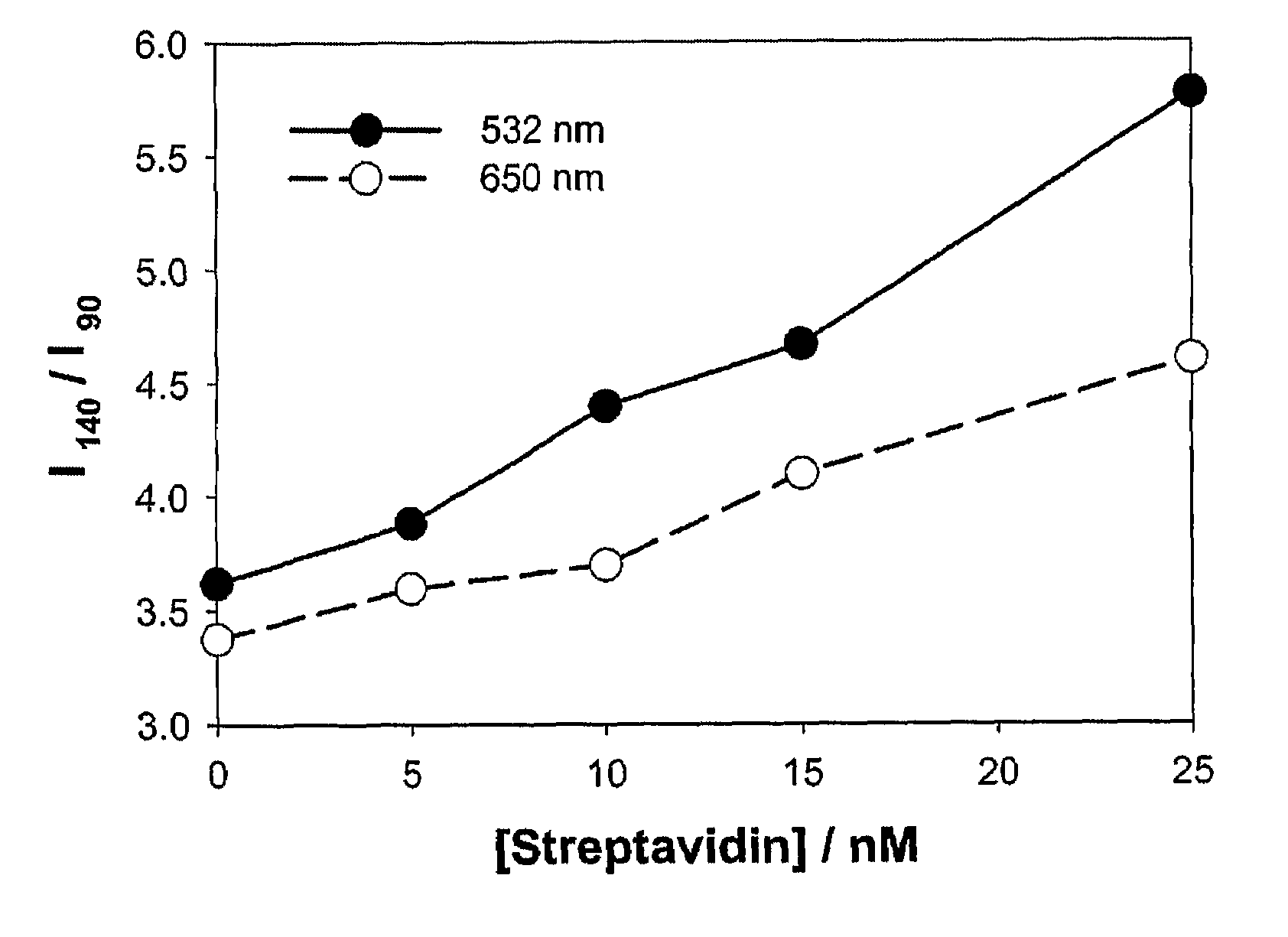
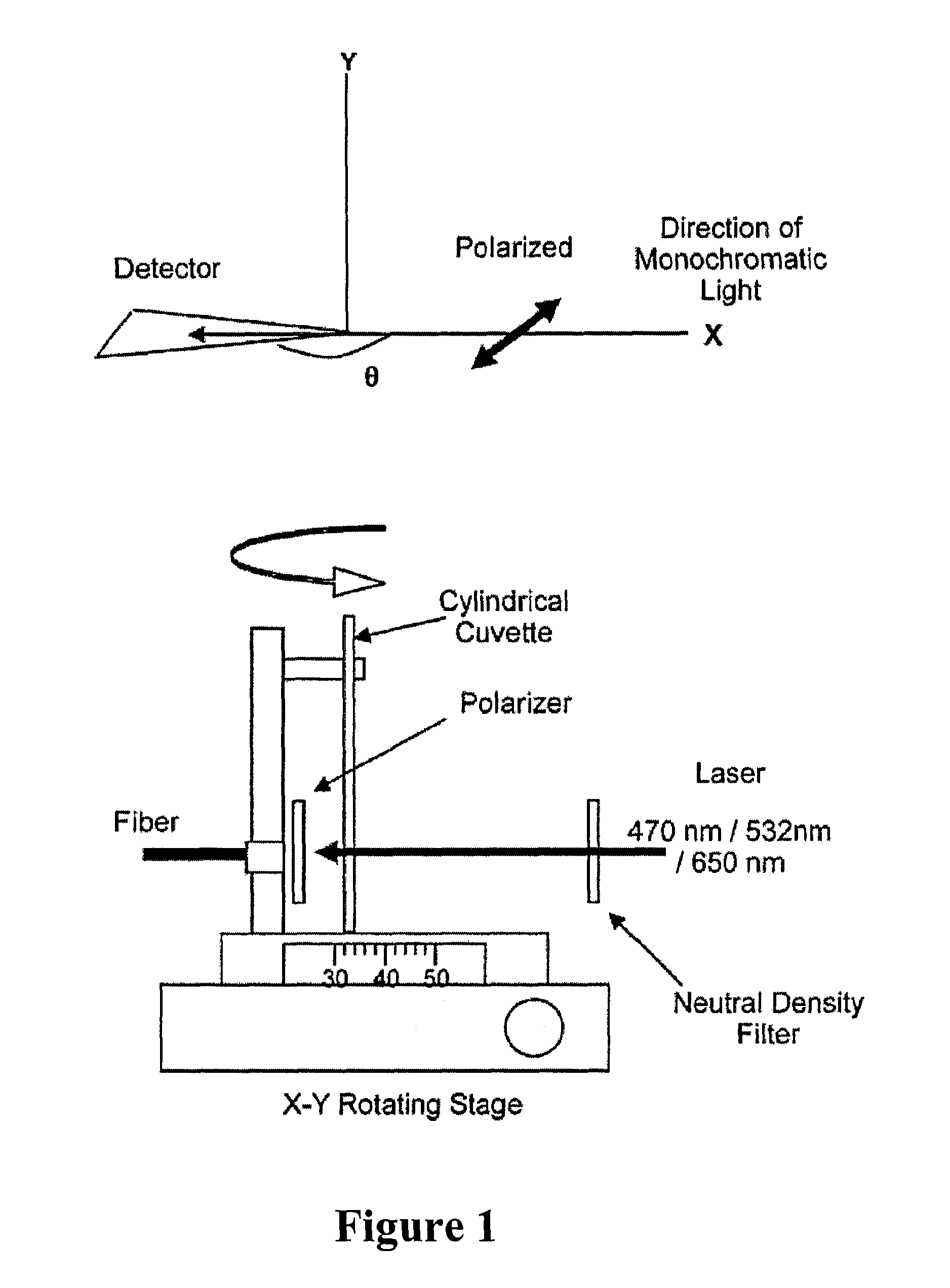
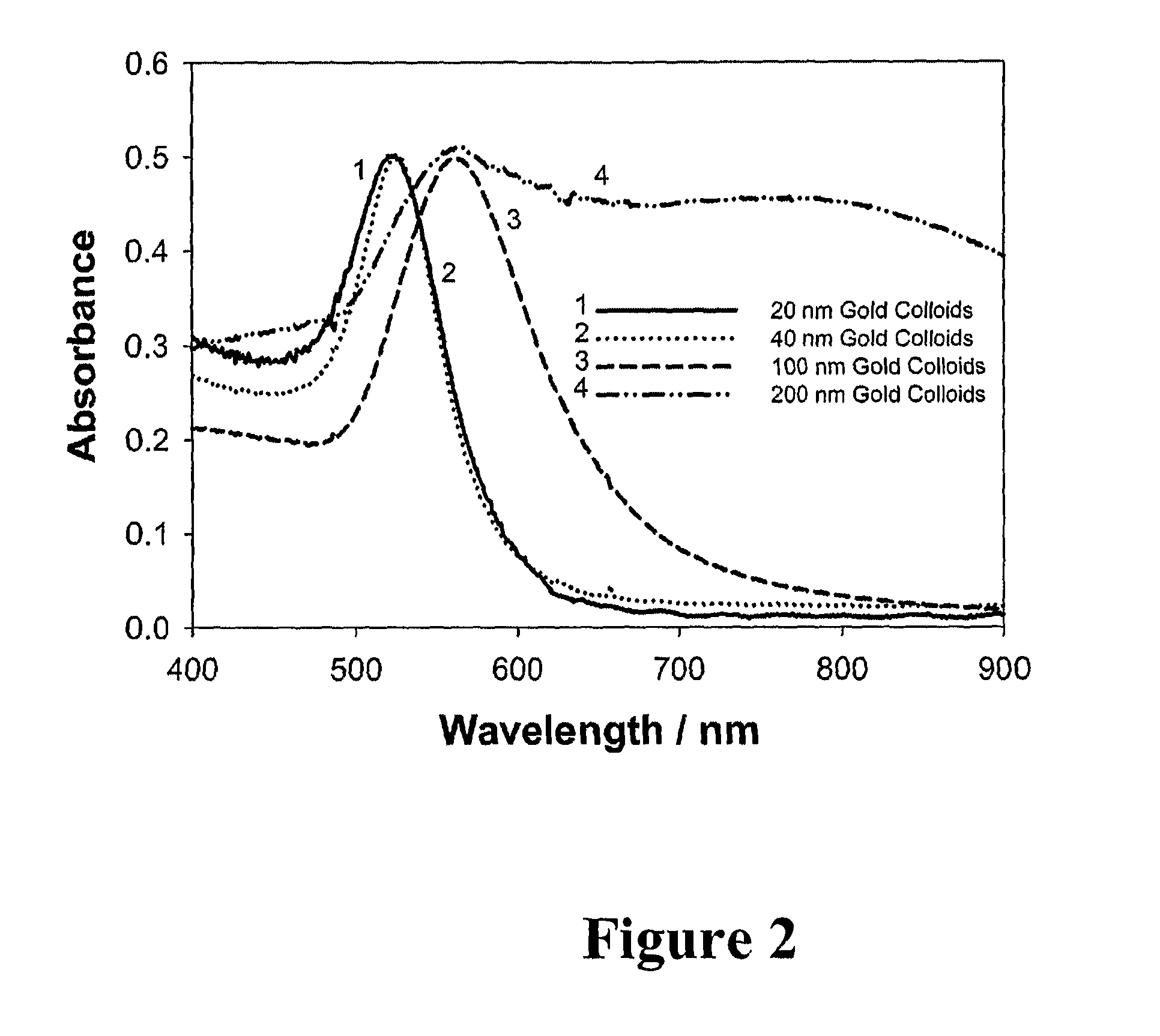
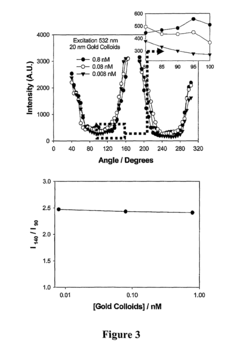
Exploring the Role of Standards in Plasmonic Biosensor Deployment
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasmonic Biosensor Standards Background and Objectives
Plasmonic biosensors represent a revolutionary advancement in sensing technology, combining the principles of plasmonics with biological recognition elements to create highly sensitive detection platforms. The evolution of this technology spans several decades, beginning with fundamental research on surface plasmon resonance (SPR) in the 1960s and advancing to the sophisticated nanoscale plasmonic biosensors of today. This technological progression has been characterized by continuous improvements in sensitivity, specificity, and miniaturization capabilities, enabling increasingly precise detection of biomolecules at ever-lower concentrations.
The current trajectory of plasmonic biosensor development is moving toward greater integration with other technologies, including microfluidics, artificial intelligence, and portable diagnostic platforms. This convergence is creating new possibilities for real-time, point-of-care diagnostics across multiple sectors including healthcare, environmental monitoring, and food safety. However, the absence of universally accepted standards has emerged as a significant barrier to widespread adoption and commercialization.
