How Plasmonic Biosensors Interface with Modern Electronic Devices
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasmonic Biosensor Evolution and Objectives
Plasmonic biosensors have evolved significantly over the past three decades, transforming from rudimentary surface plasmon resonance (SPR) devices into sophisticated integrated sensing platforms. The journey began in the early 1990s with conventional SPR sensors primarily used in laboratory settings for biomolecular interaction analysis. These early systems relied on the Kretschmann configuration, where light excites surface plasmons at a metal-dielectric interface, enabling detection of biomolecular binding events through refractive index changes.
By the early 2000s, localized surface plasmon resonance (LSPR) emerged as a pivotal advancement, utilizing noble metal nanoparticles to confine electromagnetic fields at nanoscale dimensions. This breakthrough eliminated the need for bulky optical components and paved the way for miniaturization. The mid-2000s witnessed the integration of microfluidics with plasmonic sensing, enabling multiplexed detection capabilities and reduced sample volumes.
The 2010s marked a transformative period with the development of smartphone-based plasmonic biosensors, democratizing access to sophisticated diagnostic tools beyond traditional laboratory environments. Concurrently, researchers began exploring novel plasmonic materials beyond gold and silver, including aluminum, copper, and various alloys to enhance sensitivity and expand the spectral range of operation.
Recent years have seen accelerated efforts to interface plasmonic biosensors with modern electronic architectures. The integration of CMOS technology with plasmonic elements has enabled direct signal processing and amplification, significantly improving signal-to-noise ratios. Additionally, the incorporation of machine learning algorithms has enhanced pattern recognition capabilities, allowing for more accurate interpretation of complex biosensor data.
The primary objective of current plasmonic biosensor development is to achieve seamless integration with existing electronic ecosystems while maintaining or improving sensitivity, specificity, and reliability. This includes developing standardized interfaces between plasmonic sensing elements and electronic readout systems, optimizing power consumption for portable applications, and ensuring compatibility with wireless communication protocols for remote monitoring and telemedicine applications.
Another critical goal is to reduce manufacturing complexity and cost, making these advanced sensing technologies accessible for point-of-care diagnostics in resource-limited settings. Researchers are exploring scalable fabrication techniques such as nanoimprint lithography and roll-to-roll processing to address this challenge.
Looking forward, the field aims to develop fully integrated "lab-on-a-chip" platforms where plasmonic biosensors work in concert with microprocessors, wireless communication modules, and power management systems. The ultimate vision is to create autonomous sensing platforms capable of continuous monitoring with minimal human intervention, potentially revolutionizing healthcare delivery, environmental monitoring, and food safety assessment.
By the early 2000s, localized surface plasmon resonance (LSPR) emerged as a pivotal advancement, utilizing noble metal nanoparticles to confine electromagnetic fields at nanoscale dimensions. This breakthrough eliminated the need for bulky optical components and paved the way for miniaturization. The mid-2000s witnessed the integration of microfluidics with plasmonic sensing, enabling multiplexed detection capabilities and reduced sample volumes.
The 2010s marked a transformative period with the development of smartphone-based plasmonic biosensors, democratizing access to sophisticated diagnostic tools beyond traditional laboratory environments. Concurrently, researchers began exploring novel plasmonic materials beyond gold and silver, including aluminum, copper, and various alloys to enhance sensitivity and expand the spectral range of operation.
Recent years have seen accelerated efforts to interface plasmonic biosensors with modern electronic architectures. The integration of CMOS technology with plasmonic elements has enabled direct signal processing and amplification, significantly improving signal-to-noise ratios. Additionally, the incorporation of machine learning algorithms has enhanced pattern recognition capabilities, allowing for more accurate interpretation of complex biosensor data.
The primary objective of current plasmonic biosensor development is to achieve seamless integration with existing electronic ecosystems while maintaining or improving sensitivity, specificity, and reliability. This includes developing standardized interfaces between plasmonic sensing elements and electronic readout systems, optimizing power consumption for portable applications, and ensuring compatibility with wireless communication protocols for remote monitoring and telemedicine applications.
