Application of Plasmonic Biosensors in Novel Drug Delivery Systems
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasmonic Biosensor Technology Background and Objectives
Plasmonic biosensors represent a revolutionary advancement in sensing technology, combining the principles of plasmonics with biological recognition elements to create highly sensitive detection platforms. The evolution of this technology began in the late 1990s with the discovery of surface plasmon resonance (SPR) phenomena, which has since evolved into more sophisticated localized surface plasmon resonance (LSPR) techniques. This progression has been driven by advancements in nanofabrication methods, enabling the creation of precisely engineered metallic nanostructures that exhibit unique optical properties when interacting with electromagnetic radiation.
The technological trajectory of plasmonic biosensors has been characterized by continuous improvements in sensitivity, specificity, and miniaturization. Early systems were primarily laboratory-based instruments requiring significant expertise to operate, whereas current generations feature portable, user-friendly designs suitable for point-of-care applications. This evolution reflects the broader trend toward decentralized healthcare diagnostics and personalized medicine.
In the context of drug delivery systems, plasmonic biosensors offer unprecedented capabilities for real-time monitoring of drug release kinetics, biodistribution, and therapeutic efficacy. The integration of these sensing platforms with advanced drug delivery vehicles represents a paradigm shift in pharmaceutical development, enabling precise control over drug administration and personalized treatment regimens.
The primary technical objectives for plasmonic biosensors in novel drug delivery applications include enhancing detection sensitivity to sub-nanomolar concentrations, improving specificity through advanced surface functionalization strategies, and developing multiplexed sensing capabilities for simultaneous monitoring of multiple analytes. Additionally, there is significant focus on creating biocompatible sensor designs suitable for in vivo applications, which necessitates addressing challenges related to biofouling, stability in biological environments, and long-term performance reliability.
Another critical objective involves the development of wireless, implantable plasmonic biosensors capable of continuous monitoring and feedback-controlled drug release. This approach aims to create closed-loop drug delivery systems that can autonomously adjust dosing based on real-time physiological parameters, thereby optimizing therapeutic outcomes while minimizing adverse effects.
The convergence of plasmonic biosensing with emerging technologies such as artificial intelligence, microfluidics, and advanced materials science is expected to accelerate innovation in this field. Research efforts are increasingly focused on creating integrated sensing and delivery platforms that combine diagnostic capabilities with therapeutic interventions, embodying the concept of theranostics (therapeutic diagnostics) in next-generation pharmaceutical applications.
The technological trajectory of plasmonic biosensors has been characterized by continuous improvements in sensitivity, specificity, and miniaturization. Early systems were primarily laboratory-based instruments requiring significant expertise to operate, whereas current generations feature portable, user-friendly designs suitable for point-of-care applications. This evolution reflects the broader trend toward decentralized healthcare diagnostics and personalized medicine.
In the context of drug delivery systems, plasmonic biosensors offer unprecedented capabilities for real-time monitoring of drug release kinetics, biodistribution, and therapeutic efficacy. The integration of these sensing platforms with advanced drug delivery vehicles represents a paradigm shift in pharmaceutical development, enabling precise control over drug administration and personalized treatment regimens.
The primary technical objectives for plasmonic biosensors in novel drug delivery applications include enhancing detection sensitivity to sub-nanomolar concentrations, improving specificity through advanced surface functionalization strategies, and developing multiplexed sensing capabilities for simultaneous monitoring of multiple analytes. Additionally, there is significant focus on creating biocompatible sensor designs suitable for in vivo applications, which necessitates addressing challenges related to biofouling, stability in biological environments, and long-term performance reliability.
Another critical objective involves the development of wireless, implantable plasmonic biosensors capable of continuous monitoring and feedback-controlled drug release. This approach aims to create closed-loop drug delivery systems that can autonomously adjust dosing based on real-time physiological parameters, thereby optimizing therapeutic outcomes while minimizing adverse effects.
