Role of Electrolytes in Optimizing Plasmonic Biosensor Response
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasmonic Biosensor Electrolyte Background and Objectives
Plasmonic biosensors have emerged as powerful analytical tools in the field of biomedical diagnostics, environmental monitoring, and food safety due to their exceptional sensitivity, label-free detection capabilities, and real-time response. The evolution of these sensors has been marked by significant advancements in nanofabrication techniques, optical instrumentation, and surface chemistry over the past two decades. Initially developed as simple surface plasmon resonance (SPR) platforms, these sensors have now evolved into sophisticated nanostructured systems capable of detecting biomolecular interactions at the single-molecule level.
The role of electrolytes in plasmonic biosensing has been increasingly recognized as a critical factor influencing sensor performance, yet remains relatively underexplored compared to other aspects of sensor design. Electrolytes—ionic solutions that conduct electricity—create the microenvironment in which biomolecular recognition events occur at the sensor surface. The composition, concentration, and properties of these electrolytes significantly impact the plasmon resonance conditions, biomolecular binding kinetics, and overall signal transduction efficiency.
Historical developments in plasmonic biosensing have primarily focused on improving the optical and material properties of the sensing platforms, with less attention paid to optimizing the liquid medium in which detection occurs. Early research established basic buffer requirements for maintaining biomolecule stability, but detailed understanding of how electrolyte properties modulate plasmonic responses has only gained momentum in the last decade.
Recent technological trends indicate a shift toward more sophisticated electrolyte engineering approaches, including the use of ionic strength gradients, pH-responsive electrolytes, and custom buffer formulations tailored to specific sensing applications. These developments align with the broader trend toward personalized and point-of-care diagnostic platforms that require robust performance across diverse sample matrices.
The primary objective of this technical research is to comprehensively analyze how electrolyte properties—including ionic strength, pH, specific ion effects, and temperature dependence—influence the sensitivity, specificity, and reliability of plasmonic biosensors. We aim to establish quantitative relationships between electrolyte parameters and sensor performance metrics to enable rational design of optimized sensing environments.
Secondary objectives include identifying novel electrolyte formulations that can enhance signal-to-noise ratios, reduce non-specific binding, and extend the dynamic range of plasmonic biosensors. Additionally, we seek to develop predictive models that can guide electrolyte selection for specific biosensing applications, potentially enabling automated optimization systems for next-generation plasmonic platforms.
The ultimate goal is to establish a comprehensive framework for electrolyte engineering in plasmonic biosensing that bridges the gap between fundamental plasmonics, surface chemistry, and practical biosensor implementation, thereby advancing the field toward more sensitive, reliable, and versatile detection systems for biomedical and environmental applications.
The role of electrolytes in plasmonic biosensing has been increasingly recognized as a critical factor influencing sensor performance, yet remains relatively underexplored compared to other aspects of sensor design. Electrolytes—ionic solutions that conduct electricity—create the microenvironment in which biomolecular recognition events occur at the sensor surface. The composition, concentration, and properties of these electrolytes significantly impact the plasmon resonance conditions, biomolecular binding kinetics, and overall signal transduction efficiency.
Historical developments in plasmonic biosensing have primarily focused on improving the optical and material properties of the sensing platforms, with less attention paid to optimizing the liquid medium in which detection occurs. Early research established basic buffer requirements for maintaining biomolecule stability, but detailed understanding of how electrolyte properties modulate plasmonic responses has only gained momentum in the last decade.
Recent technological trends indicate a shift toward more sophisticated electrolyte engineering approaches, including the use of ionic strength gradients, pH-responsive electrolytes, and custom buffer formulations tailored to specific sensing applications. These developments align with the broader trend toward personalized and point-of-care diagnostic platforms that require robust performance across diverse sample matrices.
The primary objective of this technical research is to comprehensively analyze how electrolyte properties—including ionic strength, pH, specific ion effects, and temperature dependence—influence the sensitivity, specificity, and reliability of plasmonic biosensors. We aim to establish quantitative relationships between electrolyte parameters and sensor performance metrics to enable rational design of optimized sensing environments.
Secondary objectives include identifying novel electrolyte formulations that can enhance signal-to-noise ratios, reduce non-specific binding, and extend the dynamic range of plasmonic biosensors. Additionally, we seek to develop predictive models that can guide electrolyte selection for specific biosensing applications, potentially enabling automated optimization systems for next-generation plasmonic platforms.
The ultimate goal is to establish a comprehensive framework for electrolyte engineering in plasmonic biosensing that bridges the gap between fundamental plasmonics, surface chemistry, and practical biosensor implementation, thereby advancing the field toward more sensitive, reliable, and versatile detection systems for biomedical and environmental applications.
Market Analysis for Electrolyte-Enhanced Biosensors
The global market for electrolyte-enhanced plasmonic biosensors is experiencing significant growth, driven by increasing demand for rapid, sensitive, and accurate diagnostic tools across healthcare, environmental monitoring, and food safety sectors. Current market valuations indicate that the biosensor market reached approximately 23 billion USD in 2022, with plasmonic biosensors representing a rapidly growing segment projected to expand at a compound annual growth rate of 8.7% through 2030.
The healthcare sector dominates the application landscape, accounting for over 60% of market share, particularly in point-of-care diagnostics, continuous health monitoring, and pharmaceutical research. The integration of optimized electrolyte solutions has created a specialized sub-segment with premium pricing potential due to enhanced sensitivity and reliability metrics that conventional biosensors cannot match.