Standards play a crucial role in technology maturation by establishing common frameworks for performance evaluation, quality assurance, and interoperability. For plasmonic biosensors, standardization objectives encompass several dimensions: technical specifications for materials and fabrication processes, performance metrics for sensitivity and specificity, validation protocols for different application scenarios, and interoperability guidelines for integration with existing diagnostic infrastructures.
The primary goal of standardization efforts in this field is to facilitate the transition of plasmonic biosensors from research laboratories to commercial applications by providing clear benchmarks for performance and reliability. This includes establishing standardized testing methodologies to enable meaningful comparisons between different sensor designs and ensuring reproducibility of results across different laboratories and manufacturing facilities.
Additionally, standards aim to address critical challenges in the field, such as the need for consistent calibration procedures, reference materials for validation, and protocols for surface functionalization that ensure optimal biomolecular recognition. These standards must balance the need for innovation with practical considerations of manufacturability, cost-effectiveness, and regulatory compliance.
International collaboration represents another key objective in the standardization process, as harmonized global standards can accelerate market acceptance and reduce barriers to entry for innovative technologies. Organizations such as ISO, ASTM International, and IEEE are increasingly focusing on developing comprehensive standards frameworks for nanoscale sensing technologies, including plasmonic biosensors.
The ultimate technical objective is to establish a robust foundation that supports the next generation of plasmonic biosensor applications, from rapid infectious disease diagnostics to continuous monitoring of biomarkers for personalized medicine, while ensuring these technologies meet the highest standards of reliability, reproducibility, and safety.
The current trajectory of plasmonic biosensor development is moving toward greater integration with other technologies, including microfluidics, artificial intelligence, and portable diagnostic platforms. This convergence is creating new possibilities for real-time, point-of-care diagnostics across multiple sectors including healthcare, environmental monitoring, and food safety. However, the absence of universally accepted standards has emerged as a significant barrier to widespread adoption and commercialization.
Standards play a crucial role in technology maturation by establishing common frameworks for performance evaluation, quality assurance, and interoperability. For plasmonic biosensors, standardization objectives encompass several dimensions: technical specifications for materials and fabrication processes, performance metrics for sensitivity and specificity, validation protocols for different application scenarios, and interoperability guidelines for integration with existing diagnostic infrastructures.
