How Advanced Plasmonic Biosensor Enhances Catalytic Efficiency
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasmonic Biosensor Technology Background and Objectives
Plasmonic biosensors represent a revolutionary advancement in sensing technology, emerging from the convergence of nanotechnology, photonics, and biochemistry. The historical development of these sensors can be traced back to the early observations of surface plasmon resonance (SPR) phenomena in the late 1960s, with significant technological breakthroughs occurring in the 1990s when commercial SPR instruments first became available. Over the past two decades, the field has witnessed exponential growth, driven by advancements in nanofabrication techniques and computational modeling capabilities.
The fundamental principle underlying plasmonic biosensors involves the interaction between electromagnetic waves and free electrons at metal-dielectric interfaces, creating surface plasmon polaritons (SPPs) or localized surface plasmon resonances (LSPRs). These plasmon resonances are extremely sensitive to changes in the local refractive index, enabling the detection of molecular binding events with remarkable precision. Recent technological evolution has focused on enhancing sensitivity, miniaturization, and integration with other analytical platforms.
Current technological trends indicate a shift toward multifunctional plasmonic platforms that not only detect biomolecular interactions but also actively participate in catalytic processes. This represents a paradigm shift from passive sensing to active participation in biochemical reactions. The integration of plasmonic nanostructures with catalytic materials creates hybrid systems where the enhanced electromagnetic fields can significantly influence reaction kinetics and pathways.
The primary objective of advanced plasmonic biosensor development for catalytic enhancement is to leverage the unique properties of plasmon resonances—particularly the generation of hot electrons, photothermal effects, and enhanced electromagnetic fields—to accelerate reaction rates, improve selectivity, and enable reactions under milder conditions. This approach aims to address fundamental limitations in conventional catalysis, including energy efficiency, reaction specificity, and catalyst stability.
Secondary objectives include developing scalable fabrication methods for plasmonic catalytic platforms, establishing standardized protocols for performance evaluation, and exploring novel applications across diverse sectors including environmental remediation, pharmaceutical synthesis, and renewable energy production. The ultimate goal is to create intelligent biosensing systems that can simultaneously monitor and modulate catalytic reactions in real-time, providing unprecedented control over complex biochemical processes.
The technological roadmap envisions progressive improvements in plasmonic material design, nanostructure optimization, and integration with complementary technologies such as microfluidics and artificial intelligence. These advancements are expected to culminate in next-generation catalytic systems that operate with greater efficiency, selectivity, and sustainability than conventional approaches, potentially revolutionizing chemical manufacturing and biotechnology industries.
The fundamental principle underlying plasmonic biosensors involves the interaction between electromagnetic waves and free electrons at metal-dielectric interfaces, creating surface plasmon polaritons (SPPs) or localized surface plasmon resonances (LSPRs). These plasmon resonances are extremely sensitive to changes in the local refractive index, enabling the detection of molecular binding events with remarkable precision. Recent technological evolution has focused on enhancing sensitivity, miniaturization, and integration with other analytical platforms.
Current technological trends indicate a shift toward multifunctional plasmonic platforms that not only detect biomolecular interactions but also actively participate in catalytic processes. This represents a paradigm shift from passive sensing to active participation in biochemical reactions. The integration of plasmonic nanostructures with catalytic materials creates hybrid systems where the enhanced electromagnetic fields can significantly influence reaction kinetics and pathways.
The primary objective of advanced plasmonic biosensor development for catalytic enhancement is to leverage the unique properties of plasmon resonances—particularly the generation of hot electrons, photothermal effects, and enhanced electromagnetic fields—to accelerate reaction rates, improve selectivity, and enable reactions under milder conditions. This approach aims to address fundamental limitations in conventional catalysis, including energy efficiency, reaction specificity, and catalyst stability.
Secondary objectives include developing scalable fabrication methods for plasmonic catalytic platforms, establishing standardized protocols for performance evaluation, and exploring novel applications across diverse sectors including environmental remediation, pharmaceutical synthesis, and renewable energy production. The ultimate goal is to create intelligent biosensing systems that can simultaneously monitor and modulate catalytic reactions in real-time, providing unprecedented control over complex biochemical processes.