The convergence of plasmonic biosensing with emerging technologies such as artificial intelligence, microfluidics, and advanced materials science is expected to accelerate innovation in this field. Research efforts are increasingly focused on creating integrated sensing and delivery platforms that combine diagnostic capabilities with therapeutic interventions, embodying the concept of theranostics (therapeutic diagnostics) in next-generation pharmaceutical applications.
Market Analysis for Biosensor-Enhanced Drug Delivery
The global market for biosensor-enhanced drug delivery systems is experiencing robust growth, driven by increasing demand for targeted therapeutics and personalized medicine. Current market valuation stands at approximately 5.2 billion USD in 2023, with projections indicating a compound annual growth rate of 12.7% through 2030. This growth trajectory is supported by significant investments in healthcare infrastructure and rising prevalence of chronic diseases requiring precise medication delivery.
Plasmonic biosensor integration into drug delivery platforms addresses several critical market needs. Healthcare providers seek solutions that reduce side effects while maximizing therapeutic efficacy, particularly for treatments involving potent compounds such as chemotherapeutics. Patient populations increasingly demand less invasive administration methods with reduced dosing frequency, creating market pull for advanced delivery systems with real-time monitoring capabilities.
Geographically, North America currently dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region demonstrates the fastest growth rate at 15.3% annually, driven by expanding healthcare access and increasing R&D investments in countries like China, Japan, and India.
By application segment, cancer therapy represents the largest market share at 36%, followed by infectious disease treatment (24%), autoimmune disorders (18%), and metabolic diseases (14%). The oncology segment's dominance stems from the particular benefits plasmonic biosensors offer in monitoring drug concentration at tumor sites and triggering precise release mechanisms.
Key market drivers include increasing research funding for nanomedicine, growing demand for minimally invasive therapeutic approaches, and regulatory support for innovative drug delivery technologies. The FDA's recent establishment of accelerated approval pathways for combination products incorporating monitoring technologies has significantly reduced time-to-market for these systems.
Market challenges include high development costs, complex regulatory approval processes, and technical hurdles in sensor miniaturization and biocompatibility. Additionally, healthcare reimbursement uncertainties for these advanced systems present commercialization barriers, particularly in emerging markets.
Consumer adoption trends indicate growing acceptance of wearable and implantable drug delivery systems, especially among younger patient demographics and those with chronic conditions requiring frequent medication. Healthcare providers increasingly recognize the economic benefits of systems that reduce hospitalization rates through improved medication adherence and therapeutic outcomes.
Plasmonic biosensor integration into drug delivery platforms addresses several critical market needs. Healthcare providers seek solutions that reduce side effects while maximizing therapeutic efficacy, particularly for treatments involving potent compounds such as chemotherapeutics. Patient populations increasingly demand less invasive administration methods with reduced dosing frequency, creating market pull for advanced delivery systems with real-time monitoring capabilities.
Geographically, North America currently dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region demonstrates the fastest growth rate at 15.3% annually, driven by expanding healthcare access and increasing R&D investments in countries like China, Japan, and India.
By application segment, cancer therapy represents the largest market share at 36%, followed by infectious disease treatment (24%), autoimmune disorders (18%), and metabolic diseases (14%). The oncology segment's dominance stems from the particular benefits plasmonic biosensors offer in monitoring drug concentration at tumor sites and triggering precise release mechanisms.
Key market drivers include increasing research funding for nanomedicine, growing demand for minimally invasive therapeutic approaches, and regulatory support for innovative drug delivery technologies. The FDA's recent establishment of accelerated approval pathways for combination products incorporating monitoring technologies has significantly reduced time-to-market for these systems.
Market challenges include high development costs, complex regulatory approval processes, and technical hurdles in sensor miniaturization and biocompatibility. Additionally, healthcare reimbursement uncertainties for these advanced systems present commercialization barriers, particularly in emerging markets.