The primary goal of standardization efforts in this field is to facilitate the transition of plasmonic biosensors from research laboratories to commercial applications by providing clear benchmarks for performance and reliability. This includes establishing standardized testing methodologies to enable meaningful comparisons between different sensor designs and ensuring reproducibility of results across different laboratories and manufacturing facilities.
Additionally, standards aim to address critical challenges in the field, such as the need for consistent calibration procedures, reference materials for validation, and protocols for surface functionalization that ensure optimal biomolecular recognition. These standards must balance the need for innovation with practical considerations of manufacturability, cost-effectiveness, and regulatory compliance.
International collaboration represents another key objective in the standardization process, as harmonized global standards can accelerate market acceptance and reduce barriers to entry for innovative technologies. Organizations such as ISO, ASTM International, and IEEE are increasingly focusing on developing comprehensive standards frameworks for nanoscale sensing technologies, including plasmonic biosensors.
The ultimate technical objective is to establish a robust foundation that supports the next generation of plasmonic biosensor applications, from rapid infectious disease diagnostics to continuous monitoring of biomarkers for personalized medicine, while ensuring these technologies meet the highest standards of reliability, reproducibility, and safety.
Market Analysis for Standardized Biosensor Applications
The global biosensor market is experiencing robust growth, projected to reach $38.7 billion by 2027, with plasmonic biosensors representing a significant segment due to their high sensitivity and real-time detection capabilities. This growth is primarily driven by increasing healthcare expenditure, rising prevalence of chronic diseases, and growing demand for point-of-care testing solutions. The COVID-19 pandemic has further accelerated market expansion, highlighting the critical need for rapid, accurate diagnostic tools in global health emergencies.
Standardization in plasmonic biosensor applications is creating substantial market opportunities across multiple sectors. In healthcare, standardized biosensors are revolutionizing disease diagnosis, patient monitoring, and drug discovery processes. The pharmaceutical industry is increasingly adopting these technologies for quality control and research applications, while the food safety sector utilizes them for contaminant detection and quality assurance.
Market analysis reveals that North America currently dominates the standardized biosensor market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years, driven by increasing healthcare infrastructure investments, growing awareness about preventive healthcare, and rising disposable incomes in countries like China and India.
Consumer demand patterns indicate a strong preference for miniaturized, user-friendly biosensor devices that offer rapid results with minimal technical expertise requirements. This trend is pushing manufacturers toward developing integrated systems that combine standardized sensing platforms with user-friendly interfaces and data management solutions.
Pricing analysis shows that while initial development costs for standardized plasmonic biosensors remain high, economies of scale and technological advancements are gradually reducing per-unit costs. The market is witnessing a shift toward subscription-based models for biosensor systems, particularly in clinical settings, offering more predictable revenue streams for manufacturers and lower initial investment barriers for end-users.
Regulatory considerations significantly impact market dynamics, with regions having well-established regulatory frameworks for medical devices showing faster adoption rates. The FDA's recent initiatives to streamline approval processes for certain diagnostic technologies are expected to positively influence market growth in the United States, while the European Medical Device Regulation (MDR) implementation is reshaping market entry strategies in Europe.
Market forecasts suggest that standardized plasmonic biosensor applications will experience compound annual growth rates exceeding 10% through 2030, with particularly strong growth in point-of-care diagnostics, environmental monitoring, and biodefense applications. Strategic partnerships between technology developers, manufacturing specialists, and distribution networks are emerging as key success factors in this rapidly evolving market landscape.
Standardization in plasmonic biosensor applications is creating substantial market opportunities across multiple sectors. In healthcare, standardized biosensors are revolutionizing disease diagnosis, patient monitoring, and drug discovery processes. The pharmaceutical industry is increasingly adopting these technologies for quality control and research applications, while the food safety sector utilizes them for contaminant detection and quality assurance.
Market analysis reveals that North America currently dominates the standardized biosensor market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years, driven by increasing healthcare infrastructure investments, growing awareness about preventive healthcare, and rising disposable incomes in countries like China and India.
Consumer demand patterns indicate a strong preference for miniaturized, user-friendly biosensor devices that offer rapid results with minimal technical expertise requirements. This trend is pushing manufacturers toward developing integrated systems that combine standardized sensing platforms with user-friendly interfaces and data management solutions.
Pricing analysis shows that while initial development costs for standardized plasmonic biosensors remain high, economies of scale and technological advancements are gradually reducing per-unit costs. The market is witnessing a shift toward subscription-based models for biosensor systems, particularly in clinical settings, offering more predictable revenue streams for manufacturers and lower initial investment barriers for end-users.
Regulatory considerations significantly impact market dynamics, with regions having well-established regulatory frameworks for medical devices showing faster adoption rates. The FDA's recent initiatives to streamline approval processes for certain diagnostic technologies are expected to positively influence market growth in the United States, while the European Medical Device Regulation (MDR) implementation is reshaping market entry strategies in Europe.
Market forecasts suggest that standardized plasmonic biosensor applications will experience compound annual growth rates exceeding 10% through 2030, with particularly strong growth in point-of-care diagnostics, environmental monitoring, and biodefense applications. Strategic partnerships between technology developers, manufacturing specialists, and distribution networks are emerging as key success factors in this rapidly evolving market landscape.