Regional analysis reveals North America as the current market leader with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is demonstrating the fastest growth trajectory, particularly in China, India, and South Korea, where substantial investments in healthcare infrastructure and biotechnology research are creating favorable market conditions.
Key customer segments include hospital laboratories, diagnostic centers, research institutions, and pharmaceutical companies. These stakeholders prioritize performance metrics such as detection limit, response time, and reproducibility—all areas where electrolyte optimization provides measurable advantages. Market research indicates willingness to pay premium prices for biosensors that can achieve detection limits in the femtomolar range, which electrolyte-enhanced plasmonic systems increasingly deliver.
Competitive analysis reveals a fragmented market landscape with several specialized players rather than dominant market leaders. This fragmentation presents opportunities for new entrants with innovative electrolyte formulations. Current pricing structures show high-performance plasmonic biosensors commanding 30-50% price premiums over conventional alternatives, with electrolyte-enhanced versions positioned at the premium end of this spectrum.
Market barriers include regulatory hurdles, particularly for clinical applications, and the technical complexity of integrating optimized electrolyte systems into existing biosensor platforms. Additionally, cost sensitivity in emerging markets remains a challenge, though this is partially offset by the improved performance-to-cost ratio that electrolyte optimization can provide.
Future market growth will likely be driven by increasing prevalence of chronic diseases requiring regular monitoring, growing emphasis on personalized medicine, and expanding applications in environmental monitoring and food safety. The development of standardized electrolyte formulations that can be easily integrated into various plasmonic biosensor platforms represents a significant market opportunity with potential to accelerate adoption across multiple industry verticals.
The healthcare sector dominates the application landscape, accounting for over 60% of market share, particularly in point-of-care diagnostics, continuous health monitoring, and pharmaceutical research. The integration of optimized electrolyte solutions has created a specialized sub-segment with premium pricing potential due to enhanced sensitivity and reliability metrics that conventional biosensors cannot match.
Regional analysis reveals North America as the current market leader with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is demonstrating the fastest growth trajectory, particularly in China, India, and South Korea, where substantial investments in healthcare infrastructure and biotechnology research are creating favorable market conditions.
Key customer segments include hospital laboratories, diagnostic centers, research institutions, and pharmaceutical companies. These stakeholders prioritize performance metrics such as detection limit, response time, and reproducibility—all areas where electrolyte optimization provides measurable advantages. Market research indicates willingness to pay premium prices for biosensors that can achieve detection limits in the femtomolar range, which electrolyte-enhanced plasmonic systems increasingly deliver.
Competitive analysis reveals a fragmented market landscape with several specialized players rather than dominant market leaders. This fragmentation presents opportunities for new entrants with innovative electrolyte formulations. Current pricing structures show high-performance plasmonic biosensors commanding 30-50% price premiums over conventional alternatives, with electrolyte-enhanced versions positioned at the premium end of this spectrum.
Market barriers include regulatory hurdles, particularly for clinical applications, and the technical complexity of integrating optimized electrolyte systems into existing biosensor platforms. Additionally, cost sensitivity in emerging markets remains a challenge, though this is partially offset by the improved performance-to-cost ratio that electrolyte optimization can provide.
Future market growth will likely be driven by increasing prevalence of chronic diseases requiring regular monitoring, growing emphasis on personalized medicine, and expanding applications in environmental monitoring and food safety. The development of standardized electrolyte formulations that can be easily integrated into various plasmonic biosensor platforms represents a significant market opportunity with potential to accelerate adoption across multiple industry verticals.
Current Electrolyte Technologies and Challenges in Biosensing
Current electrolyte technologies in plasmonic biosensing primarily revolve around aqueous solutions containing various ionic species that facilitate charge transfer and signal transduction at the sensor-analyte interface. Traditional electrolyte systems employ simple salt solutions such as phosphate-buffered saline (PBS), which provides stable pH and ionic strength for biomolecular interactions. These conventional electrolytes have served as the foundation for many biosensing applications due to their biocompatibility and well-characterized properties.
Advanced electrolyte formulations have emerged in recent years, incorporating specific ionic components designed to enhance plasmonic responses. These include electrolytes with carefully controlled ionic strength gradients that can modulate the electrical double layer at metal-solution interfaces, directly affecting localized surface plasmon resonance (LSPR) characteristics. Additionally, specialized electrolytes containing redox-active species have been developed to amplify electrochemical-plasmonic coupling effects.
Despite these advancements, significant challenges persist in electrolyte optimization for plasmonic biosensing. A primary limitation is the Debye screening effect, where high ionic strengths can shield charge-based interactions between biomolecules and sensor surfaces, potentially reducing sensitivity. This creates a fundamental trade-off between maintaining physiological conditions for biomolecules and achieving optimal sensing performance.
Another critical challenge involves electrolyte stability during sensing operations. Temperature fluctuations, evaporation, and electrochemical reactions at electrode surfaces can alter electrolyte composition over time, leading to drift in sensor response and reduced reproducibility. This is particularly problematic for continuous monitoring applications where long-term stability is essential.
The integration of electrolytes with complex biological samples presents additional complications. Blood, serum, and other biological fluids contain numerous interfering components that can interact with both the electrolyte and sensor surface, potentially masking the target analyte signal. Current electrolyte technologies struggle to maintain performance in these complex matrices without extensive sample preparation.