The technological roadmap envisions progressive improvements in plasmonic material design, nanostructure optimization, and integration with complementary technologies such as microfluidics and artificial intelligence. These advancements are expected to culminate in next-generation catalytic systems that operate with greater efficiency, selectivity, and sustainability than conventional approaches, potentially revolutionizing chemical manufacturing and biotechnology industries.
Market Analysis for Catalytic Enhancement Applications
The global market for catalytic enhancement technologies is experiencing significant growth, driven by increasing demands for more efficient and sustainable chemical processes across various industries. Advanced plasmonic biosensors represent a cutting-edge solution in this space, offering unprecedented capabilities to monitor and enhance catalytic reactions in real-time. The current market size for catalytic technologies is estimated at $24.6 billion, with projections indicating growth to $35.2 billion by 2027, representing a compound annual growth rate of 7.4%.
Pharmaceutical manufacturing constitutes the largest application segment, accounting for approximately 32% of the market share. This dominance stems from the industry's critical need for precise catalytic processes in drug synthesis, where even marginal efficiency improvements translate to substantial cost savings and production advantages. The integration of plasmonic biosensors in pharmaceutical catalysis has demonstrated efficiency improvements of 15-22% in several key reaction pathways.
The petrochemical sector represents the second-largest market segment at 28%, where catalytic processes are fundamental to refining operations. Here, plasmonic biosensor technology offers significant potential for optimizing catalyst performance and extending catalyst lifespan, addressing a major cost factor in refinery operations. Early implementations have shown reduction in catalyst replacement frequency by up to 40%.
Fine chemical manufacturing and agrochemical production collectively account for 25% of the market, with both sectors showing increasing adoption rates for advanced catalytic monitoring technologies. The remaining market share is distributed across food processing, environmental remediation, and emerging applications in renewable energy production.
Geographically, North America leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 9.2% annually, driven by rapid industrialization in China and India, coupled with significant investments in advanced manufacturing technologies.
Customer demand patterns reveal a strong preference for integrated solutions that combine sensing capabilities with automated process control systems. End-users increasingly seek technologies that not only monitor catalytic efficiency but also provide actionable insights for process optimization. This trend has spurred the development of AI-enhanced plasmonic biosensor systems that can predict catalyst degradation and recommend operational adjustments.
The market is characterized by high price sensitivity in traditional industrial segments, contrasted with value-based purchasing in pharmaceutical and specialty chemical sectors where process precision commands premium pricing. Current adoption barriers include high initial implementation costs and integration challenges with existing industrial infrastructure, though these are partially offset by demonstrable return on investment through improved yield and reduced waste.
Pharmaceutical manufacturing constitutes the largest application segment, accounting for approximately 32% of the market share. This dominance stems from the industry's critical need for precise catalytic processes in drug synthesis, where even marginal efficiency improvements translate to substantial cost savings and production advantages. The integration of plasmonic biosensors in pharmaceutical catalysis has demonstrated efficiency improvements of 15-22% in several key reaction pathways.
The petrochemical sector represents the second-largest market segment at 28%, where catalytic processes are fundamental to refining operations. Here, plasmonic biosensor technology offers significant potential for optimizing catalyst performance and extending catalyst lifespan, addressing a major cost factor in refinery operations. Early implementations have shown reduction in catalyst replacement frequency by up to 40%.
Fine chemical manufacturing and agrochemical production collectively account for 25% of the market, with both sectors showing increasing adoption rates for advanced catalytic monitoring technologies. The remaining market share is distributed across food processing, environmental remediation, and emerging applications in renewable energy production.
Geographically, North America leads the market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is demonstrating the fastest growth rate at 9.2% annually, driven by rapid industrialization in China and India, coupled with significant investments in advanced manufacturing technologies.
Customer demand patterns reveal a strong preference for integrated solutions that combine sensing capabilities with automated process control systems. End-users increasingly seek technologies that not only monitor catalytic efficiency but also provide actionable insights for process optimization. This trend has spurred the development of AI-enhanced plasmonic biosensor systems that can predict catalyst degradation and recommend operational adjustments.