Another critical goal is to reduce manufacturing complexity and cost, making these advanced sensing technologies accessible for point-of-care diagnostics in resource-limited settings. Researchers are exploring scalable fabrication techniques such as nanoimprint lithography and roll-to-roll processing to address this challenge.
Looking forward, the field aims to develop fully integrated "lab-on-a-chip" platforms where plasmonic biosensors work in concert with microprocessors, wireless communication modules, and power management systems. The ultimate vision is to create autonomous sensing platforms capable of continuous monitoring with minimal human intervention, potentially revolutionizing healthcare delivery, environmental monitoring, and food safety assessment.
Market Analysis for Integrated Biosensing Solutions
The global market for integrated biosensing solutions is experiencing robust growth, driven by increasing demand for rapid, accurate, and portable diagnostic technologies. The plasmonic biosensor market, valued at approximately $21.2 billion in 2022, is projected to reach $38.7 billion by 2028, representing a compound annual growth rate of 10.6%. This growth trajectory is particularly pronounced in healthcare applications, where point-of-care testing and continuous health monitoring are revolutionizing patient care paradigms.
North America currently dominates the market with a 42% share, followed by Europe at 28% and Asia-Pacific at 23%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 12.3% annually through 2028, primarily due to increasing healthcare expenditure, growing awareness about early disease detection, and substantial government investments in healthcare infrastructure.
The integration of plasmonic biosensors with electronic devices has created several distinct market segments. The medical diagnostics segment holds the largest share at 36%, followed by environmental monitoring (22%), food safety testing (18%), and pharmaceutical research (15%). The remaining 9% encompasses emerging applications such as wearable health monitors and IoT-enabled sensing platforms.
Key market drivers include the rising prevalence of chronic diseases necessitating continuous monitoring, growing consumer preference for personalized healthcare solutions, and increasing adoption of telemedicine platforms requiring remote diagnostic capabilities. The COVID-19 pandemic has significantly accelerated market growth by highlighting the critical importance of rapid, accurate diagnostic technologies deployable outside traditional laboratory settings.
Consumer demand patterns indicate a strong preference for miniaturized, user-friendly devices with smartphone connectivity, cloud data storage capabilities, and AI-powered analytics. The market for smartphone-compatible biosensors alone is growing at 14.2% annually, reflecting the convergence of consumer electronics and medical diagnostics.
Regulatory landscapes significantly impact market dynamics, with FDA and CE mark approvals serving as critical milestones for commercial success. Recent regulatory frameworks focusing on digital health technologies have created more streamlined pathways for innovative biosensing solutions, particularly those integrating with existing electronic platforms.
Market challenges include price sensitivity in emerging economies, interoperability issues between different electronic platforms, and concerns regarding data privacy and security. Additionally, reimbursement uncertainties for novel diagnostic approaches present commercialization barriers that companies must navigate strategically.
North America currently dominates the market with a 42% share, followed by Europe at 28% and Asia-Pacific at 23%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 12.3% annually through 2028, primarily due to increasing healthcare expenditure, growing awareness about early disease detection, and substantial government investments in healthcare infrastructure.
The integration of plasmonic biosensors with electronic devices has created several distinct market segments. The medical diagnostics segment holds the largest share at 36%, followed by environmental monitoring (22%), food safety testing (18%), and pharmaceutical research (15%). The remaining 9% encompasses emerging applications such as wearable health monitors and IoT-enabled sensing platforms.
Key market drivers include the rising prevalence of chronic diseases necessitating continuous monitoring, growing consumer preference for personalized healthcare solutions, and increasing adoption of telemedicine platforms requiring remote diagnostic capabilities. The COVID-19 pandemic has significantly accelerated market growth by highlighting the critical importance of rapid, accurate diagnostic technologies deployable outside traditional laboratory settings.