Miniaturization of biosensing platforms introduces further constraints on electrolyte design. Microfluidic systems require electrolytes with appropriate viscosity and surface tension properties, while maintaining ionic characteristics that support plasmonic enhancement. The limited volume in such systems also amplifies concerns about electrolyte depletion and local concentration changes during sensing operations.
Emerging research directions include stimuli-responsive electrolytes that can dynamically adjust their properties in response to external triggers, potentially enabling adaptive sensing protocols. Additionally, ionic liquid-based electrolytes offer promising alternatives to aqueous systems, providing wider electrochemical windows and enhanced stability, though their higher viscosity and cost remain limiting factors for widespread adoption.
Advanced electrolyte formulations have emerged in recent years, incorporating specific ionic components designed to enhance plasmonic responses. These include electrolytes with carefully controlled ionic strength gradients that can modulate the electrical double layer at metal-solution interfaces, directly affecting localized surface plasmon resonance (LSPR) characteristics. Additionally, specialized electrolytes containing redox-active species have been developed to amplify electrochemical-plasmonic coupling effects.
Despite these advancements, significant challenges persist in electrolyte optimization for plasmonic biosensing. A primary limitation is the Debye screening effect, where high ionic strengths can shield charge-based interactions between biomolecules and sensor surfaces, potentially reducing sensitivity. This creates a fundamental trade-off between maintaining physiological conditions for biomolecules and achieving optimal sensing performance.
Another critical challenge involves electrolyte stability during sensing operations. Temperature fluctuations, evaporation, and electrochemical reactions at electrode surfaces can alter electrolyte composition over time, leading to drift in sensor response and reduced reproducibility. This is particularly problematic for continuous monitoring applications where long-term stability is essential.
The integration of electrolytes with complex biological samples presents additional complications. Blood, serum, and other biological fluids contain numerous interfering components that can interact with both the electrolyte and sensor surface, potentially masking the target analyte signal. Current electrolyte technologies struggle to maintain performance in these complex matrices without extensive sample preparation.
Miniaturization of biosensing platforms introduces further constraints on electrolyte design. Microfluidic systems require electrolytes with appropriate viscosity and surface tension properties, while maintaining ionic characteristics that support plasmonic enhancement. The limited volume in such systems also amplifies concerns about electrolyte depletion and local concentration changes during sensing operations.
Emerging research directions include stimuli-responsive electrolytes that can dynamically adjust their properties in response to external triggers, potentially enabling adaptive sensing protocols. Additionally, ionic liquid-based electrolytes offer promising alternatives to aqueous systems, providing wider electrochemical windows and enhanced stability, though their higher viscosity and cost remain limiting factors for widespread adoption.
Current Electrolyte Formulations for Biosensor Optimization
01 Electrolyte composition effects on plasmonic biosensor sensitivity
The composition of electrolytes significantly impacts the sensitivity and performance of plasmonic biosensors. Different ionic species and concentrations can alter the local refractive index near the sensor surface, affecting the surface plasmon resonance (SPR) signal. Optimizing electrolyte composition can enhance the detection limit and dynamic range of biosensors by modulating the electrical double layer at the sensor-analyte interface, thereby improving the overall biosensor response to target analytes.- Electrolyte composition effects on plasmonic biosensor sensitivity: The composition of electrolytes significantly impacts the sensitivity and response of plasmonic biosensors. Specific ionic species and concentrations can enhance the localized surface plasmon resonance (LSPR) effect, improving detection limits. Optimized electrolyte formulations can reduce background noise and increase signal-to-noise ratios, enabling more accurate detection of target analytes. The interaction between electrolytes and the metallic nanostructures affects the refractive index at the sensing interface, which directly influences biosensor performance.
- Ionic strength modulation for enhanced biosensor response: Modulating the ionic strength of electrolyte solutions can significantly enhance plasmonic biosensor response. By carefully controlling the concentration of ions in the sensing environment, researchers can optimize the electrical double layer formation at the sensor surface, which affects analyte binding efficiency. This approach enables tuning of the Debye length, critical for biomolecular detection in complex media. Strategic ionic strength adjustment can minimize non-specific binding while maximizing target capture, leading to improved sensitivity and specificity in biosensing applications.
- Integration of electrolyte-gated transistors with plasmonic elements: Combining electrolyte-gated transistors with plasmonic sensing elements creates highly sensitive hybrid biosensor platforms. These integrated systems leverage the amplification capabilities of transistors with the optical sensitivity of plasmonic structures. The electrolyte serves as both the gate medium for the transistor and the environment for plasmonic sensing, enabling dual-mode detection. This approach allows for electrical readout of plasmonic events, simplifying signal processing and potentially enabling point-of-care applications with enhanced sensitivity and reduced complexity.
- pH-responsive plasmonic biosensors utilizing electrolyte interactions: pH-responsive plasmonic biosensors exploit the interaction between electrolytes and sensing surfaces to detect subtle changes in solution acidity. These systems often incorporate pH-sensitive polymers or functional groups that undergo conformational changes with pH variations, altering the plasmonic response. The electrolyte composition influences the stability and sensitivity of these pH-dependent responses. Applications include monitoring biological processes, environmental sensing, and medical diagnostics where pH changes indicate specific conditions or disease states.