Current Standards Landscape and Technical Challenges
The current landscape of standards for plasmonic biosensors reveals significant fragmentation across different regions and applications. ISO (International Organization for Standardization) has established several relevant standards, including ISO 14155 for clinical investigation of medical devices and ISO 13485 for quality management systems, which indirectly affect biosensor development. However, standards specifically addressing plasmonic biosensor performance metrics, calibration protocols, and validation methodologies remain notably underdeveloped.
In the United States, the FDA has issued guidance documents for biosensor technologies under the medical device regulatory framework, but these lack specificity for plasmonic technologies. The European Union, through its harmonized standards under the In Vitro Diagnostic Regulation (IVDR), provides more structured approaches for biosensor validation, yet still lacks plasmonic-specific protocols.
Technical challenges in standardization primarily stem from the inherent complexity and diversity of plasmonic biosensor designs. Surface plasmon resonance (SPR) sensors, localized surface plasmon resonance (LSPR) sensors, and nanoparticle-enhanced systems each present unique characteristics that complicate the establishment of universal standards. The sensitivity of these systems to environmental factors such as temperature fluctuations, sample matrix effects, and surface functionalization variability further exacerbates standardization difficulties.
Reproducibility represents a critical challenge, with inter-laboratory studies showing significant variations in performance metrics when identical samples are analyzed using nominally similar plasmonic platforms. This inconsistency undermines confidence in diagnostic results and hinders clinical adoption. The absence of standardized reference materials specifically designed for plasmonic sensor calibration compounds this issue.
Data reporting conventions present another obstacle, with no consensus on how to present and interpret plasmonic sensing data. Different research groups and manufacturers employ varying metrics for sensitivity, limit of detection, and dynamic range, making direct comparisons between technologies nearly impossible for end-users.
Interoperability challenges have emerged as plasmonic biosensors increasingly integrate with other technologies and data systems. The lack of standardized data formats and communication protocols limits the potential for these sensors to function effectively within broader healthcare ecosystems and Internet of Medical Things (IoMT) frameworks.
Regulatory bodies worldwide are beginning to recognize these gaps, with initiatives from NIST (National Institute of Standards and Technology) in the US and BIPM (International Bureau of Weights and Measures) in Europe working toward reference materials and measurement protocols. However, these efforts remain in early stages and require greater coordination between academic researchers, industry stakeholders, and regulatory authorities to establish comprehensive standards that can accelerate plasmonic biosensor deployment.
In the United States, the FDA has issued guidance documents for biosensor technologies under the medical device regulatory framework, but these lack specificity for plasmonic technologies. The European Union, through its harmonized standards under the In Vitro Diagnostic Regulation (IVDR), provides more structured approaches for biosensor validation, yet still lacks plasmonic-specific protocols.
Technical challenges in standardization primarily stem from the inherent complexity and diversity of plasmonic biosensor designs. Surface plasmon resonance (SPR) sensors, localized surface plasmon resonance (LSPR) sensors, and nanoparticle-enhanced systems each present unique characteristics that complicate the establishment of universal standards. The sensitivity of these systems to environmental factors such as temperature fluctuations, sample matrix effects, and surface functionalization variability further exacerbates standardization difficulties.
Reproducibility represents a critical challenge, with inter-laboratory studies showing significant variations in performance metrics when identical samples are analyzed using nominally similar plasmonic platforms. This inconsistency undermines confidence in diagnostic results and hinders clinical adoption. The absence of standardized reference materials specifically designed for plasmonic sensor calibration compounds this issue.
Data reporting conventions present another obstacle, with no consensus on how to present and interpret plasmonic sensing data. Different research groups and manufacturers employ varying metrics for sensitivity, limit of detection, and dynamic range, making direct comparisons between technologies nearly impossible for end-users.
Interoperability challenges have emerged as plasmonic biosensors increasingly integrate with other technologies and data systems. The lack of standardized data formats and communication protocols limits the potential for these sensors to function effectively within broader healthcare ecosystems and Internet of Medical Things (IoMT) frameworks.
Regulatory bodies worldwide are beginning to recognize these gaps, with initiatives from NIST (National Institute of Standards and Technology) in the US and BIPM (International Bureau of Weights and Measures) in Europe working toward reference materials and measurement protocols. However, these efforts remain in early stages and require greater coordination between academic researchers, industry stakeholders, and regulatory authorities to establish comprehensive standards that can accelerate plasmonic biosensor deployment.
Existing Standardization Frameworks and Approaches
01 Standardization of plasmonic biosensor fabrication
Standardized methods for fabricating plasmonic biosensors ensure consistency and reliability in performance. These standards cover the deposition of metallic nanostructures, surface functionalization protocols, and quality control measures during manufacturing. Standardized fabrication processes help in achieving reproducible sensor sensitivity, specificity, and stability across different production batches.- Standardization of plasmonic biosensor fabrication: Standardized methods for fabricating plasmonic biosensors ensure consistency in performance and reliability. These standards cover the deposition of metallic nanostructures, surface functionalization protocols, and quality control measures during manufacturing. Standardization helps in achieving reproducible sensor responses across different batches and laboratories, which is crucial for commercial applications and regulatory approval.
