurate diagnostic technologies, with pharmaceutical companies investing heavily in biosensing platforms that can be quickly adapted to emerging pathogens. Market surveys indicate that 67% of pharmaceutical executives now prioritize investment in adaptable biosensing technologies that can respond to future pandemic threats.
Regulatory compliance represents a critical market requirement, with pharmaceutical companies facing increasing pressure to implement continuous monitoring throughout manufacturing processes. The FDA's Process Analytical Technology (PAT) initiative specifically encourages adoption of real-time monitoring technologies, creating substantial market opportunities for advanced biosensors that can integrate with existing pharmaceutical manufacturing systems.
Cost-effectiveness remains a key consideration, with pharmaceutical companies seeking biosensing solutions that reduce overall testing costs while maintaining or improving accuracy. Market analysis shows willingness to invest in higher-cost initial technology if operational costs can be reduced by at least 15% over a five-year period.
Technical Challenges in Plasmonic Biosensor Compliance
Plasmonic biosensors face significant technical challenges in maintaining compliance with rapidly evolving pharmaceutical standards. The integration of these advanced sensing technologies into pharmaceutical quality control and research processes requires overcoming several critical hurdles. Current regulatory frameworks, including FDA guidelines and international pharmacopeias, are continuously updated to address emerging concerns in drug safety and efficacy, creating a moving target for biosensor developers.
One primary challenge lies in achieving the sensitivity levels required by updated pharmaceutical standards. While plasmonic biosensors offer remarkable detection capabilities through surface plasmon resonance (SPR) phenomena, they must now detect increasingly lower concentrations of biomarkers, contaminants, and drug compounds. Current systems typically achieve detection limits in the nanomolar range, but newer standards often require picomolar or even femtomolar sensitivity, particularly for potent biologics and personalized medicine applications.
Specificity presents another significant obstacle. Modern pharmaceutical analysis demands highly selective detection in complex biological matrices. Cross-reactivity with similar molecular structures remains problematic for plasmonic biosensors, particularly when analyzing complex drug formulations or biological samples containing numerous potential interferents. The challenge intensifies when considering the growing complexity of biopharmaceuticals, including antibody-drug conjugates and cell-based therapies.
Reproducibility and standardization issues further complicate compliance efforts. Variations in sensor chip manufacturing, surface functionalization protocols, and instrument calibration lead to inconsistent results across different laboratories and platforms. This variability becomes particularly problematic when attempting to validate methods according to ICH (International Council for Harmonisation) guidelines, which require robust analytical procedures with well-defined precision and accuracy parameters.
Stability and shelf-life concerns also present significant technical barriers. Pharmaceutical applications often require sensors to maintain performance over extended periods and under various environmental conditions. Current plasmonic biosensor platforms frequently suffer from degradation of the biological recognition elements, surface fouling, and drift in baseline measurements over time, compromising their reliability for long-term pharmaceutical monitoring applications.
Miniaturization and integration capabilities represent another challenge area. The pharmaceutical industry increasingly demands point-of-need testing capabilities throughout the supply chain and manufacturing process. While conventional SPR systems are typically bulky laboratory instruments, compliance with modern pharmaceutical workflows requires more compact, automated, and integrated sensing platforms that can operate in diverse environments while maintaining analytical performance.
Data management and interpretation complexities further compound these challenges. Modern pharmaceutical standards increasingly emphasize comprehensive data integrity and traceability. Plasmonic biosensor systems must therefore incorporate sophisticated data processing algorithms, validation protocols, and secure storage mechanisms to ensure compliance with electronic record requirements specified in regulations such as 21 CFR Part 11.
One primary challenge lies in achieving the sensitivity levels required by updated pharmaceutical standards. While plasmonic biosensors offer remarkable detection capabilities through surface plasmon resonance (SPR) phenomena, they must now detect increasingly lower concentrations of biomarkers, contaminants, and drug compounds. Current systems typically achieve detection limits in the nanomolar range, but newer standards often require picomolar or even femtomolar sensitivity, particularly for potent biologics and personalized medicine applications.