- Temperature effects on electrolyte-mediated plasmonic sensing: Temperature significantly impacts electrolyte behavior in plasmonic biosensors, affecting ionic mobility, diffusion rates, and binding kinetics. Controlling temperature is essential for reliable biosensor response, as thermal fluctuations can alter the refractive index of the sensing medium and change the plasmonic resonance conditions. Some advanced biosensor designs incorporate temperature compensation mechanisms or utilize the temperature dependence as an additional sensing parameter. Understanding and managing these thermal effects is crucial for developing robust plasmonic biosensors that maintain accuracy across varying environmental conditions.
02 Integration of electrolyte-gated field-effect in plasmonic biosensing
Combining electrolyte-gated field-effect transistors with plasmonic structures creates highly sensitive biosensing platforms. The electrolyte serves as both a conductive medium and a gate dielectric, allowing electrical modulation of the plasmonic response. This integration enables dual-mode sensing where both electrical and optical signals can be measured simultaneously, providing complementary information about biomolecular interactions and reducing false positives in complex biological samples.Expand Specific Solutions03 Electrolyte-mediated signal amplification mechanisms
Specific electrolyte formulations can amplify biosensor signals through various mechanisms including ionic strength modulation, redox cycling, and electrochemical enhancement of plasmonic effects. By carefully selecting electrolyte components that participate in charge transfer processes near the sensor surface, the sensitivity of plasmonic biosensors can be significantly improved. These amplification strategies are particularly valuable for detecting low-abundance biomarkers in clinical diagnostics and environmental monitoring applications.Expand Specific Solutions04 Temperature and pH stability of electrolyte systems in biosensors
The stability of electrolyte systems under varying temperature and pH conditions is crucial for reliable plasmonic biosensor operation. Specialized buffer formulations can maintain consistent ionic strength and conductivity across environmental fluctuations, ensuring reproducible biosensor responses. Advanced electrolyte systems incorporate pH-responsive components that can compensate for changes in sample acidity, maintaining optimal conditions for biomolecular recognition events and preserving the integrity of the plasmonic sensing elements.Expand Specific Solutions05 Microfluidic delivery of electrolytes for dynamic biosensing
Microfluidic systems enable precise control over electrolyte delivery to plasmonic biosensor surfaces, allowing for dynamic sensing protocols and real-time response monitoring. These systems can introduce electrolyte gradients or pulses that enhance binding kinetics analysis and improve discrimination between specific and non-specific interactions. Integration of microfluidic electrolyte handling with plasmonic sensing elements facilitates multiplexed detection and automated sample processing, making these biosensors suitable for point-of-care diagnostics and high-throughput screening applications.Expand Specific Solutions
Leading Companies and Research Institutions in Plasmonic Biosensing
The field of plasmonic biosensor optimization through electrolytes is currently in a growth phase, with the market expanding rapidly due to increasing applications in healthcare diagnostics and environmental monitoring. The global market is projected to reach significant value as biosensing technologies become more integrated into point-of-care devices. Technologically, the field shows moderate maturity with established players like PHC Holdings, Koninklijke Philips, and Siemens AG leading commercial development, while academic institutions such as Washington University in St. Louis, Boston University, and Imperial College London drive fundamental research. Universities like EPFL and KAUST are advancing novel electrolyte formulations, while companies including Nitto Denko and Infineon Technologies focus on sensor miniaturization and integration. The ecosystem demonstrates a productive balance between academic innovation and industrial commercialization, with cross-sector collaborations accelerating technological advancement.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has developed advanced electrolyte-optimized plasmonic biosensing platforms that leverage their expertise in chemical engineering and materials science. Their approach focuses on the systematic design of electrolyte compositions to enhance the performance of surface plasmon resonance (SPR) and localized surface plasmon resonance (LSPR) biosensors. The university's research team has created specialized buffer systems incorporating ionic liquids and crown ethers that modify the electrical double layer at the metal-solution interface, significantly improving sensor sensitivity. Their proprietary electrolyte formulations enable precise control over charge screening effects, allowing for the detection of both small molecules and large biomolecules with equal efficiency. Recent publications demonstrate their technology achieving sub-nanomolar detection limits for various biomarkers, with particularly strong performance in challenging sample matrices. The team has also pioneered the use of stimuli-responsive electrolytes that can dynamically adjust their properties during sensing operations, enabling adaptive detection protocols for complex biological samples.
Strengths: Versatile detection capabilities across different analyte sizes; excellent performance in complex sample matrices; innovative use of ionic liquids for enhanced sensitivity. Weaknesses: More complex preparation procedures compared to standard buffers; potential challenges in long-term stability of specialized electrolyte components; higher cost of implementation for routine diagnostic applications.
Zhejiang University
Technical Solution: Zhejiang University has developed innovative electrolyte-enhanced plasmonic biosensing platforms that significantly improve detection sensitivity and specificity. Their research focuses on the strategic manipulation of electrolyte composition to optimize the interaction between target biomolecules and plasmonic nanostructures. The university's approach involves using gradient electrolyte systems with precisely controlled ionic strength and pH to create favorable conditions for biomolecular recognition events at the sensor surface. Their proprietary electrolyte formulations incorporate specific ion combinations that minimize non-specific binding while enhancing target capture efficiency. Zhejiang researchers have demonstrated that their optimized electrolyte environments can extend the effective sensing range of plasmonic biosensors by modulating the electrical double layer thickness. Recent publications show their system achieving detection limits in the picomolar range for various protein biomarkers, representing a 10-fold improvement over conventional buffer systems. The technology has been successfully applied to point-of-care diagnostic platforms for infectious disease detection.