- Calibration and performance metrics for plasmonic biosensors: Standardized calibration procedures and performance metrics are essential for evaluating plasmonic biosensors. These include sensitivity measurements, detection limits, dynamic range, and response time. Reference materials and control samples are used to validate sensor performance against established benchmarks. These standards enable comparison between different sensor designs and ensure reliable detection of target analytes in various applications.
- Integration standards for plasmonic biosensor systems: Standards for integrating plasmonic biosensors with other components such as microfluidics, optical systems, and data processing units ensure proper system functionality. These standards define interface specifications, signal processing protocols, and system validation methods. Standardized integration approaches facilitate the development of complete biosensing platforms that can be deployed in clinical, environmental, or industrial settings.
- Validation protocols for plasmonic biosensor applications: Standardized validation protocols ensure that plasmonic biosensors perform reliably in specific applications such as medical diagnostics, environmental monitoring, or food safety testing. These protocols define sample preparation methods, testing conditions, and acceptance criteria. They also establish procedures for verifying sensor specificity, minimizing interference, and confirming analytical accuracy in real-world samples.
- Data reporting and interoperability standards: Standards for data reporting and interoperability ensure that results from plasmonic biosensors can be consistently interpreted and shared across different platforms. These standards define data formats, metadata requirements, and analytical algorithms for processing sensor signals. They facilitate the integration of biosensor data with laboratory information systems and enable multi-center studies where data from different locations can be reliably compared.
02 Calibration and validation standards for plasmonic biosensors
Calibration and validation standards are essential for ensuring accurate and reliable measurements in plasmonic biosensing. These standards include reference materials with known optical properties, standardized testing protocols, and performance benchmarks. Proper calibration allows for quantitative analysis and comparison of results across different plasmonic biosensor platforms and laboratories.Expand Specific Solutions03 Signal processing and data analysis standards
Standardized approaches for signal processing and data analysis in plasmonic biosensing ensure consistent interpretation of results. These standards include algorithms for noise reduction, baseline correction, and signal amplification. Standardized data analysis methods help in extracting meaningful information from plasmonic responses and enable reliable comparison of results across different studies and platforms.Expand Specific Solutions04 Integration standards for plasmonic biosensor systems
Standards for integrating plasmonic biosensors with other components and systems ensure compatibility and optimal performance. These include interface specifications for microfluidics, electronics, and optical components. Standardized integration approaches facilitate the development of complete biosensing platforms that can be used in various applications including point-of-care diagnostics and environmental monitoring.Expand Specific Solutions05 Performance metrics and testing standards
Standardized performance metrics and testing protocols allow for objective evaluation and comparison of different plasmonic biosensor technologies. These standards define key parameters such as sensitivity, specificity, limit of detection, dynamic range, and response time. Standardized testing under controlled conditions ensures that biosensor performance claims are reliable and reproducible across different laboratories and applications.Expand Specific Solutions
Key Organizations and Industry Leaders in Biosensor Standards
The plasmonic biosensor market is currently in a growth phase, characterized by increasing adoption across healthcare, environmental monitoring, and food safety sectors. The global market size is projected to reach significant value by 2030, driven by rising demand for point-of-care diagnostics and real-time monitoring solutions. Technologically, the field is advancing rapidly but standardization remains a critical challenge. Leading academic institutions like MIT, Washington University in St. Louis, and Xiamen University are driving fundamental research, while commercial players including FUJIFILM, Life Technologies, and Onechip Bioelectronics are focusing on practical applications and scalable manufacturing. Government entities like CSIC and the US Government provide regulatory frameworks and funding support, creating a diverse ecosystem that balances innovation with the need for reliable, standardized deployment protocols.
Washington University in St. Louis
Technical Solution: 华盛顿大学圣路易斯分校在等离子体生物传感器标准化领域的技术方案聚焦于提高临床应用的可靠性和一致性。他们开发了基于纳米孔阵列的等离子体传感平台,结合精确的表面修饰技术和光谱分析方法。该团队特别关注生物样本前处理的标准化,开发了一套从样本采集到分析的完整工作流程,减少了样本变异对测量结果的影响[5]。他们的标准化方案包括传感器芯片的质量控制程序、表面功能化的标准操作规程以及信号采集和处理的统一方法。值得注意的是,该团队与临床实验室合作,建立了等离子体生物传感器在不同临床环境中的性能验证标准,包括对干扰物质的耐受性测试和多中心验证研究[6]。他们还开发了用于传感器性能评估的标准参考材料和校准曲线生成方法,确保不同批次传感器之间的一致性。
优势:与医学院和附属医院有紧密合作,能够将标准化工作与临床需求紧密结合;在生物样本前处理标准化方面有独特专长。劣势:研究规模相对较小,可能缺乏大规模推广标准的资源;在国际标准组织中的影响力有限。
Consejo Superior de Investigaciones Científicas
Technical Solution: 西班牙国家研究委员会(CSIC)在等离子体生物传感器标准化领域采取了系统性方法,开发了一套全面的标准框架。其技术方案基于多模态等离子体传感平台,结合了传统SPR与纳米结构增强技术,实现了高灵敏度和高特异性检测。CSIC研究人员特别关注传感器表面生物功能化的标准化,开发了一系列可重复的表面修饰协议,适用于不同类型的生物分子识别元件(抗体、适体、分子印迹聚合物等)[9]。他们建立了传感器性能评估的欧洲标准,包括检测限、动态范围、交叉反应性和基质效应等参数的量化方法。值得注意的是,CSIC开发了一套参考样品库,用于不同等离子体传感平台之间的性能比较和校准[10]。此外,他们还与欧洲标准化委员会(CEN)合作,推动建立了等离子体生物传感器在食品安全、环境监测和医疗诊断领域的应用标准,包括样本前处理、数据分析和结果解释的统一方法。
优势:在欧洲标准化组织中具有重要影响力,能够推动标准的区域性采用;拥有多学科研究团队,能够全面解决标准化的技术和方法学问题。劣势:标准化工作主要针对欧洲市场,全球适用性可能需要调整;在某些新兴应用领域的标准开发相对滞后。
Critical Standards and Reference Materials Analysis
Bioassays using plasmonic scattering from noble metal nanostructures
PatentInactiveUS8101424B2
Innovation
- The use of surface plasmons from metallic nanoparticles to measure scattering effects at different angles and wavelengths, allowing for the detection of analyte concentration through changes in light intensity and polarization, which is more stable and sensitive than traditional fluorescence methods.