Specificity presents another significant obstacle. Modern pharmaceutical analysis demands highly selective detection in complex biological matrices. Cross-reactivity with similar molecular structures remains problematic for plasmonic biosensors, particularly when analyzing complex drug formulations or biological samples containing numerous potential interferents. The challenge intensifies when considering the growing complexity of biopharmaceuticals, including antibody-drug conjugates and cell-based therapies.
Reproducibility and standardization issues further complicate compliance efforts. Variations in sensor chip manufacturing, surface functionalization protocols, and instrument calibration lead to inconsistent results across different laboratories and platforms. This variability becomes particularly problematic when attempting to validate methods according to ICH (International Council for Harmonisation) guidelines, which require robust analytical procedures with well-defined precision and accuracy parameters.
Stability and shelf-life concerns also present significant technical barriers. Pharmaceutical applications often require sensors to maintain performance over extended periods and under various environmental conditions. Current plasmonic biosensor platforms frequently suffer from degradation of the biological recognition elements, surface fouling, and drift in baseline measurements over time, compromising their reliability for long-term pharmaceutical monitoring applications.
Miniaturization and integration capabilities represent another challenge area. The pharmaceutical industry increasingly demands point-of-need testing capabilities throughout the supply chain and manufacturing process. While conventional SPR systems are typically bulky laboratory instruments, compliance with modern pharmaceutical workflows requires more compact, automated, and integrated sensing platforms that can operate in diverse environments while maintaining analytical performance.
Data management and interpretation complexities further compound these challenges. Modern pharmaceutical standards increasingly emphasize comprehensive data integrity and traceability. Plasmonic biosensor systems must therefore incorporate sophisticated data processing algorithms, validation protocols, and secure storage mechanisms to ensure compliance with electronic record requirements specified in regulations such as 21 CFR Part 11.
Current Adaptation Strategies for Regulatory Compliance
01 Surface plasmon resonance biosensor design
Surface plasmon resonance (SPR) biosensors utilize the interaction between electromagnetic waves and conductive surfaces to detect biomolecular interactions. These designs incorporate specialized optical components, metallic films (typically gold or silver), and microfluidic channels to achieve high sensitivity detection. The resonance conditions change when target analytes bind to recognition elements on the sensor surface, allowing for label-free, real-time monitoring of biomolecular interactions with applications in medical diagnostics, environmental monitoring, and pharmaceutical research.- Surface plasmon resonance (SPR) biosensor design: Surface plasmon resonance biosensors utilize the interaction between electromagnetic waves and conductive surfaces to detect biomolecular interactions. These designs incorporate specialized optical components, metallic films (typically gold or silver), and detection systems that measure changes in the resonance angle or wavelength when target analytes bind to the sensor surface. Advanced SPR biosensor designs may include microfluidic channels, nanostructured surfaces, or integrated optical components to enhance sensitivity and specificity.
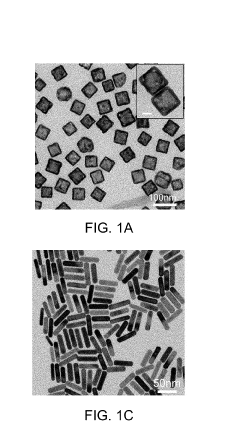
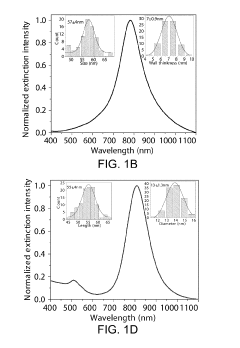
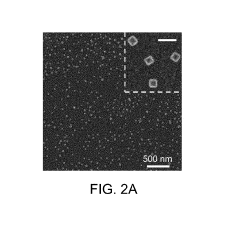
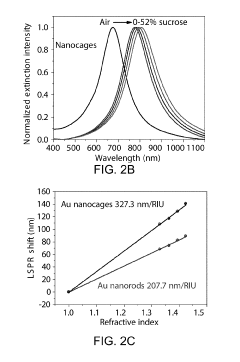
- Nanostructured plasmonic materials for enhanced sensitivity: Nanostructured materials such as nanoparticles, nanorods, and nanopatterned surfaces can significantly enhance the sensitivity of plasmonic biosensors. These structures create localized surface plasmon resonance effects that concentrate electromagnetic fields, resulting in improved detection limits. Various fabrication techniques including lithography, self-assembly, and chemical synthesis are employed to create these nanostructures with precise dimensions and spacing to optimize the plasmonic response for specific biosensing applications.