Strengths: Excellent sensitivity with detection limits in picomolar range; reduced non-specific binding through specialized electrolyte formulations; practical implementation in point-of-care diagnostic platforms. Weaknesses: Performance may vary across different target analytes; requires precise control of environmental conditions; potential challenges in long-term stability of specialized electrolyte formulations.
Key Electrolyte-Plasmon Interaction Mechanisms and Patents
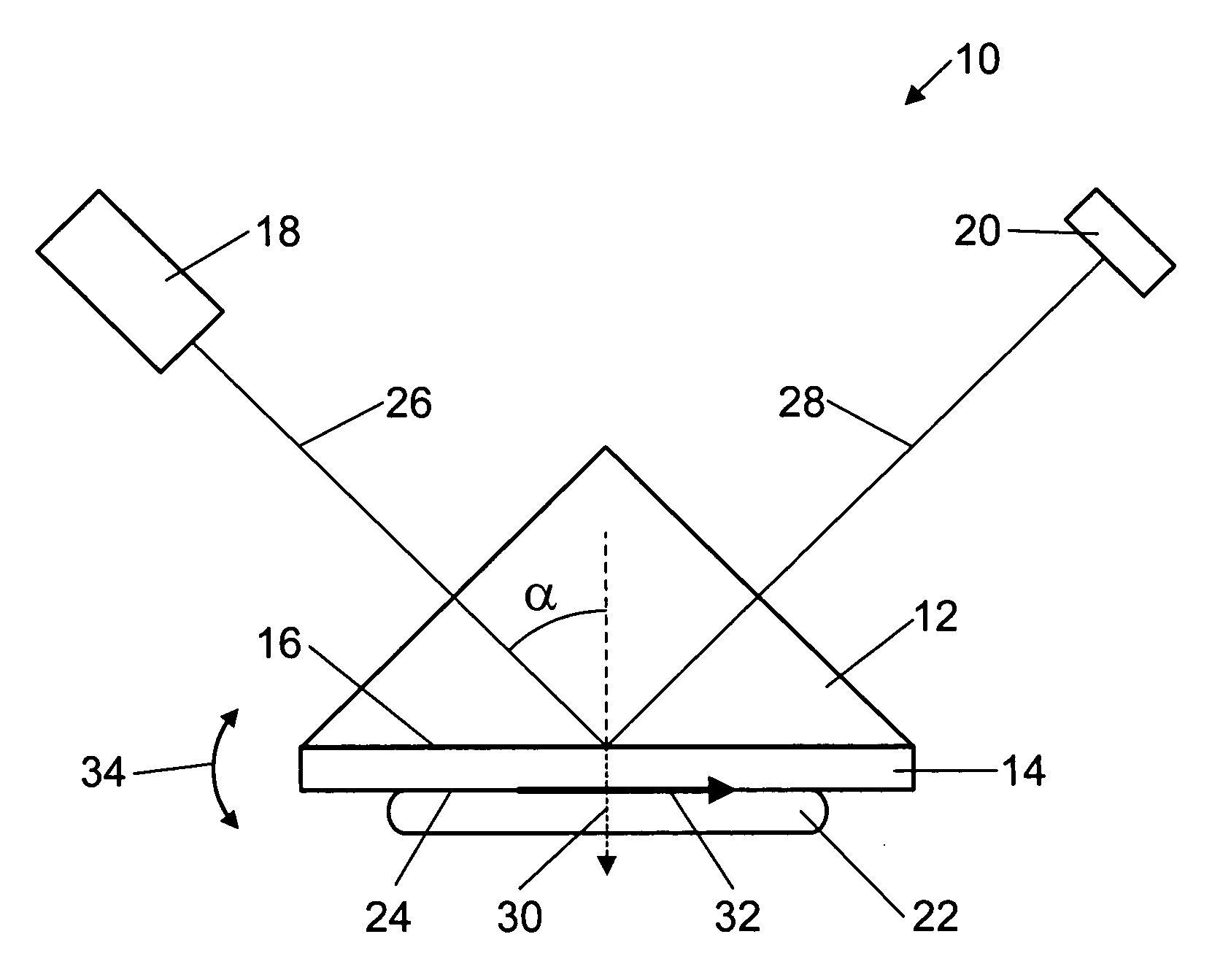
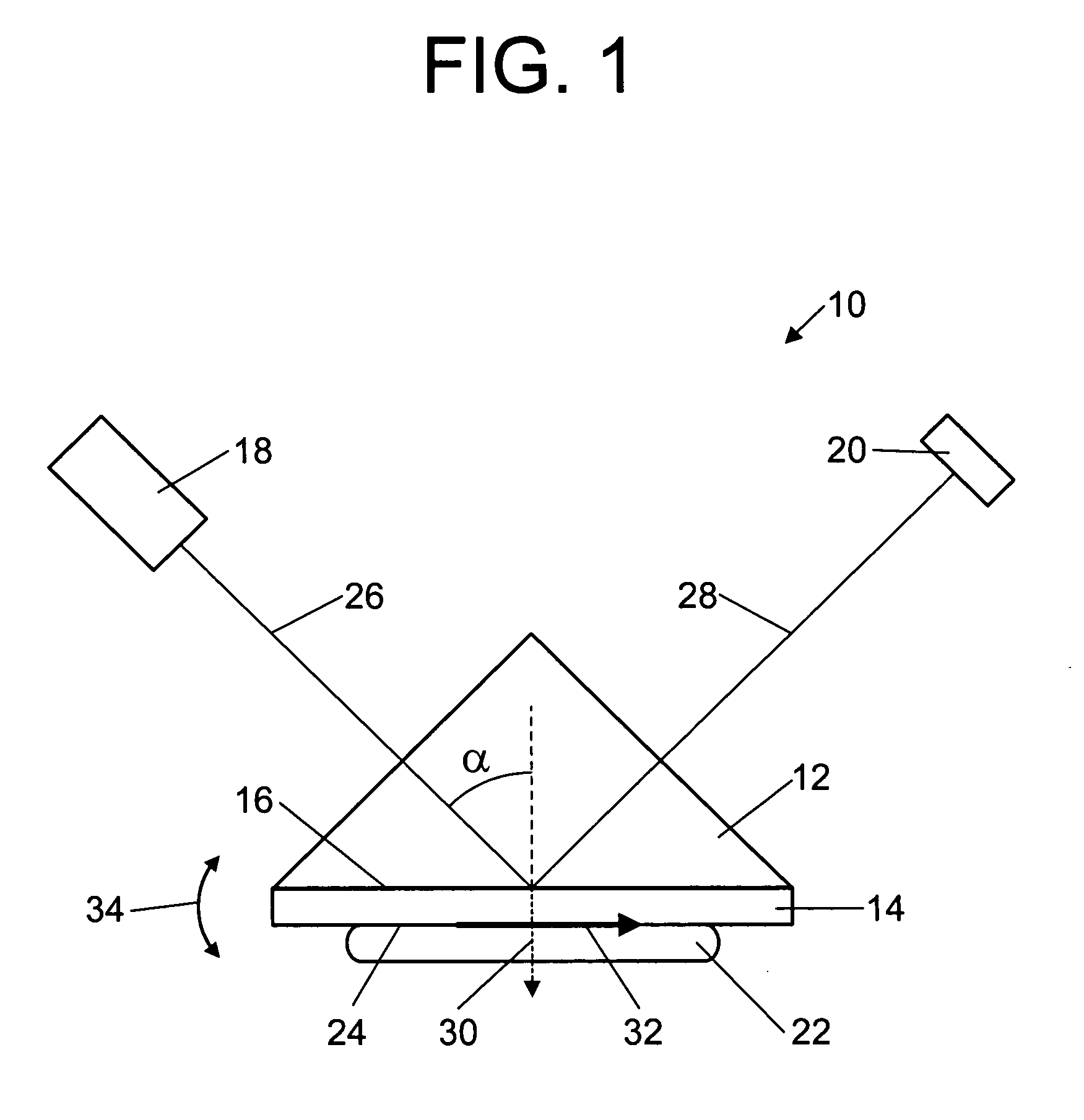
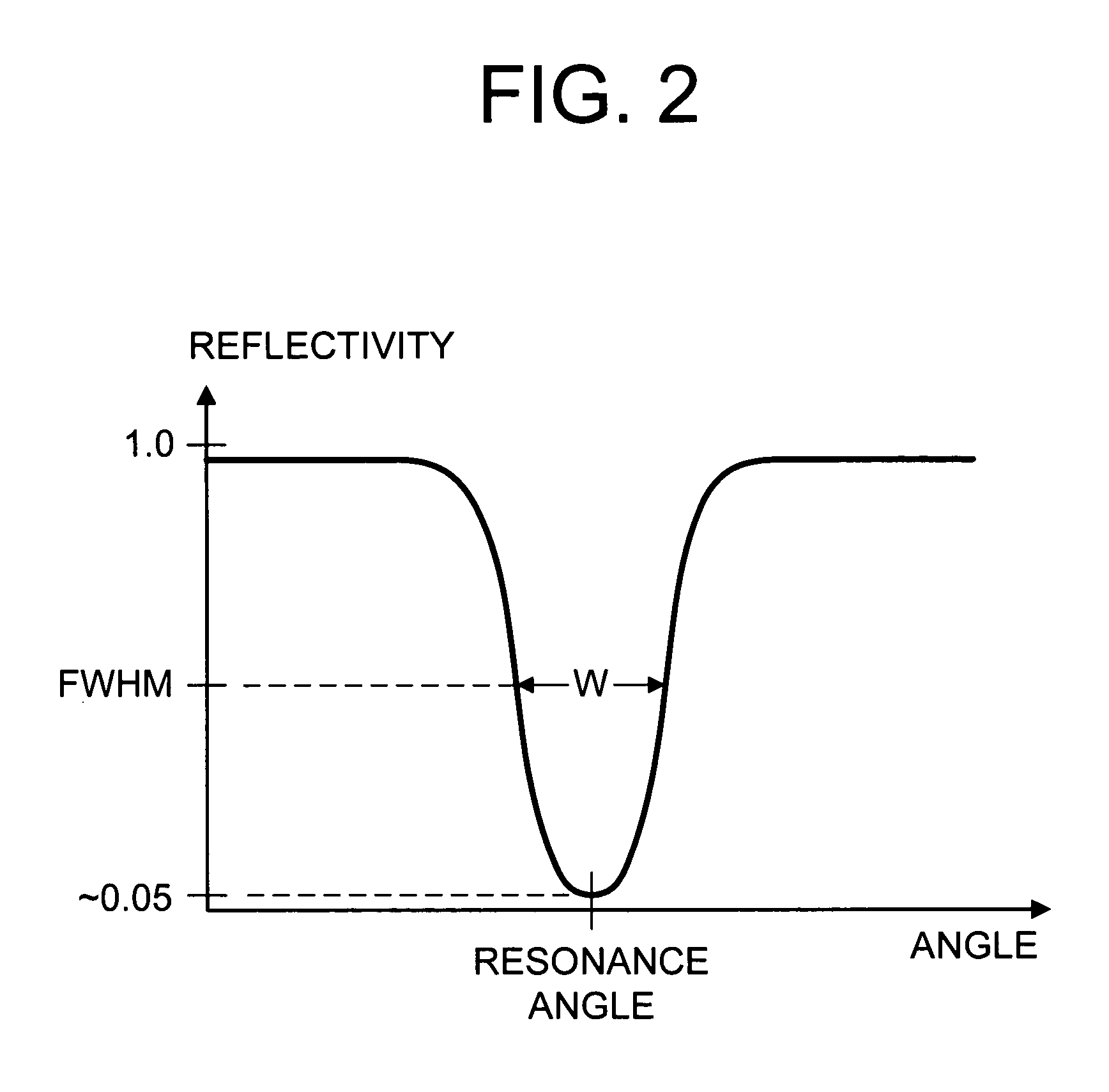
Surface plasmon resonance biosensor using coupled surface plasmons to decrease width of reflectivity dip
PatentInactiveUS20070177150A1
Innovation
- The use of coupled surface plasmons, specifically long-range coupled surface plasmons (LRCSPs) and slightly asymmetric LRCSPs, is employed to decrease the width of the reflectivity dip by reducing absorption losses and increasing the lifetime of surface plasmons, achieved through optimized layer configurations and indices of refraction, allowing for more precise measurement of changes in the sample's index of refraction.
Surface plasmon resonance sensor, localized plasmon resonance sensor, and method for manufacturing same
PatentActiveUS8699032B2
Innovation
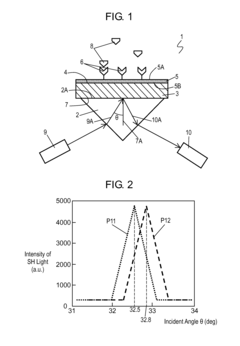
- A surface plasmon resonance sensor is designed with a substrate and a dielectric film having a nonlinear optical effect, where the surface plasmon resonance is detected by measuring changes in the second-order harmonic component of reflected light, enhancing sensitivity through the use of lead zirconate titanate (PZT) or non-lead inorganic nonlinear optical materials.