Consumer demand patterns indicate a strong preference for miniaturized, user-friendly devices with smartphone connectivity, cloud data storage capabilities, and AI-powered analytics. The market for smartphone-compatible biosensors alone is growing at 14.2% annually, reflecting the convergence of consumer electronics and medical diagnostics.
Regulatory landscapes significantly impact market dynamics, with FDA and CE mark approvals serving as critical milestones for commercial success. Recent regulatory frameworks focusing on digital health technologies have created more streamlined pathways for innovative biosensing solutions, particularly those integrating with existing electronic platforms.
Market challenges include price sensitivity in emerging economies, interoperability issues between different electronic platforms, and concerns regarding data privacy and security. Additionally, reimbursement uncertainties for novel diagnostic approaches present commercialization barriers that companies must navigate strategically.
Current Challenges in Biosensor-Electronics Integration
Despite significant advancements in plasmonic biosensor technology, the integration with modern electronic devices presents several formidable challenges. The miniaturization requirements of contemporary electronics often conflict with the optical components necessary for plasmonic sensing, creating a fundamental design tension. Current plasmonic biosensors typically require specialized optical setups including light sources, detectors, and coupling mechanisms that are difficult to incorporate into compact electronic devices without compromising performance.
Signal transduction represents another major hurdle, as converting the optical resonance shifts characteristic of plasmonic sensing into electronic signals requires sophisticated interface circuitry. The sensitivity of these conversion mechanisms often suffers from noise interference, particularly in portable or wearable applications where environmental factors can significantly impact measurement accuracy.
Power consumption remains a critical constraint, especially for implantable or remote sensing applications. Traditional plasmonic measurement systems demand substantial energy for light generation and detection, creating a bottleneck for battery-powered electronic integration. Current solutions frequently sacrifice either sensing performance or operational longevity to address this limitation.
Data processing capabilities present additional complications, as real-time analysis of plasmonic signals requires substantial computational resources. The complex algorithms necessary for signal deconvolution and noise filtering exceed the processing capacity of many portable electronic platforms, necessitating compromises in analytical depth or response time.
Biocompatibility issues arise at the interface between biological samples and sensing surfaces. The materials used in electronic components often interact unfavorably with biological specimens, potentially causing degradation of both the sample and the sensor over time. Current surface functionalization approaches frequently fail to maintain stability in complex biological environments while preserving electronic functionality.
Manufacturing scalability poses significant challenges, as the precision required for plasmonic structures (typically at nanometer scales) conflicts with mass-production techniques used for consumer electronics. The integration of these disparate manufacturing processes results in low yields and high costs, limiting commercial viability.
Standardization remains underdeveloped, with various proprietary solutions emerging without cohesive protocols for data formatting, calibration, or cross-platform compatibility. This fragmentation impedes the development of universal interfaces between plasmonic biosensors and mainstream electronic ecosystems, restricting their adoption in consumer applications and healthcare systems.
Signal transduction represents another major hurdle, as converting the optical resonance shifts characteristic of plasmonic sensing into electronic signals requires sophisticated interface circuitry. The sensitivity of these conversion mechanisms often suffers from noise interference, particularly in portable or wearable applications where environmental factors can significantly impact measurement accuracy.
Power consumption remains a critical constraint, especially for implantable or remote sensing applications. Traditional plasmonic measurement systems demand substantial energy for light generation and detection, creating a bottleneck for battery-powered electronic integration. Current solutions frequently sacrifice either sensing performance or operational longevity to address this limitation.
Data processing capabilities present additional complications, as real-time analysis of plasmonic signals requires substantial computational resources. The complex algorithms necessary for signal deconvolution and noise filtering exceed the processing capacity of many portable electronic platforms, necessitating compromises in analytical depth or response time.