Consumer adoption trends indicate growing acceptance of wearable and implantable drug delivery systems, especially among younger patient demographics and those with chronic conditions requiring frequent medication. Healthcare providers increasingly recognize the economic benefits of systems that reduce hospitalization rates through improved medication adherence and therapeutic outcomes.
Current Challenges in Plasmonic Biosensor Integration
Despite the promising potential of plasmonic biosensors in drug delivery systems, several significant technical challenges impede their widespread integration and clinical application. The primary obstacle remains the complexity of fabricating reproducible plasmonic nanostructures with consistent optical properties at scale. Current manufacturing processes often result in batch-to-batch variations that affect sensor performance and reliability, creating barriers for pharmaceutical quality control standards.
Signal-to-noise ratio optimization presents another substantial challenge, particularly when detecting biomolecules in complex biological matrices such as blood, cerebrospinal fluid, or intracellular environments. The presence of proteins, electrolytes, and other biomolecules can cause non-specific binding and interfere with plasmonic resonance signals, reducing detection accuracy in real-world drug delivery applications.
Biocompatibility issues continue to hinder integration into drug delivery platforms. While gold nanoparticles are generally considered biocompatible, long-term stability and potential toxicity of functionalized plasmonic materials within biological systems remain inadequately characterized. Surface modifications necessary for specific molecular recognition may alter the toxicological profile of these materials, requiring extensive safety evaluation before clinical implementation.
The translation from laboratory proof-of-concept to integrated systems faces significant engineering hurdles. Miniaturization of optical components, integration with microfluidics, and development of user-friendly interfaces that don't require specialized training represent major technical barriers. Current plasmonic biosensor systems often require complex instrumentation and expertise for operation and data interpretation.
Real-time monitoring capabilities, essential for controlled drug release systems, are limited by current plasmonic sensor designs. The challenge lies in developing sensors that can continuously function in biological environments without degradation or biofouling while maintaining sensitivity over extended periods. Most existing systems demonstrate proof-of-concept functionality but fail under prolonged exposure to biological conditions.
Regulatory pathways for combined diagnostic-therapeutic systems remain unclear, creating uncertainty for developers. The novel nature of plasmonic biosensor-integrated drug delivery systems means they often fall between established regulatory categories, complicating approval processes and extending development timelines.
Cost considerations present practical barriers to commercialization. Current fabrication methods for high-quality plasmonic structures often involve expensive equipment and materials, making mass production economically challenging. Reducing production costs while maintaining performance specifications represents a critical challenge for market viability.
Signal-to-noise ratio optimization presents another substantial challenge, particularly when detecting biomolecules in complex biological matrices such as blood, cerebrospinal fluid, or intracellular environments. The presence of proteins, electrolytes, and other biomolecules can cause non-specific binding and interfere with plasmonic resonance signals, reducing detection accuracy in real-world drug delivery applications.
Biocompatibility issues continue to hinder integration into drug delivery platforms. While gold nanoparticles are generally considered biocompatible, long-term stability and potential toxicity of functionalized plasmonic materials within biological systems remain inadequately characterized. Surface modifications necessary for specific molecular recognition may alter the toxicological profile of these materials, requiring extensive safety evaluation before clinical implementation.
The translation from laboratory proof-of-concept to integrated systems faces significant engineering hurdles. Miniaturization of optical components, integration with microfluidics, and development of user-friendly interfaces that don't require specialized training represent major technical barriers. Current plasmonic biosensor systems often require complex instrumentation and expertise for operation and data interpretation.
Real-time monitoring capabilities, essential for controlled drug release systems, are limited by current plasmonic sensor designs. The challenge lies in developing sensors that can continuously function in biological environments without degradation or biofouling while maintaining sensitivity over extended periods. Most existing systems demonstrate proof-of-concept functionality but fail under prolonged exposure to biological conditions.
Regulatory pathways for combined diagnostic-therapeutic systems remain unclear, creating uncertainty for developers. The novel nature of plasmonic biosensor-integrated drug delivery systems means they often fall between established regulatory categories, complicating approval processes and extending development timelines.