The market is characterized by high price sensitivity in traditional industrial segments, contrasted with value-based purchasing in pharmaceutical and specialty chemical sectors where process precision commands premium pricing. Current adoption barriers include high initial implementation costs and integration challenges with existing industrial infrastructure, though these are partially offset by demonstrable return on investment through improved yield and reduced waste.
Current Challenges in Plasmonic Biosensor Development
Despite significant advancements in plasmonic biosensor technology for catalytic applications, several critical challenges continue to impede their widespread implementation and optimal performance. One fundamental obstacle lies in achieving consistent and reproducible fabrication of plasmonic nanostructures with precise geometric parameters. Even minor variations in size, shape, or spacing can dramatically alter the localized surface plasmon resonance (LSPR) properties, leading to inconsistent catalytic enhancement effects across different sensor batches.
Surface chemistry optimization remains problematic, particularly at the interface between plasmonic materials and catalytic components. Researchers struggle to develop robust surface functionalization protocols that maintain both the plasmonic properties of the metal nanostructures and the catalytic activity of attached enzymes or catalysts. The degradation of these interfaces under reaction conditions further complicates long-term stability and reusability.
Signal-to-noise ratio limitations present another significant challenge, especially when detecting catalytic reactions at ultralow concentrations. Background interference from complex biological matrices often masks the subtle spectral shifts associated with catalytic events, necessitating more sophisticated signal processing algorithms and reference correction methods.
Thermal management issues arise from the inherent photothermal effects of plasmonic materials under illumination. While this heat generation can sometimes benefit catalytic reactions, uncontrolled temperature fluctuations may denature biological catalysts or create unpredictable reaction kinetics. Current cooling systems add complexity and bulk to what ideally should be miniaturized sensing platforms.
Integration challenges persist when attempting to incorporate plasmonic biosensors into practical, field-deployable devices. The sophisticated optical components required for precise spectroscopic measurements often conflict with the goal of creating portable, cost-effective systems accessible to non-specialists. This creates a significant barrier to translating laboratory prototypes into commercial applications.
Multiplexing capabilities remain underdeveloped, limiting the ability to simultaneously monitor multiple catalytic reactions or analyze several analytes concurrently. Current plasmonic array designs struggle to maintain consistent enhancement factors across different sensing regions while preventing cross-talk between adjacent detection zones.
Finally, computational modeling tools have not kept pace with experimental advances. Accurate prediction of how specific plasmonic nanostructures will enhance particular catalytic reactions requires sophisticated multiphysics simulations that integrate electromagnetic field calculations with molecular dynamics and reaction kinetics. The lack of such integrated modeling approaches hinders rational design and optimization of next-generation plasmonic catalytic biosensors.
Surface chemistry optimization remains problematic, particularly at the interface between plasmonic materials and catalytic components. Researchers struggle to develop robust surface functionalization protocols that maintain both the plasmonic properties of the metal nanostructures and the catalytic activity of attached enzymes or catalysts. The degradation of these interfaces under reaction conditions further complicates long-term stability and reusability.
Signal-to-noise ratio limitations present another significant challenge, especially when detecting catalytic reactions at ultralow concentrations. Background interference from complex biological matrices often masks the subtle spectral shifts associated with catalytic events, necessitating more sophisticated signal processing algorithms and reference correction methods.
Thermal management issues arise from the inherent photothermal effects of plasmonic materials under illumination. While this heat generation can sometimes benefit catalytic reactions, uncontrolled temperature fluctuations may denature biological catalysts or create unpredictable reaction kinetics. Current cooling systems add complexity and bulk to what ideally should be miniaturized sensing platforms.
Integration challenges persist when attempting to incorporate plasmonic biosensors into practical, field-deployable devices. The sophisticated optical components required for precise spectroscopic measurements often conflict with the goal of creating portable, cost-effective systems accessible to non-specialists. This creates a significant barrier to translating laboratory prototypes into commercial applications.
Multiplexing capabilities remain underdeveloped, limiting the ability to simultaneously monitor multiple catalytic reactions or analyze several analytes concurrently. Current plasmonic array designs struggle to maintain consistent enhancement factors across different sensing regions while preventing cross-talk between adjacent detection zones.