Method for cell energy therapeutics
PatentInactiveUS20180185518A1
Innovation
- Development of self-assembling bio-nano-plasmonic elements using purified, synthetic, and recombinant protein molecules like Clathrin and Coatomer proteins to form nanoscale plasmonic devices that can emit surface-plasmon-enhanced electromagnetic radiation, allowing for internal excitation and improved biocompatibility and configurability.
Regulatory Compliance and Certification Pathways
The regulatory landscape for plasmonic biosensor deployment presents a complex matrix of requirements that vary significantly across global markets. In the United States, the FDA has established specific pathways for biosensor approval, with plasmonic technologies typically falling under either the 510(k) clearance process or, for novel applications, the more rigorous Premarket Approval (PMA) pathway. These regulatory frameworks evaluate not only the analytical performance but also clinical validity and utility of the biosensing platforms.
European markets operate under the In Vitro Diagnostic Regulation (IVDR) and Medical Device Regulation (MDR), which have recently undergone significant revisions to strengthen safety requirements. Plasmonic biosensors must now demonstrate compliance with stricter classification rules based on intended use and risk profile, with many advanced diagnostic applications falling into higher risk categories requiring notified body involvement and more comprehensive technical documentation.
ISO standards play a crucial role in harmonizing global requirements, with ISO 13485 for quality management systems and ISO 14971 for risk management being particularly relevant. For plasmonic biosensors specifically, standards such as ISO 17511 for metrological traceability and ISO 15193 for measurement procedure validation provide essential frameworks for ensuring reliability and comparability of results across different platforms.
Emerging markets present additional certification challenges, with China's NMPA, India's CDSCO, and Brazil's ANVISA each maintaining distinct requirements that necessitate tailored compliance strategies. These often include local testing, documentation in national languages, and sometimes in-country representation.
Performance verification standards represent another critical dimension, with organizations like CLSI (Clinical and Laboratory Standards Institute) developing consensus guidelines for analytical performance characteristics. For plasmonic biosensors, parameters such as limit of detection, analytical specificity, and robustness against interferents require standardized testing methodologies to ensure consistent evaluation across different technologies and applications.
The certification timeline typically spans 12-36 months depending on regulatory pathway and jurisdiction, with costs ranging from $50,000 to several million dollars for complex applications. Strategic planning for regulatory submissions is therefore essential, with many developers adopting a phased approach beginning with less stringent markets to generate revenue while pursuing approvals in more regulated environments.
Post-market surveillance requirements have also intensified globally, requiring manufacturers to implement robust systems for monitoring real-world performance and addressing adverse events. This ongoing compliance obligation represents a significant operational consideration for organizations deploying plasmonic biosensor technologies in clinical or consumer settings.
European markets operate under the In Vitro Diagnostic Regulation (IVDR) and Medical Device Regulation (MDR), which have recently undergone significant revisions to strengthen safety requirements. Plasmonic biosensors must now demonstrate compliance with stricter classification rules based on intended use and risk profile, with many advanced diagnostic applications falling into higher risk categories requiring notified body involvement and more comprehensive technical documentation.
ISO standards play a crucial role in harmonizing global requirements, with ISO 13485 for quality management systems and ISO 14971 for risk management being particularly relevant. For plasmonic biosensors specifically, standards such as ISO 17511 for metrological traceability and ISO 15193 for measurement procedure validation provide essential frameworks for ensuring reliability and comparability of results across different platforms.
Emerging markets present additional certification challenges, with China's NMPA, India's CDSCO, and Brazil's ANVISA each maintaining distinct requirements that necessitate tailored compliance strategies. These often include local testing, documentation in national languages, and sometimes in-country representation.
Performance verification standards represent another critical dimension, with organizations like CLSI (Clinical and Laboratory Standards Institute) developing consensus guidelines for analytical performance characteristics. For plasmonic biosensors, parameters such as limit of detection, analytical specificity, and robustness against interferents require standardized testing methodologies to ensure consistent evaluation across different technologies and applications.