Cost considerations present practical barriers to commercialization. Current fabrication methods for high-quality plasmonic structures often involve expensive equipment and materials, making mass production economically challenging. Reducing production costs while maintaining performance specifications represents a critical challenge for market viability.
Current Plasmonic Biosensor Implementation Strategies
01 Plasmonic nanostructures for enhanced biosensing
Plasmonic biosensors utilize nanostructures such as gold or silver nanoparticles that exhibit localized surface plasmon resonance (LSPR) for highly sensitive detection of biomolecules. These nanostructures can be engineered with specific shapes and sizes to optimize the sensing performance. The plasmonic effect creates strong electromagnetic field enhancement at the metal-dielectric interface, enabling detection of biomolecular interactions with high sensitivity and specificity.- Plasmonic nanostructures for biosensing: Plasmonic biosensors utilize nanostructures such as gold or silver nanoparticles that exhibit localized surface plasmon resonance (LSPR) for highly sensitive detection of biomolecules. These nanostructures can be engineered with specific shapes and sizes to enhance the sensing performance. The interaction between target analytes and the plasmonic nanostructures causes measurable shifts in the resonance wavelength, allowing for label-free detection of various biomarkers with high sensitivity and specificity.
- Surface plasmon resonance (SPR) based detection systems: Surface plasmon resonance biosensors utilize the phenomenon of SPR occurring at the interface between a metal film and a dielectric medium. When biomolecules bind to the functionalized sensor surface, they cause changes in the refractive index that can be detected as shifts in the resonance angle or wavelength. These systems often incorporate microfluidic channels for sample delivery and optical components for precise measurement of the SPR signal, enabling real-time, label-free detection of biomolecular interactions with high sensitivity.
- Integration of plasmonic biosensors with waveguides and optical fibers: Plasmonic biosensors can be integrated with optical waveguides and fibers to create compact and versatile sensing platforms. These integrated systems guide light to interact with plasmonic structures, enhancing the sensing capabilities and allowing for remote sensing applications. The combination of plasmonic elements with waveguide technology enables miniaturization of sensing devices while maintaining high sensitivity, making them suitable for point-of-care diagnostics and field-deployable biosensing applications.
- Plasmonic biosensors with enhanced sensitivity through signal amplification: Various techniques are employed to enhance the sensitivity of plasmonic biosensors, including signal amplification strategies. These may involve secondary binding events, enzymatic reactions, or coupling with other detection methods. Advanced plasmonic structures such as nanogaps, nanoholes, or nanoantenna arrays can create electromagnetic hot spots that significantly amplify the local field, leading to improved detection limits. These enhancements enable the detection of biomolecules at extremely low concentrations, potentially down to the single-molecule level.
- Plasmonic biosensors for specific biomedical applications: Plasmonic biosensors are tailored for specific biomedical applications such as disease diagnosis, drug discovery, and environmental monitoring. These sensors can be functionalized with specific recognition elements like antibodies, aptamers, or molecularly imprinted polymers to target particular biomarkers. The design considerations include selectivity, stability in biological fluids, and compatibility with existing analytical platforms. Recent developments focus on multiplexed detection capabilities and integration with portable devices for point-of-care diagnostics.