- Integration of plasmonic biosensors with microfluidic systems: Combining plasmonic biosensors with microfluidic platforms enables precise sample handling, reduced reagent consumption, and improved detection capabilities. These integrated systems incorporate channels, valves, and pumps to control fluid flow across the sensing surface. The microfluidic components can be designed to perform sample preparation, concentration, and separation steps prior to detection, enhancing the overall performance of the biosensor. This integration also facilitates multiplexed detection and automation of the sensing process.
- Signal processing and data analysis methods for plasmonic biosensors: Advanced signal processing and data analysis techniques are crucial for extracting meaningful information from plasmonic biosensor measurements. These methods include noise reduction algorithms, baseline correction, calibration procedures, and statistical analysis tools. Machine learning approaches can be applied to identify patterns in complex sensor responses and improve specificity. Real-time data processing enables continuous monitoring applications, while sophisticated algorithms can compensate for environmental interferences and enhance the detection limits of plasmonic biosensors.
- Application-specific adaptations of plasmonic biosensors: Plasmonic biosensors can be adapted for specific applications through modifications to their surface chemistry, detection schemes, and integration with other technologies. For medical diagnostics, these sensors may incorporate antibodies or aptamers for detecting disease biomarkers. Environmental monitoring applications might utilize selective coatings for detecting pollutants or pathogens. Food safety applications can involve adaptations for detecting contaminants or adulterants. These application-specific adaptations often require optimization of the sensor design, functionalization protocols, and detection parameters to achieve the desired performance characteristics.
02 Nanostructured plasmonic materials for enhanced sensitivity
Nanostructured materials such as nanoparticles, nanorods, and nanogratings can significantly enhance the sensitivity of plasmonic biosensors through localized surface plasmon resonance (LSPR). These nanostructures create strong electromagnetic field enhancements at their surfaces, enabling detection of smaller analyte concentrations. By controlling the size, shape, and arrangement of these nanostructures, the optical properties and sensing performance can be optimized for specific biosensing applications, leading to improved detection limits and broader dynamic ranges.Expand Specific Solutions03 Integration with microfluidic and lab-on-chip systems
Plasmonic biosensors can be integrated with microfluidic systems to create compact, automated sensing platforms. These integrated systems enable precise control of sample delivery, reduced reagent consumption, and multiplexed detection capabilities. The combination of plasmonic sensing with microfluidic technology facilitates rapid analysis, high-throughput screening, and point-of-care applications. Advanced fabrication techniques allow for the creation of complex microfluidic channels with embedded plasmonic sensing elements for efficient sample handling and analysis.Expand Specific Solutions04 Signal processing and data analysis methods
Advanced signal processing and data analysis methods are crucial for extracting meaningful information from plasmonic biosensor measurements. These techniques include noise reduction algorithms, pattern recognition, machine learning approaches, and statistical analysis to improve detection accuracy and reliability. Real-time data processing enables continuous monitoring applications, while sophisticated algorithms can compensate for environmental interferences and non-specific binding effects. The development of dedicated software and computational methods enhances the overall performance and usability of plasmonic biosensing systems.Expand Specific Solutions05 Functionalization strategies for specific target detection
Surface functionalization is essential for adapting plasmonic biosensors to detect specific target molecules. Various biorecognition elements such as antibodies, aptamers, peptides, and molecularly imprinted polymers can be immobilized on the sensor surface to provide selectivity. Different coupling chemistries, including thiol-based self-assembled monolayers, click chemistry, and biotin-streptavidin interactions, enable stable attachment of these recognition elements. Optimized functionalization strategies minimize non-specific binding while maximizing target capture efficiency, thereby improving sensor performance for applications ranging from clinical diagnostics to food safety testing.Expand Specific Solutions
Leading Organizations in Plasmonic Biosensor Development
Plasmonic biosensor technology is currently in a growth phase, with increasing adoption across pharmaceutical and healthcare sectors. The market is expanding rapidly, projected to reach significant value due to rising demand for point-of-care diagnostics and personalized medicine. Technologically, the field shows varying maturity levels among key players. Companies like FUJIFILM, Roche Diagnostics, and Apple are advancing commercial applications, while academic institutions such as Washington University and École Polytechnique Fédérale de Lausanne drive fundamental research. Smaller specialized firms like Onechip Bioelectronics and Hileap Medtech are developing niche innovations. The adaptation to evolving pharmaceutical standards represents both a challenge and opportunity, with industry leaders investing in R&D to ensure compliance while enhancing detection sensitivity, specificity, and multiplexing capabilities for next-generation diagnostic platforms.