Biocompatibility issues arise at the interface between biological samples and sensing surfaces. The materials used in electronic components often interact unfavorably with biological specimens, potentially causing degradation of both the sample and the sensor over time. Current surface functionalization approaches frequently fail to maintain stability in complex biological environments while preserving electronic functionality.
Manufacturing scalability poses significant challenges, as the precision required for plasmonic structures (typically at nanometer scales) conflicts with mass-production techniques used for consumer electronics. The integration of these disparate manufacturing processes results in low yields and high costs, limiting commercial viability.
Standardization remains underdeveloped, with various proprietary solutions emerging without cohesive protocols for data formatting, calibration, or cross-platform compatibility. This fragmentation impedes the development of universal interfaces between plasmonic biosensors and mainstream electronic ecosystems, restricting their adoption in consumer applications and healthcare systems.
Interface Architectures Between Biosensors and Electronics
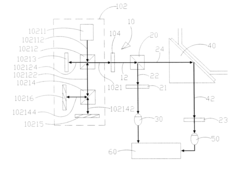
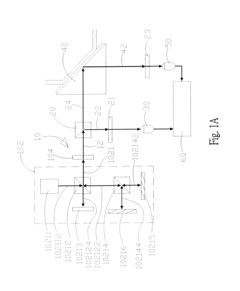
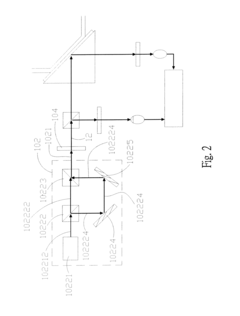
01 Surface plasmon resonance biosensor design
Surface plasmon resonance (SPR) biosensors utilize the interaction between electromagnetic waves and metal surfaces to detect biomolecular interactions. These biosensors are designed with specialized interfaces that enhance sensitivity and specificity for target analytes. The interface typically consists of a metal film (often gold) on which biomolecular recognition elements are immobilized. This design allows for label-free, real-time detection of biomolecular interactions by measuring changes in the refractive index near the sensor surface.- Surface plasmon resonance biosensor interfaces: Surface plasmon resonance (SPR) technology forms the foundation of many plasmonic biosensors, utilizing the interaction between electromagnetic waves and metal surfaces to detect biomolecular binding events. These interfaces typically consist of a thin metal film (often gold) on which biomolecular recognition elements are immobilized. When target analytes bind to these recognition elements, the resulting change in refractive index at the metal-dielectric interface causes a measurable shift in the resonance conditions, enabling highly sensitive detection of biomolecules without labels.
- Nanostructured plasmonic interfaces for enhanced sensitivity: Nanostructured plasmonic interfaces incorporate engineered metallic nanostructures such as nanoparticles, nanorods, or nanoholes to enhance biosensor performance. These nanostructures create localized surface plasmon resonance (LSPR) effects that concentrate electromagnetic fields into nanoscale volumes, dramatically increasing sensitivity. The geometry, size, and arrangement of these nanostructures can be optimized to tune the resonance wavelength and maximize the sensor response to specific biomarkers, enabling detection at extremely low concentrations.
- Integrated optical waveguide plasmonic biosensor interfaces: Integrated optical waveguide plasmonic biosensor interfaces combine traditional waveguide technology with plasmonic elements to create compact, high-performance sensing platforms. These hybrid systems guide light through optical waveguides that couple to plasmonic structures at specific sensing regions. This integration enables efficient light delivery to multiple sensing spots on a single chip, facilitating multiplexed detection while maintaining the sensitivity advantages of plasmonic sensing. Such interfaces are particularly valuable for lab-on-a-chip applications and point-of-care diagnostics.
- Functionalization strategies for plasmonic biosensor interfaces: Functionalization strategies for plasmonic biosensor interfaces involve chemical modification of the metal surface to enable specific and efficient capture of target biomolecules. These approaches include self-assembled monolayers, polymer brushes, and various bioconjugation chemistries that immobilize recognition elements such as antibodies, aptamers, or complementary DNA strands. Advanced functionalization techniques focus on minimizing non-specific binding, optimizing receptor orientation, and maintaining the biological activity of immobilized biomolecules to enhance sensor specificity and sensitivity.