02 Integration of plasmonic biosensors with microfluidic systems
Combining plasmonic biosensors with microfluidic platforms enables precise sample handling, reduced reagent consumption, and improved detection efficiency. These integrated systems allow for real-time monitoring of biomolecular interactions in a controlled environment. Microfluidic channels can be designed to optimize the interaction between the analyte and the plasmonic sensing elements, enhancing sensitivity and enabling multiplexed detection of multiple biomarkers simultaneously.Expand Specific Solutions03 Waveguide-coupled plasmonic biosensors
Optical waveguides can be coupled with plasmonic structures to create highly sensitive biosensing platforms. These systems guide light to interact with plasmonic elements where biomolecular binding occurs, allowing for efficient excitation of surface plasmons and detection of binding events. Waveguide-coupled plasmonic biosensors offer advantages such as miniaturization, integration capabilities with other photonic components, and enhanced sensitivity through optimized light-matter interactions.Expand Specific Solutions04 Signal processing and readout methods for plasmonic biosensors
Advanced signal processing techniques and readout methods are essential for extracting meaningful data from plasmonic biosensors. These include spectroscopic analysis, imaging techniques, and electronic signal processing algorithms that can enhance the signal-to-noise ratio and improve detection limits. Real-time data processing enables continuous monitoring of biomolecular interactions, while machine learning algorithms can be applied to analyze complex sensor responses for improved diagnostic accuracy.Expand Specific Solutions05 Novel materials and fabrication techniques for plasmonic biosensors
Innovative materials and fabrication methods are being developed to enhance the performance of plasmonic biosensors. These include the use of alternative plasmonic materials beyond gold and silver, such as aluminum or copper, as well as hybrid materials that combine plasmonic elements with other functional components. Advanced nanofabrication techniques like electron beam lithography, nanoimprint lithography, and self-assembly methods enable precise control over the structure and properties of plasmonic sensing elements.Expand Specific Solutions
Key Industry Players in Biosensor-Based Drug Delivery
The field of plasmonic biosensors in novel drug delivery systems is currently in an early growth phase, characterized by significant research activity but limited commercial deployment. The global market for this technology is projected to reach approximately $2.5 billion by 2027, driven by increasing demand for targeted drug delivery solutions. While the technology shows promising applications in personalized medicine and nanotherapeutics, it remains in the developmental stage with varying degrees of maturity across applications. Leading academic institutions like MIT, Washington University, and Johns Hopkins University are advancing fundamental research, while pharmaceutical companies including Pfizer, Sanofi, and PharmaIN are focusing on translational applications. Smaller specialized firms such as Lyra Therapeutics and XTuit Pharmaceuticals are developing niche applications, indicating a competitive landscape balanced between established players and innovative startups.
Sanofi
Technical Solution: Sanofi has pioneered the development of PlasmoDelivery™, an innovative drug delivery platform that integrates plasmonic biosensing capabilities with smart release mechanisms. Their technology utilizes gold nanorods with tunable aspect ratios to achieve specific plasmonic responses across different wavelengths, enabling multiplexed detection of biomarkers and drug molecules. The platform incorporates a layer-by-layer assembly approach to create responsive polymer shells around the plasmonic core, which can be triggered to release therapeutic payloads upon specific biological or external stimuli. Sanofi has successfully applied this technology to insulin delivery systems, where glucose-responsive plasmonic sensors trigger insulin release only when blood glucose levels exceed predetermined thresholds. This approach has demonstrated superior glycemic control in preclinical diabetes models compared to conventional insulin delivery methods. The company has also expanded this technology to autoimmune disease treatments, where plasmonic detection of inflammatory biomarkers can modulate the release of anti-inflammatory compounds with high spatiotemporal precision.
Strengths: Highly specific glucose-responsive insulin delivery with improved glycemic control; versatile platform applicable to multiple therapeutic areas; tunable plasmonic properties for different sensing and release requirements. Weaknesses: Complex manufacturing process affecting scalability; potential immunogenicity concerns with repeated administration; higher cost compared to traditional drug delivery approaches.