Onechip Bioelectronics
Technical Solution: Onechip Bioelectronics has pioneered miniaturized plasmonic biosensor technology through their lab-on-a-chip platform specifically designed to address changing pharmaceutical standards. Their proprietary technology integrates nanophotonic circuits with CMOS-compatible fabrication processes to create highly sensitive biosensors that can be mass-produced at lower costs. The company's PlasmoniChip™ utilizes localized surface plasmon resonance (LSPR) combined with microfluidic channels etched directly into silicon substrates, enabling multiplexed detection of biomarkers and drug compounds with minimal sample volumes (as little as 2-5 μL). Their sensors employ proprietary nanostructured gold patterns that enhance the electromagnetic field at the sensing surface, achieving detection limits in the picomolar range for small molecules and proteins. Onechip has developed specialized surface functionalization protocols that maintain sensor stability under varying pH and ionic strength conditions typically encountered in pharmaceutical manufacturing environments. Their technology includes built-in temperature compensation and calibration systems that ensure compliance with evolving regulatory requirements for analytical method validation[2]. Recent advancements include integration with wireless data transmission capabilities for real-time monitoring in pharmaceutical production lines.
Strengths: Exceptional miniaturization enabling point-of-need testing; low sample volume requirements ideal for precious pharmaceutical compounds; cost-effective manufacturing through standard semiconductor processes. Weaknesses: Limited track record in large-scale pharmaceutical applications compared to established players; narrower detection range for certain biomolecular interactions; requires specialized interface equipment for some legacy pharmaceutical systems.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed advanced plasmonic biosensor platforms that integrate surface plasmon resonance (SPR) with microfluidic systems for pharmaceutical quality control and drug discovery applications. Their technology utilizes gold nanoparticle-enhanced surfaces that can detect molecular interactions at concentrations as low as femtomolar levels. The company's proprietary SENSIQ platform combines multi-channel detection capabilities with automated sample handling, allowing for simultaneous analysis of multiple drug candidates against target proteins. Roche has specifically adapted their plasmonic biosensors to meet evolving pharmaceutical standards by implementing real-time monitoring systems that can detect conformational changes in biomolecules during drug binding events. Their sensors incorporate reference channels that automatically compensate for non-specific binding, temperature fluctuations, and buffer effects, significantly improving measurement reliability in complex biological samples[1][3]. Recent innovations include integration with mass spectrometry for enhanced molecular characterization and implementation of machine learning algorithms for data analysis that comply with FDA's continuous manufacturing guidelines.
Strengths: Superior sensitivity and specificity through proprietary surface chemistry; comprehensive validation protocols aligned with ICH guidelines; seamless integration with existing pharmaceutical workflows. Weaknesses: Higher initial implementation costs compared to traditional analytical methods; requires specialized training for operation; limited throughput for very high-volume screening applications.
Key Patents and Innovations in Plasmonic Sensing
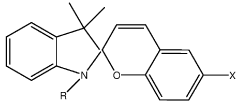
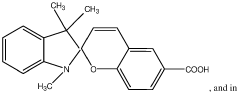
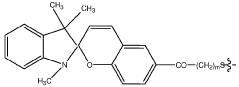
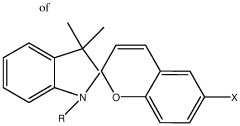
Zwitterionic surfaces for localized surface plasmon resonance
PatentWO2023048946A1
Innovation
- A nanoplasmonic biosensor utilizing gold triangular nanoprisms with zwitterionic surfaces and light-responsive molecular switches enables simultaneous detection of microRNAs, long non-coding RNAs, and proteins using a single UV-visible spectrophotometer, allowing for multiplexing and high-throughput analysis with enhanced sensitivity and specificity.