- Microfluidic integration with plasmonic biosensor interfaces: Microfluidic integration with plasmonic biosensor interfaces combines precise fluid handling capabilities with sensitive plasmonic detection to create comprehensive analytical systems. These integrated platforms incorporate microchannels, mixers, and valves that deliver samples and reagents to plasmonic sensing elements in a controlled manner. The microfluidic components enable sample preparation, concentration, and sequential delivery of multiple reagents, while also reducing sample volume requirements and enhancing reaction kinetics at the sensor surface, resulting in improved detection limits and faster response times.
02 Nanostructured plasmonic interfaces
Nanostructured plasmonic interfaces incorporate engineered nanoscale features to enhance biosensor performance. These structures include nanoparticles, nanoholes, nanorods, and other geometries that can significantly amplify the local electromagnetic field, leading to improved sensitivity. The nanostructured interfaces create localized surface plasmon resonance (LSPR) effects that are highly sensitive to changes in the surrounding environment, making them excellent platforms for detecting biomolecules at low concentrations.Expand Specific Solutions03 Functionalization of plasmonic biosensor surfaces
The functionalization of plasmonic biosensor surfaces involves modifying the sensor interface with specific biomolecular recognition elements such as antibodies, aptamers, or other binding proteins. These modifications create selective binding sites for target analytes, improving specificity and reducing non-specific interactions. Various surface chemistry techniques are employed to attach these recognition elements while maintaining their biological activity and optimizing their orientation for maximum binding efficiency.Expand Specific Solutions04 Integrated optical waveguide plasmonic biosensors
Integrated optical waveguide plasmonic biosensors combine traditional waveguide technology with plasmonic sensing elements to create compact, sensitive detection platforms. These systems guide light through specialized structures that interact with plasmonic interfaces at precise locations. The integration allows for miniaturization of the sensing platform while maintaining high sensitivity, making these biosensors suitable for lab-on-chip applications and portable diagnostic devices.Expand Specific Solutions05 Signal processing and readout systems for plasmonic biosensors
Advanced signal processing and readout systems are essential components of plasmonic biosensor interfaces. These systems include specialized optics, detectors, and data analysis algorithms that convert the physical changes at the sensor interface into measurable signals. Techniques such as phase detection, intensity measurement, and spectral analysis are employed to extract meaningful information from plasmonic responses. Modern plasmonic biosensor interfaces often incorporate digital signal processing and machine learning algorithms to improve detection limits and reduce noise.Expand Specific Solutions
Leading Companies in Plasmonic Biosensor Development
Plasmonic biosensor technology is currently in a growth phase, with the market expanding due to increasing applications in healthcare, environmental monitoring, and diagnostics. The global market is projected to reach significant value as integration with electronic devices accelerates. Technologically, the field shows varying maturity levels across players: established corporations like FUJIFILM, Canon, and Apple are leveraging their electronics expertise to develop commercial applications, while research institutions such as Boston University, National University of Singapore, and Georgia Tech are driving fundamental innovations. Companies like Onechip Bioelectronics and X-Body are emerging as specialized players focusing on semiconductor-integrated biosensors. The competitive landscape reveals a collaborative ecosystem where academic-industrial partnerships are accelerating the transition from laboratory research to practical electronic device integration.