Case Western Reserve University
Technical Solution: Case Western Reserve University has developed a groundbreaking plasmonic biosensor platform for drug delivery applications called NanoPlasmo-Rx. Their approach utilizes silver nanocube arrays with exceptionally strong plasmonic coupling effects, creating "hot spots" with dramatically enhanced electromagnetic fields for ultrasensitive detection. The technology incorporates stimuli-responsive hydrogel matrices that encapsulate therapeutic compounds and respond to plasmonic heating for controlled release. A key innovation is their dual-mode sensing capability, which simultaneously monitors both drug concentration and relevant biomarkers to create adaptive delivery profiles. The research team has demonstrated particular success in ocular drug delivery applications, where the plasmonic platform can be integrated into contact lens materials for sustained release of ophthalmic medications while continuously monitoring intraocular pressure and inflammatory markers. Their system also incorporates machine learning algorithms that analyze plasmonic sensing data to optimize drug release profiles based on individual patient responses, representing an important step toward personalized medicine. Recent collaborations with pharmaceutical companies have focused on extending this technology to transdermal delivery systems for chronic disease management.
Strengths: Exceptional sensitivity through plasmonic coupling effects; adaptive drug delivery based on real-time biomarker monitoring; innovative applications in ocular therapeutics with demonstrated efficacy. Weaknesses: Complex fabrication process for precisely arranged plasmonic arrays; potential biocompatibility concerns with silver nanostructures; limited scalability of current manufacturing approaches.
Critical Patents and Research in Plasmonic Biosensing
Plasmonic biosensor
PatentActiveUS20210048435A1
Innovation
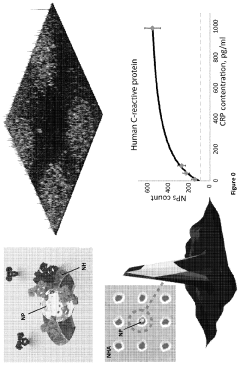
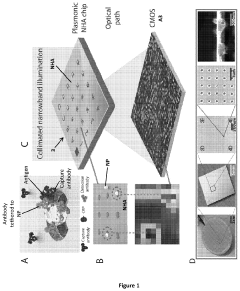
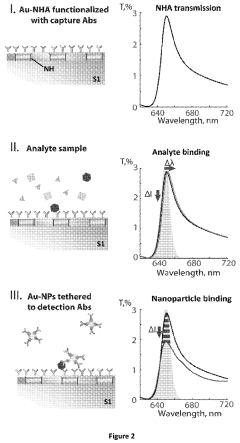
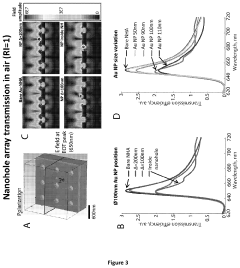
- A nanoparticle-enhanced plasmonic biosensor system using gold nano-hole arrays (Au-NHAs) that visualizes single sub-wavelength nanoparticles under bright-field imaging, enabling digital quantification and localization of individual nanoparticle-labeled molecules through local extraordinary optical transmission quenching, allowing for the detection of biomarkers at much lower concentrations, such as 10 pg/ml for biotinylated bovine serum albumin and 27 pg/ml for human C-reactive protein.
Method and device to provide a microfluidic flow
PatentActiveUS20220228094A1
Innovation
- A microfluidic flow system is developed where biological cells and solid particles, such as nanoparticles or microparticles, are maintained at a predetermined minimal distance from each other, allowing for controlled interaction without direct contact, using a central and outer flow configuration with adjustable flow rates and angles to optimize particle positioning and ensure cell viability.
Regulatory Framework for Nanobiosensor Medical Applications
The regulatory landscape for nanobiosensor medical applications, including plasmonic biosensors in drug delivery systems, presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA has established a dedicated Nanotechnology Task Force to address the unique challenges posed by nanoscale medical devices and drug delivery systems. These regulatory pathways typically classify plasmonic biosensor-based drug delivery systems as combination products, requiring comprehensive evaluation under both medical device regulations (21 CFR Part 820) and pharmaceutical guidelines.
The European Medicines Agency (EMA) has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which specifically address nanomaterials in medical applications. These regulations mandate rigorous safety assessments, including evaluation of potential nanoparticle accumulation in tissues and long-term biocompatibility. For plasmonic biosensors integrated into drug delivery systems, manufacturers must demonstrate compliance with both pharmaceutical and medical device directives.