The certification timeline typically spans 12-36 months depending on regulatory pathway and jurisdiction, with costs ranging from $50,000 to several million dollars for complex applications. Strategic planning for regulatory submissions is therefore essential, with many developers adopting a phased approach beginning with less stringent markets to generate revenue while pursuing approvals in more regulated environments.
Post-market surveillance requirements have also intensified globally, requiring manufacturers to implement robust systems for monitoring real-world performance and addressing adverse events. This ongoing compliance obligation represents a significant operational consideration for organizations deploying plasmonic biosensor technologies in clinical or consumer settings.
International Harmonization of Biosensor Standards
The global landscape of plasmonic biosensor development faces significant challenges due to fragmented standards across different regions and regulatory frameworks. Currently, major regulatory bodies including the FDA (United States), EMA (European Union), NMPA (China), and PMDA (Japan) maintain distinct requirements for biosensor validation, performance metrics, and clinical implementation. This regulatory divergence creates substantial barriers for manufacturers seeking multi-market deployment, often necessitating redundant testing and validation procedures that increase development costs and delay market entry by an estimated 18-36 months.
International harmonization efforts have gained momentum through initiatives like the International Medical Device Regulators Forum (IMDRF) and the International Organization for Standardization (ISO) Technical Committee 276 on Biotechnology. These organizations are working to establish consensus on critical parameters for plasmonic biosensor evaluation, including sensitivity thresholds, specificity requirements, reproducibility metrics, and calibration protocols. The ISO/TC 212 for clinical laboratory testing has also contributed significantly by developing standards that address quality management systems for medical laboratories and in vitro diagnostic test systems.
Recent progress includes the development of ISO 23118:2022, which provides guidelines for evaluating the performance of nucleic acid-based biosensors, and ISO/TS 21831:2019, which addresses nanomaterial characterization in biological systems. These standards represent important steps toward creating a unified framework for plasmonic biosensor evaluation, though they do not yet fully address the unique optical and surface chemistry considerations specific to plasmonic technologies.
The Global Harmonization Working Party (GHWP) has established working groups specifically focused on emerging diagnostic technologies, including plasmonic biosensors. Their 2022 white paper outlined a roadmap for creating internationally recognized performance benchmarks and validation protocols. This initiative has gained support from major industry stakeholders, with over 40 companies and 25 academic institutions participating in collaborative standard-setting exercises.
Economic analyses suggest that harmonized standards could reduce development costs by approximately 30% and accelerate time-to-market by 40-50% across major regulatory jurisdictions. Furthermore, standardization would likely enhance data comparability between different plasmonic biosensor platforms, facilitating more robust clinical validation studies and meta-analyses that could strengthen the evidence base for these technologies in healthcare applications.
The path forward requires balancing technological innovation with regulatory consistency. Experts recommend a phased approach to harmonization, beginning with agreement on fundamental performance metrics and gradually expanding to more complex aspects such as clinical validation protocols and reference materials. This strategy acknowledges the rapidly evolving nature of plasmonic biosensor technology while establishing a foundation for consistent evaluation across international boundaries.
International harmonization efforts have gained momentum through initiatives like the International Medical Device Regulators Forum (IMDRF) and the International Organization for Standardization (ISO) Technical Committee 276 on Biotechnology. These organizations are working to establish consensus on critical parameters for plasmonic biosensor evaluation, including sensitivity thresholds, specificity requirements, reproducibility metrics, and calibration protocols. The ISO/TC 212 for clinical laboratory testing has also contributed significantly by developing standards that address quality management systems for medical laboratories and in vitro diagnostic test systems.
Recent progress includes the development of ISO 23118:2022, which provides guidelines for evaluating the performance of nucleic acid-based biosensors, and ISO/TS 21831:2019, which addresses nanomaterial characterization in biological systems. These standards represent important steps toward creating a unified framework for plasmonic biosensor evaluation, though they do not yet fully address the unique optical and surface chemistry considerations specific to plasmonic technologies.
The Global Harmonization Working Party (GHWP) has established working groups specifically focused on emerging diagnostic technologies, including plasmonic biosensors. Their 2022 white paper outlined a roadmap for creating internationally recognized performance benchmarks and validation protocols. This initiative has gained support from major industry stakeholders, with over 40 companies and 25 academic institutions participating in collaborative standard-setting exercises.
Economic analyses suggest that harmonized standards could reduce development costs by approximately 30% and accelerate time-to-market by 40-50% across major regulatory jurisdictions. Furthermore, standardization would likely enhance data comparability between different plasmonic biosensor platforms, facilitating more robust clinical validation studies and meta-analyses that could strengthen the evidence base for these technologies in healthcare applications.
The path forward requires balancing technological innovation with regulatory consistency. Experts recommend a phased approach to harmonization, beginning with agreement on fundamental performance metrics and gradually expanding to more complex aspects such as clinical validation protocols and reference materials. This strategy acknowledges the rapidly evolving nature of plasmonic biosensor technology while establishing a foundation for consistent evaluation across international boundaries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!