Onechip Bioelectronics
Technical Solution: Onechip Bioelectronics has pioneered fully integrated plasmonic biosensor systems on single silicon chips. Their technology combines plasmonic nanostructures directly with CMOS electronics, creating true "lab-on-a-chip" devices for point-of-care diagnostics. The company's approach utilizes nanoimprint lithography to create large arrays of plasmonic nanostructures with precisely controlled dimensions, coupled with custom-designed readout electronics. Their biosensors incorporate microfluidic channels fabricated directly on the silicon substrate, enabling automated sample handling and preparation. Onechip's technology features real-time data processing capabilities through integrated microcontrollers that can perform signal filtering, calibration, and analysis without external computing resources. The company has developed specialized surface chemistry protocols for immobilizing various biorecognition elements on their plasmonic structures, enabling detection of proteins, nucleic acids, and small molecules.
Strengths: True single-chip integration reducing size and cost; automated sample handling capabilities; versatile detection modalities for different biomarkers. Weaknesses: Limited production scale compared to larger manufacturers; relatively new technology with less field validation; potential challenges in mass manufacturing consistency.
Electronics & Telecommunications Research Institute
Technical Solution: ETRI has developed advanced plasmonic biosensor platforms that integrate with modern electronic systems for healthcare and environmental monitoring applications. Their technology utilizes nanopatterned metallic films with precisely engineered plasmonic resonances, coupled with custom-designed electronic readout systems. ETRI's approach incorporates silicon photonics technology to guide light to and from the plasmonic sensing regions, enabling efficient optical coupling and signal collection. Their biosensors feature integrated temperature control systems to maintain optimal operating conditions and compensate for thermal drift effects. ETRI has developed specialized signal processing algorithms implemented on field-programmable gate arrays (FPGAs) for real-time data analysis and feature extraction. The institute's technology includes wireless communication modules for remote monitoring applications, with low-power operation modes suitable for battery-powered portable devices.
Strengths: Strong integration of optical and electronic components; robust temperature compensation systems; advanced signal processing capabilities. Weaknesses: Complex fabrication process potentially increasing costs; requires specialized expertise for operation and maintenance; limited commercial deployment compared to industry leaders.
Key Patents in Plasmonic-Electronic Integration
Sensors and sensing methods
PatentInactiveUS20230266291A1
Innovation
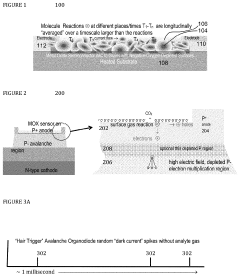
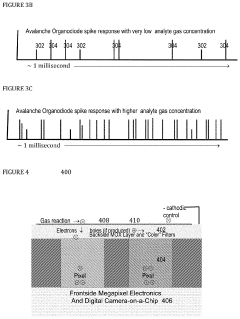
- The development of organodiode detectors and multisample sensors with nanoscale metal oxide surfaces that allow for direct, immediate, and real-time detection of individual gas reaction events, utilizing statistical processing and high-field gradients for enhanced sensitivity and rapid analyte capture, and the integration of plasmonic arrays for optical interaction with analytes.
Surface plasmon resonance measuring device
PatentInactiveUS20110273716A1
Innovation
- A phase differential SPR measuring device utilizing a circularly polarized heterodyne light source, beam splitting elements, and processing circuits to enhance phase change sensitivity, allowing for improved detection of tiny changes in physical quantities of analytes by amplifying and calculating phase differences in the reflected light signals.
Miniaturization Strategies for Portable Diagnostic Systems
The miniaturization of plasmonic biosensor systems represents a critical frontier in developing portable diagnostic technologies that can interface effectively with modern electronic devices. Current miniaturization strategies focus on reducing the overall footprint while maintaining or enhancing sensitivity and specificity of detection mechanisms.
Microfluidic integration stands as a cornerstone approach, where plasmonic sensing elements are incorporated into lab-on-chip platforms. These systems utilize precisely engineered channels with dimensions in the micrometer range to control fluid flow across sensing surfaces, dramatically reducing sample volume requirements from milliliters to microliters or even nanoliters. This integration enables multiplexed detection capabilities while minimizing reagent consumption.