In Asia, regulatory frameworks show considerable variation. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for nanomedicine evaluation, while China's National Medical Products Administration (NMPA) has recently updated its regulations to include nanoscale medical technologies, requiring additional safety data for plasmonic materials that may interact with biological systems.
International harmonization efforts are being led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the International Medical Device Regulators Forum (IMDRF). These organizations are working to develop standardized approaches for evaluating nanobiosensors, though significant regulatory gaps remain regarding the long-term safety monitoring of these advanced systems.
A critical regulatory consideration for plasmonic biosensors in drug delivery is the demonstration of controlled release mechanisms and targeting specificity. Regulatory bodies increasingly require manufacturers to provide data on the precision of drug release triggered by plasmonic detection events, as well as evidence of minimal off-target effects. This includes validation of the sensing mechanisms and their correlation with therapeutic delivery.
Emerging regulatory trends indicate a shift toward adaptive licensing pathways for innovative nanobiosensor technologies, allowing for phased approval based on risk-benefit assessments throughout the product lifecycle. This approach may accelerate the clinical translation of plasmonic biosensor-based drug delivery systems while maintaining rigorous safety standards through enhanced post-market surveillance requirements and real-world evidence collection.
The European Medicines Agency (EMA) has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which specifically address nanomaterials in medical applications. These regulations mandate rigorous safety assessments, including evaluation of potential nanoparticle accumulation in tissues and long-term biocompatibility. For plasmonic biosensors integrated into drug delivery systems, manufacturers must demonstrate compliance with both pharmaceutical and medical device directives.
In Asia, regulatory frameworks show considerable variation. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for nanomedicine evaluation, while China's National Medical Products Administration (NMPA) has recently updated its regulations to include nanoscale medical technologies, requiring additional safety data for plasmonic materials that may interact with biological systems.
International harmonization efforts are being led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the International Medical Device Regulators Forum (IMDRF). These organizations are working to develop standardized approaches for evaluating nanobiosensors, though significant regulatory gaps remain regarding the long-term safety monitoring of these advanced systems.
A critical regulatory consideration for plasmonic biosensors in drug delivery is the demonstration of controlled release mechanisms and targeting specificity. Regulatory bodies increasingly require manufacturers to provide data on the precision of drug release triggered by plasmonic detection events, as well as evidence of minimal off-target effects. This includes validation of the sensing mechanisms and their correlation with therapeutic delivery.
Emerging regulatory trends indicate a shift toward adaptive licensing pathways for innovative nanobiosensor technologies, allowing for phased approval based on risk-benefit assessments throughout the product lifecycle. This approach may accelerate the clinical translation of plasmonic biosensor-based drug delivery systems while maintaining rigorous safety standards through enhanced post-market surveillance requirements and real-world evidence collection.
Biocompatibility and Safety Considerations
The integration of plasmonic biosensors with drug delivery systems necessitates rigorous evaluation of biocompatibility and safety profiles. Metallic nanoparticles, particularly gold and silver, which form the core components of plasmonic biosensors, exhibit unique interactions with biological systems that must be thoroughly characterized before clinical implementation.
Surface chemistry plays a critical role in determining the biocompatibility of plasmonic materials. Unmodified metallic nanoparticles may trigger immune responses or exhibit cytotoxicity at certain concentrations. Research indicates that surface functionalization with biocompatible polymers such as polyethylene glycol (PEG) significantly reduces immunogenicity and extends circulation time in vivo, enhancing the therapeutic index of drug delivery systems incorporating plasmonic elements.
Cellular uptake mechanisms and intracellular fate of plasmonic materials represent another crucial safety consideration. Studies demonstrate that size, shape, and surface charge dramatically influence cellular internalization pathways and subsequent biological responses. Particles below 50 nm typically undergo clathrin-mediated endocytosis, while larger structures may trigger phagocytosis or macropinocytosis, potentially altering therapeutic efficacy and safety profiles.