Regulatory Considerations for Biosensor Electrolyte Solutions
The regulatory landscape for electrolyte solutions used in plasmonic biosensors presents a complex framework that manufacturers and researchers must navigate carefully. In the United States, the FDA classifies most biosensor systems as medical devices, with electrolyte solutions potentially falling under either device components or in vitro diagnostic reagents depending on their specific function and claims. This classification significantly impacts the regulatory pathway, documentation requirements, and time-to-market considerations.
European regulations under the IVDR (In Vitro Diagnostic Regulation) and MDR (Medical Device Regulation) impose stringent requirements for biosensor components, including electrolyte solutions. These regulations emphasize risk classification based on intended use, with higher-risk applications facing more comprehensive conformity assessment procedures. Manufacturers must demonstrate both safety and performance through technical documentation and clinical evidence.
Quality control standards for electrolyte solutions are particularly rigorous, with ISO 13485 serving as the foundational quality management system standard. For plasmonic biosensors specifically, electrolyte composition must be precisely controlled and documented, with batch-to-batch consistency verified through validated analytical methods. Stability testing under various environmental conditions is mandatory to establish shelf-life claims.
Environmental and safety regulations present additional compliance challenges. REACH regulations in Europe require registration of chemical substances in electrolyte formulations, while RoHS directives limit the use of certain hazardous substances. In laboratory settings, biosafety regulations govern handling procedures, particularly when electrolytes are used with biological samples potentially containing infectious agents.
International harmonization efforts through organizations like the International Medical Device Regulators Forum (IMDRF) are gradually standardizing requirements across major markets, though significant regional variations persist. Manufacturers developing novel electrolyte formulations for plasmonic biosensors must consider these regulatory frameworks early in development to avoid costly redesigns or delays.
Regulatory trends indicate increasing scrutiny of nanomaterials and novel chemical compositions in biosensor components, with particular attention to biocompatibility and potential environmental impacts. The growing emphasis on real-world performance data is driving requirements for more comprehensive validation studies demonstrating electrolyte stability and performance across the intended operational range of biosensor devices.
European regulations under the IVDR (In Vitro Diagnostic Regulation) and MDR (Medical Device Regulation) impose stringent requirements for biosensor components, including electrolyte solutions. These regulations emphasize risk classification based on intended use, with higher-risk applications facing more comprehensive conformity assessment procedures. Manufacturers must demonstrate both safety and performance through technical documentation and clinical evidence.