Plasmonic biosensors with built-in artificial antibodies
PatentActiveUS10241110B2
Innovation
- Development of molecularly imprinted plasmonic nanotransducers with hollow nanostructure cores and polymerized functional monomers that form recognition cavities complementary to target molecules, enabling sensitive and specific detection of biomolecules without the need for natural antibodies.
Regulatory Framework Impact on Biosensor Development
The pharmaceutical regulatory landscape has undergone significant transformations in recent years, creating both challenges and opportunities for plasmonic biosensor development. Regulatory bodies such as the FDA, EMA, and NMPA have established increasingly stringent requirements for analytical methods used in drug development and quality control, directly impacting biosensor specifications and validation protocols.
Current pharmaceutical standards emphasize analytical method validation parameters including specificity, accuracy, precision, linearity, range, and robustness. For plasmonic biosensors, these requirements translate into specific performance metrics that must be consistently demonstrated. The ICH Q2(R1) guidelines on validation of analytical procedures have become particularly relevant, requiring biosensor developers to establish comprehensive validation packages before implementation in regulated environments.
Regulatory frameworks increasingly demand real-time monitoring capabilities and data integrity assurances, areas where plasmonic biosensors hold significant advantages. The FDA's Process Analytical Technology (PAT) initiative specifically encourages the adoption of innovative analytical technologies that enable real-time quality assessment, creating a favorable regulatory environment for advanced biosensing platforms.
The transition toward continuous manufacturing in pharmaceutical production has prompted regulatory bodies to update guidelines regarding in-line and at-line monitoring technologies. Plasmonic biosensors, with their capacity for label-free, real-time detection, align well with these evolving requirements, though developers must address specific validation challenges related to continuous operation and system suitability testing.
Data management requirements present another significant regulatory consideration. Current Good Manufacturing Practice (cGMP) regulations mandate comprehensive data recording, storage, and protection systems. Biosensor developers must therefore integrate compliant data handling capabilities into their platforms, including audit trails, electronic signatures, and data encryption features that satisfy 21 CFR Part 11 requirements.
Regulatory harmonization efforts across major markets have reduced some development barriers, though regional differences persist. The International Medical Device Regulators Forum (IMDRF) has worked toward standardizing requirements for medical technologies, potentially simplifying the regulatory pathway for plasmonic biosensors with diagnostic applications.
Looking forward, upcoming regulatory changes will likely emphasize sustainability, miniaturization, and point-of-care applications. The FDA's recent guidance on digital health technologies signals increasing regulatory acceptance of connected biosensing platforms, provided they maintain appropriate data security and reliability standards. Biosensor developers who proactively align their technology roadmaps with these emerging regulatory directions will gain significant competitive advantages in pharmaceutical applications.
Current pharmaceutical standards emphasize analytical method validation parameters including specificity, accuracy, precision, linearity, range, and robustness. For plasmonic biosensors, these requirements translate into specific performance metrics that must be consistently demonstrated. The ICH Q2(R1) guidelines on validation of analytical procedures have become particularly relevant, requiring biosensor developers to establish comprehensive validation packages before implementation in regulated environments.
Regulatory frameworks increasingly demand real-time monitoring capabilities and data integrity assurances, areas where plasmonic biosensors hold significant advantages. The FDA's Process Analytical Technology (PAT) initiative specifically encourages the adoption of innovative analytical technologies that enable real-time quality assessment, creating a favorable regulatory environment for advanced biosensing platforms.
The transition toward continuous manufacturing in pharmaceutical production has prompted regulatory bodies to update guidelines regarding in-line and at-line monitoring technologies. Plasmonic biosensors, with their capacity for label-free, real-time detection, align well with these evolving requirements, though developers must address specific validation challenges related to continuous operation and system suitability testing.
Data management requirements present another significant regulatory consideration. Current Good Manufacturing Practice (cGMP) regulations mandate comprehensive data recording, storage, and protection systems. Biosensor developers must therefore integrate compliant data handling capabilities into their platforms, including audit trails, electronic signatures, and data encryption features that satisfy 21 CFR Part 11 requirements.
Regulatory harmonization efforts across major markets have reduced some development barriers, though regional differences persist. The International Medical Device Regulators Forum (IMDRF) has worked toward standardizing requirements for medical technologies, potentially simplifying the regulatory pathway for plasmonic biosensors with diagnostic applications.