Long-term toxicity remains a significant concern for plasmonic biosensor-integrated drug delivery systems. Recent investigations reveal that while acute toxicity may be minimal, chronic exposure could lead to accumulation in organs such as the liver and spleen. Comprehensive biodistribution studies utilizing advanced imaging techniques have shown that plasmonic nanoparticles can persist in tissues for extended periods, necessitating thorough evaluation of potential long-term effects.
Regulatory considerations for plasmonic biosensor-based drug delivery systems present unique challenges. Current frameworks for nanomedicine evaluation may not adequately address the specific properties of plasmonic materials. The FDA and EMA have begun developing specialized guidelines for evaluating nanomaterial-based therapeutics, emphasizing the need for standardized protocols to assess biocompatibility, immunogenicity, and genotoxicity of these advanced delivery platforms.
Heat generation during plasmonic resonance represents both a therapeutic opportunity and safety concern. While controlled hyperthermia can enhance drug release and cellular uptake, excessive local heating may damage surrounding tissues. Engineering approaches incorporating thermal-responsive polymers with precise temperature thresholds have emerged as promising strategies to harness plasmonic heating while maintaining safety margins in biological environments.
Standardization of safety assessment protocols specifically tailored for plasmonic biosensor-integrated drug delivery systems remains an evolving field. Multi-parametric approaches combining in vitro cytotoxicity assays, advanced 3D tissue models, and sophisticated in vivo imaging techniques are increasingly recognized as essential for comprehensive safety evaluation prior to clinical translation.
Surface chemistry plays a critical role in determining the biocompatibility of plasmonic materials. Unmodified metallic nanoparticles may trigger immune responses or exhibit cytotoxicity at certain concentrations. Research indicates that surface functionalization with biocompatible polymers such as polyethylene glycol (PEG) significantly reduces immunogenicity and extends circulation time in vivo, enhancing the therapeutic index of drug delivery systems incorporating plasmonic elements.
Cellular uptake mechanisms and intracellular fate of plasmonic materials represent another crucial safety consideration. Studies demonstrate that size, shape, and surface charge dramatically influence cellular internalization pathways and subsequent biological responses. Particles below 50 nm typically undergo clathrin-mediated endocytosis, while larger structures may trigger phagocytosis or macropinocytosis, potentially altering therapeutic efficacy and safety profiles.
Long-term toxicity remains a significant concern for plasmonic biosensor-integrated drug delivery systems. Recent investigations reveal that while acute toxicity may be minimal, chronic exposure could lead to accumulation in organs such as the liver and spleen. Comprehensive biodistribution studies utilizing advanced imaging techniques have shown that plasmonic nanoparticles can persist in tissues for extended periods, necessitating thorough evaluation of potential long-term effects.
Regulatory considerations for plasmonic biosensor-based drug delivery systems present unique challenges. Current frameworks for nanomedicine evaluation may not adequately address the specific properties of plasmonic materials. The FDA and EMA have begun developing specialized guidelines for evaluating nanomaterial-based therapeutics, emphasizing the need for standardized protocols to assess biocompatibility, immunogenicity, and genotoxicity of these advanced delivery platforms.
Heat generation during plasmonic resonance represents both a therapeutic opportunity and safety concern. While controlled hyperthermia can enhance drug release and cellular uptake, excessive local heating may damage surrounding tissues. Engineering approaches incorporating thermal-responsive polymers with precise temperature thresholds have emerged as promising strategies to harness plasmonic heating while maintaining safety margins in biological environments.
Standardization of safety assessment protocols specifically tailored for plasmonic biosensor-integrated drug delivery systems remains an evolving field. Multi-parametric approaches combining in vitro cytotoxicity assays, advanced 3D tissue models, and sophisticated in vivo imaging techniques are increasingly recognized as essential for comprehensive safety evaluation prior to clinical translation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!