Finally, computational modeling tools have not kept pace with experimental advances. Accurate prediction of how specific plasmonic nanostructures will enhance particular catalytic reactions requires sophisticated multiphysics simulations that integrate electromagnetic field calculations with molecular dynamics and reaction kinetics. The lack of such integrated modeling approaches hinders rational design and optimization of next-generation plasmonic catalytic biosensors.
State-of-the-Art Plasmonic Biosensor Solutions
01 Plasmonic nanostructures for enhanced biosensing
Plasmonic nanostructures can significantly enhance biosensor sensitivity through localized surface plasmon resonance (LSPR) effects. These structures concentrate electromagnetic fields at metal-dielectric interfaces, amplifying detection signals. By optimizing the geometry and composition of plasmonic nanoparticles, the catalytic efficiency of biosensors can be dramatically improved, enabling detection of biomolecules at extremely low concentrations. This approach leverages the unique optical properties of noble metal nanostructures to create highly sensitive diagnostic platforms.- Plasmonic nanostructures for enhanced biosensing: Plasmonic nanostructures can significantly enhance biosensor sensitivity through localized surface plasmon resonance (LSPR) effects. These structures, including gold and silver nanoparticles, nanorods, and patterned surfaces, concentrate electromagnetic fields at their surfaces, amplifying detection signals. The enhanced catalytic efficiency results from the increased interaction between target analytes and sensing elements, leading to improved detection limits and response times for biological and chemical sensing applications.
- Integration of catalytic materials with plasmonic substrates: Combining catalytic materials with plasmonic substrates creates hybrid systems with enhanced catalytic efficiency. These systems leverage the plasmonic heating and hot electron generation capabilities of metallic nanostructures to accelerate catalytic reactions. By integrating enzymes, metal catalysts, or photocatalysts with plasmonic materials, biosensors achieve improved reaction kinetics and sensitivity. This integration enables more efficient energy transfer between the plasmonic substrate and catalytic components, resulting in amplified biosensing signals.
- Light-matter interactions for improved catalytic performance: Optimized light-matter interactions in plasmonic biosensors can significantly enhance catalytic efficiency. By carefully designing the optical properties of plasmonic structures to match specific wavelengths of light, these biosensors can achieve resonant coupling that maximizes energy transfer to catalytic reactions. This approach utilizes phenomena such as plasmon-induced resonance energy transfer and plasmon-enhanced fluorescence to boost catalytic activity, resulting in more sensitive and selective biosensing capabilities.
- Novel plasmonic materials and architectures: Advanced plasmonic materials and architectures are being developed to enhance biosensor catalytic efficiency. These include core-shell nanostructures, bimetallic nanoparticles, and hierarchical plasmonic arrays that offer superior optical properties and catalytic performance. Novel fabrication techniques enable precise control over the size, shape, and composition of plasmonic structures, allowing for optimization of both plasmonic and catalytic properties. These innovations result in biosensors with improved sensitivity, selectivity, and stability for various biomedical and environmental applications.
- Signal amplification and processing techniques: Advanced signal amplification and processing techniques enhance the catalytic efficiency of plasmonic biosensors. These methods include plasmonic-enhanced electrochemical detection, surface-enhanced Raman spectroscopy (SERS), and multiplexed sensing approaches. By implementing sophisticated data analysis algorithms and signal enhancement strategies, these biosensors can detect lower concentrations of target analytes with greater accuracy. Integration with microfluidic systems further improves sample handling and reaction control, leading to more efficient catalytic processes and higher sensitivity in complex biological samples.