Advanced nanofabrication techniques have enabled the development of ultra-compact plasmonic structures. Techniques such as electron beam lithography and nanoimprint lithography allow for the creation of precisely engineered nanostructures with feature sizes below 100 nm. These structures can be optimized for maximum field enhancement and resonance sensitivity, improving detection limits while reducing the physical size of sensing elements.
CMOS-compatible fabrication processes represent another significant advancement, allowing plasmonic sensing elements to be directly integrated with electronic readout circuitry. This approach eliminates bulky optical components traditionally required for signal transduction and readout, resulting in fully integrated sensing platforms. Silicon photonics integration further enables on-chip light manipulation and detection, creating truly miniaturized systems-on-chip for biosensing applications.
Smartphone-based readout systems have emerged as a particularly promising direction, leveraging the ubiquitous computing power and connectivity of mobile devices. Compact adapters containing minimal optical components can transform smartphones into portable analytical instruments, with the phone's camera serving as the detector and its processor handling signal analysis and data interpretation.
Energy efficiency considerations have driven innovations in low-power operation modes and energy harvesting techniques. These developments allow portable plasmonic biosensors to operate for extended periods without requiring frequent battery replacement or recharging, essential for field deployment in resource-limited settings.
The convergence of these miniaturization strategies is enabling a new generation of portable diagnostic systems that maintain laboratory-grade analytical performance while achieving unprecedented levels of portability, accessibility, and user-friendliness. These advances are particularly valuable for point-of-care applications in remote or resource-limited settings where conventional laboratory infrastructure is unavailable.
Microfluidic integration stands as a cornerstone approach, where plasmonic sensing elements are incorporated into lab-on-chip platforms. These systems utilize precisely engineered channels with dimensions in the micrometer range to control fluid flow across sensing surfaces, dramatically reducing sample volume requirements from milliliters to microliters or even nanoliters. This integration enables multiplexed detection capabilities while minimizing reagent consumption.
Advanced nanofabrication techniques have enabled the development of ultra-compact plasmonic structures. Techniques such as electron beam lithography and nanoimprint lithography allow for the creation of precisely engineered nanostructures with feature sizes below 100 nm. These structures can be optimized for maximum field enhancement and resonance sensitivity, improving detection limits while reducing the physical size of sensing elements.
CMOS-compatible fabrication processes represent another significant advancement, allowing plasmonic sensing elements to be directly integrated with electronic readout circuitry. This approach eliminates bulky optical components traditionally required for signal transduction and readout, resulting in fully integrated sensing platforms. Silicon photonics integration further enables on-chip light manipulation and detection, creating truly miniaturized systems-on-chip for biosensing applications.
Smartphone-based readout systems have emerged as a particularly promising direction, leveraging the ubiquitous computing power and connectivity of mobile devices. Compact adapters containing minimal optical components can transform smartphones into portable analytical instruments, with the phone's camera serving as the detector and its processor handling signal analysis and data interpretation.
Energy efficiency considerations have driven innovations in low-power operation modes and energy harvesting techniques. These developments allow portable plasmonic biosensors to operate for extended periods without requiring frequent battery replacement or recharging, essential for field deployment in resource-limited settings.
The convergence of these miniaturization strategies is enabling a new generation of portable diagnostic systems that maintain laboratory-grade analytical performance while achieving unprecedented levels of portability, accessibility, and user-friendliness. These advances are particularly valuable for point-of-care applications in remote or resource-limited settings where conventional laboratory infrastructure is unavailable.
Data Processing Algorithms for Real-time Biosensing
Real-time data processing represents a critical component in the integration of plasmonic biosensors with modern electronic devices. The algorithms employed must efficiently handle the complex optical signals generated when biomolecules interact with plasmonic surfaces, translating these interactions into meaningful electronic outputs with minimal latency.
Signal filtering algorithms form the foundation of real-time biosensing data processing. These algorithms effectively separate the plasmonic resonance shifts caused by target analyte binding from background noise, environmental fluctuations, and non-specific interactions. Advanced adaptive filtering techniques, particularly Kalman filters and wavelet transforms, have demonstrated superior performance in maintaining signal integrity while operating under varying environmental conditions.