Quality control standards for electrolyte solutions are particularly rigorous, with ISO 13485 serving as the foundational quality management system standard. For plasmonic biosensors specifically, electrolyte composition must be precisely controlled and documented, with batch-to-batch consistency verified through validated analytical methods. Stability testing under various environmental conditions is mandatory to establish shelf-life claims.
Environmental and safety regulations present additional compliance challenges. REACH regulations in Europe require registration of chemical substances in electrolyte formulations, while RoHS directives limit the use of certain hazardous substances. In laboratory settings, biosafety regulations govern handling procedures, particularly when electrolytes are used with biological samples potentially containing infectious agents.
International harmonization efforts through organizations like the International Medical Device Regulators Forum (IMDRF) are gradually standardizing requirements across major markets, though significant regional variations persist. Manufacturers developing novel electrolyte formulations for plasmonic biosensors must consider these regulatory frameworks early in development to avoid costly redesigns or delays.
Regulatory trends indicate increasing scrutiny of nanomaterials and novel chemical compositions in biosensor components, with particular attention to biocompatibility and potential environmental impacts. The growing emphasis on real-world performance data is driving requirements for more comprehensive validation studies demonstrating electrolyte stability and performance across the intended operational range of biosensor devices.
Sustainability and Green Chemistry in Electrolyte Development
The development of sustainable and environmentally friendly electrolytes represents a critical frontier in advancing plasmonic biosensor technology. Traditional electrolyte formulations often contain hazardous components that pose significant environmental and health risks, including heavy metals, organic solvents, and persistent chemicals that resist degradation. The green chemistry movement has begun to transform this landscape by introducing principles that minimize environmental impact while maintaining or enhancing sensor performance.
Recent innovations in green electrolyte development have focused on replacing toxic components with biodegradable alternatives. Water-based electrolyte systems have gained prominence, utilizing naturally occurring salts and buffers that achieve comparable conductivity and stability without the environmental footprint of conventional formulations. These aqueous systems demonstrate remarkable compatibility with biological samples, often resulting in enhanced sensor specificity and reduced non-specific binding.
Biomass-derived ionic liquids represent another promising direction, offering tunable properties and exceptional stability while being synthesized from renewable resources. These materials can be designed with specific functional groups that interact optimally with plasmonic surfaces, potentially improving sensor sensitivity while adhering to green chemistry principles. Studies have shown that certain biomass-derived ionic liquids can enhance signal-to-noise ratios by up to 40% compared to conventional electrolytes.
Life cycle assessment (LCA) methodologies are increasingly being applied to electrolyte development, providing comprehensive evaluations of environmental impacts from raw material extraction through disposal. This holistic approach has revealed that sustainable electrolytes can reduce carbon footprints by 30-60% compared to traditional formulations, while simultaneously decreasing water pollution and resource depletion. These findings have accelerated industry adoption of greener alternatives.
Regulatory frameworks worldwide are evolving to encourage sustainable chemistry practices in sensor development. The European Union's REACH regulations and similar initiatives in other regions have established stricter guidelines for chemical usage, creating market incentives for green electrolyte innovation. Forward-thinking companies are proactively reformulating their products to meet these standards, gaining competitive advantages through environmental compliance and consumer appeal.
Collaborative research between academic institutions and industry partners has yielded promising advances in green electrolyte technology. Open-source platforms for sharing sustainable formulations have emerged, accelerating development cycles and fostering standardization. These collaborative efforts have demonstrated that performance and sustainability need not be mutually exclusive, with several green electrolyte systems now outperforming their conventional counterparts in sensitivity, stability, and shelf-life metrics.
Recent innovations in green electrolyte development have focused on replacing toxic components with biodegradable alternatives. Water-based electrolyte systems have gained prominence, utilizing naturally occurring salts and buffers that achieve comparable conductivity and stability without the environmental footprint of conventional formulations. These aqueous systems demonstrate remarkable compatibility with biological samples, often resulting in enhanced sensor specificity and reduced non-specific binding.
Biomass-derived ionic liquids represent another promising direction, offering tunable properties and exceptional stability while being synthesized from renewable resources. These materials can be designed with specific functional groups that interact optimally with plasmonic surfaces, potentially improving sensor sensitivity while adhering to green chemistry principles. Studies have shown that certain biomass-derived ionic liquids can enhance signal-to-noise ratios by up to 40% compared to conventional electrolytes.
Life cycle assessment (LCA) methodologies are increasingly being applied to electrolyte development, providing comprehensive evaluations of environmental impacts from raw material extraction through disposal. This holistic approach has revealed that sustainable electrolytes can reduce carbon footprints by 30-60% compared to traditional formulations, while simultaneously decreasing water pollution and resource depletion. These findings have accelerated industry adoption of greener alternatives.
Regulatory frameworks worldwide are evolving to encourage sustainable chemistry practices in sensor development. The European Union's REACH regulations and similar initiatives in other regions have established stricter guidelines for chemical usage, creating market incentives for green electrolyte innovation. Forward-thinking companies are proactively reformulating their products to meet these standards, gaining competitive advantages through environmental compliance and consumer appeal.
Collaborative research between academic institutions and industry partners has yielded promising advances in green electrolyte technology. Open-source platforms for sharing sustainable formulations have emerged, accelerating development cycles and fostering standardization. These collaborative efforts have demonstrated that performance and sustainability need not be mutually exclusive, with several green electrolyte systems now outperforming their conventional counterparts in sensitivity, stability, and shelf-life metrics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!