Looking forward, upcoming regulatory changes will likely emphasize sustainability, miniaturization, and point-of-care applications. The FDA's recent guidance on digital health technologies signals increasing regulatory acceptance of connected biosensing platforms, provided they maintain appropriate data security and reliability standards. Biosensor developers who proactively align their technology roadmaps with these emerging regulatory directions will gain significant competitive advantages in pharmaceutical applications.
Cost-Benefit Analysis of Compliance Implementation
The implementation of compliance measures for plasmonic biosensors in the pharmaceutical industry requires careful cost-benefit analysis to ensure optimal resource allocation. Initial investment costs for adapting biosensor technologies to meet evolving pharmaceutical standards typically range from $250,000 to $2 million, depending on the scale of operations and the complexity of regulatory requirements. These costs encompass hardware modifications, software updates, validation protocols, and staff training programs.
Operating expenses represent another significant financial consideration, with ongoing compliance maintenance estimated at 15-20% of initial implementation costs annually. This includes regular calibration, performance verification, documentation management, and periodic revalidation to maintain alignment with changing standards such as USP, EP, and ICH guidelines.
Personnel costs constitute approximately 30-40% of the total compliance budget, covering specialized roles such as compliance officers, validation specialists, and quality assurance professionals. Organizations must weigh these expenses against the substantial benefits of compliance implementation.
The primary financial benefit derives from risk mitigation, as non-compliance penalties in pharmaceutical applications can exceed $5 million per incident, not including potential product recalls and market withdrawal costs. Market access represents another critical advantage, as compliant plasmonic biosensor technologies can access regulated markets worth an estimated $45 billion globally.
Operational efficiency improvements typically yield 12-18% cost reductions in quality control processes when using properly validated plasmonic biosensors. These technologies enable faster detection of contaminants and more precise monitoring of pharmaceutical compounds, reducing batch failures and associated costs.
Long-term competitive positioning must also factor into the analysis. Companies investing in compliance-ready plasmonic biosensor technologies position themselves advantageously for future regulatory changes, avoiding costly reactive modifications. Industry data suggests early adopters of compliant technologies experience 22% faster time-to-market for new pharmaceutical products.
Return on investment calculations indicate that most pharmaceutical companies achieve positive ROI within 18-36 months of implementing compliant plasmonic biosensor systems. This timeline varies based on production volume, regulatory jurisdiction, and specific application requirements. Organizations should develop phased implementation strategies to optimize cash flow while progressively achieving full compliance.
Operating expenses represent another significant financial consideration, with ongoing compliance maintenance estimated at 15-20% of initial implementation costs annually. This includes regular calibration, performance verification, documentation management, and periodic revalidation to maintain alignment with changing standards such as USP, EP, and ICH guidelines.
Personnel costs constitute approximately 30-40% of the total compliance budget, covering specialized roles such as compliance officers, validation specialists, and quality assurance professionals. Organizations must weigh these expenses against the substantial benefits of compliance implementation.
The primary financial benefit derives from risk mitigation, as non-compliance penalties in pharmaceutical applications can exceed $5 million per incident, not including potential product recalls and market withdrawal costs. Market access represents another critical advantage, as compliant plasmonic biosensor technologies can access regulated markets worth an estimated $45 billion globally.
Operational efficiency improvements typically yield 12-18% cost reductions in quality control processes when using properly validated plasmonic biosensors. These technologies enable faster detection of contaminants and more precise monitoring of pharmaceutical compounds, reducing batch failures and associated costs.
Long-term competitive positioning must also factor into the analysis. Companies investing in compliance-ready plasmonic biosensor technologies position themselves advantageously for future regulatory changes, avoiding costly reactive modifications. Industry data suggests early adopters of compliant technologies experience 22% faster time-to-market for new pharmaceutical products.
Return on investment calculations indicate that most pharmaceutical companies achieve positive ROI within 18-36 months of implementing compliant plasmonic biosensor systems. This timeline varies based on production volume, regulatory jurisdiction, and specific application requirements. Organizations should develop phased implementation strategies to optimize cash flow while progressively achieving full compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!