Machine learning approaches have revolutionized biosensor data interpretation. Convolutional neural networks (CNNs) excel at pattern recognition within spectral data, while recurrent neural networks (RNNs) effectively model the temporal dynamics of binding events. These algorithms enable the detection of subtle changes in plasmonic resonance that might otherwise be overlooked by traditional threshold-based methods, significantly enhancing sensitivity and specificity.
Parallel processing architectures have emerged as essential for handling the high data throughput required in multi-analyte plasmonic sensing arrays. Field-programmable gate arrays (FPGAs) and graphics processing units (GPUs) provide the computational infrastructure necessary for real-time analysis of complex plasmonic data. These hardware accelerators enable simultaneous processing of signals from multiple sensing elements, facilitating multiplexed detection capabilities.
Edge computing paradigms are increasingly being implemented to reduce latency in plasmonic biosensing systems. By performing preliminary data processing directly on sensor-integrated microcontrollers, these algorithms minimize the bandwidth requirements for data transmission while enabling rapid response in point-of-care applications. This distributed computing approach balances processing load between local and cloud resources, optimizing both speed and analytical depth.
Drift compensation algorithms address the challenge of baseline shifts in long-term monitoring applications. These adaptive algorithms continuously recalibrate sensor responses against reference channels or internal standards, ensuring measurement stability over extended periods. Statistical methods such as principal component analysis (PCA) effectively isolate and correct for systematic variations unrelated to analyte binding events.
The integration of these algorithmic approaches with modern electronic interfaces has enabled unprecedented capabilities in real-time biosensing, transforming plasmonic sensors from laboratory instruments into practical tools for clinical diagnostics, environmental monitoring, and food safety applications.
Signal filtering algorithms form the foundation of real-time biosensing data processing. These algorithms effectively separate the plasmonic resonance shifts caused by target analyte binding from background noise, environmental fluctuations, and non-specific interactions. Advanced adaptive filtering techniques, particularly Kalman filters and wavelet transforms, have demonstrated superior performance in maintaining signal integrity while operating under varying environmental conditions.
Machine learning approaches have revolutionized biosensor data interpretation. Convolutional neural networks (CNNs) excel at pattern recognition within spectral data, while recurrent neural networks (RNNs) effectively model the temporal dynamics of binding events. These algorithms enable the detection of subtle changes in plasmonic resonance that might otherwise be overlooked by traditional threshold-based methods, significantly enhancing sensitivity and specificity.
Parallel processing architectures have emerged as essential for handling the high data throughput required in multi-analyte plasmonic sensing arrays. Field-programmable gate arrays (FPGAs) and graphics processing units (GPUs) provide the computational infrastructure necessary for real-time analysis of complex plasmonic data. These hardware accelerators enable simultaneous processing of signals from multiple sensing elements, facilitating multiplexed detection capabilities.
Edge computing paradigms are increasingly being implemented to reduce latency in plasmonic biosensing systems. By performing preliminary data processing directly on sensor-integrated microcontrollers, these algorithms minimize the bandwidth requirements for data transmission while enabling rapid response in point-of-care applications. This distributed computing approach balances processing load between local and cloud resources, optimizing both speed and analytical depth.
Drift compensation algorithms address the challenge of baseline shifts in long-term monitoring applications. These adaptive algorithms continuously recalibrate sensor responses against reference channels or internal standards, ensuring measurement stability over extended periods. Statistical methods such as principal component analysis (PCA) effectively isolate and correct for systematic variations unrelated to analyte binding events.
The integration of these algorithmic approaches with modern electronic interfaces has enabled unprecedented capabilities in real-time biosensing, transforming plasmonic sensors from laboratory instruments into practical tools for clinical diagnostics, environmental monitoring, and food safety applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!