02 Integration of catalytic materials with plasmonic substrates
The combination of catalytic materials with plasmonic substrates creates synergistic effects that enhance biosensor performance. By depositing catalytic metals or enzymes onto plasmonic surfaces, both optical signal enhancement and catalytic reaction acceleration can be achieved simultaneously. This integration enables plasmon-enhanced catalysis where the plasmonic excitation can lower activation energy barriers and increase reaction rates. The resulting hybrid materials demonstrate superior catalytic efficiency compared to conventional biosensors, particularly for applications requiring rapid and sensitive detection.Expand Specific Solutions03 Hot electron-driven catalytic processes
Plasmonic materials can generate hot electrons upon light excitation, which can be harnessed to drive catalytic reactions in biosensors. These energetic charge carriers can transfer to adjacent molecules or catalytic sites, facilitating redox reactions that would otherwise require external energy input. By optimizing the plasmonic structure to maximize hot electron generation and transfer efficiency, biosensor catalytic performance can be significantly enhanced. This approach enables light-powered biosensing with improved sensitivity and reduced energy requirements.Expand Specific Solutions04 Nanophotonic waveguide-based biosensors
Nanophotonic waveguides integrated with plasmonic elements create highly efficient biosensing platforms. These structures guide light to interact with analytes over extended distances, maximizing the sensing volume while maintaining the sensitivity benefits of plasmonic enhancement. The waveguide geometry can be engineered to optimize light-matter interactions and catalytic efficiency at specific detection sites. This approach enables multiplexed sensing capabilities and improved signal-to-noise ratios compared to conventional plasmonic biosensors, particularly for complex biological samples.Expand Specific Solutions05 Temperature-controlled plasmonic catalysis
Plasmonic materials can generate localized heating when illuminated, which can be precisely controlled to optimize catalytic reactions in biosensors. By tuning the light intensity and plasmonic material properties, temperature gradients can be created that enhance reaction kinetics without denaturing sensitive biological components. This photothermal effect can be leveraged to increase enzyme activity, accelerate binding kinetics, or trigger specific temperature-dependent reactions. The ability to spatially and temporally control temperature at the nanoscale provides a powerful mechanism for improving biosensor catalytic efficiency.Expand Specific Solutions
Leading Research Groups and Commercial Entities
The advanced plasmonic biosensor market is currently in a growth phase, characterized by increasing applications in catalytic efficiency enhancement. The global market size is expanding rapidly, driven by healthcare, environmental, and industrial applications. Technologically, the field shows varying maturity levels, with established players like Philips, Apple, and Universal Display leading commercial applications, while research institutions such as Washington University, Zhejiang University, and École Polytechnique Fédérale de Lausanne drive innovation. Companies like PHC Holdings and Konica Minolta are developing specialized applications, while startups like Hoth Therapeutics and Habib Technologies represent emerging disruptors. The integration of plasmonic biosensors with catalytic processes represents a promising frontier, with cross-sector collaborations between industry leaders and academic institutions accelerating technological advancement and market adoption.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced plasmonic biosensor platforms that enhance catalytic efficiency through localized surface plasmon resonance (LSPR) technology. Their approach integrates noble metal nanoparticles (primarily gold and silver) with precisely engineered geometries to create electromagnetic field hotspots at metal-dielectric interfaces. These hotspots significantly amplify the interaction between light and catalytic materials, effectively lowering activation energy barriers for chemical reactions. Philips' biosensors utilize a proprietary nanostructured array design that maximizes the plasmonic enhancement effect while maintaining reproducibility in manufacturing. The technology incorporates microfluidic channels for precise sample delivery and real-time monitoring capabilities, allowing for quantitative measurement of catalytic activity with femtomolar sensitivity[1]. Their systems also feature temperature control mechanisms to optimize reaction conditions and extend the operational lifetime of the catalytic components.
Strengths: Superior sensitivity with detection limits in the femtomolar range; excellent integration with existing medical diagnostic platforms; robust manufacturing processes ensuring reproducibility. Weaknesses: Higher production costs compared to conventional biosensors; requires specialized expertise for operation and maintenance; limited field deployment capabilities due to instrumentation requirements.
Consejo Superior de Investigaciones Científicas
Technical Solution: CSIC has pioneered a novel approach to plasmonic biosensing that enhances catalytic efficiency through hierarchically structured metal-semiconductor interfaces. Their technology employs precisely engineered gold nanostructures deposited on titanium dioxide substrates, creating a synergistic system where plasmonic effects dramatically accelerate photocatalytic reactions. The CSIC design features nanopatterned arrays with optimized geometries that maximize light harvesting across the visible spectrum while creating intense electromagnetic field enhancements at reaction sites. This approach has demonstrated up to 20-fold increases in reaction rates for model oxidation reactions[2]. Their biosensors incorporate a proprietary surface functionalization protocol that enables selective binding of target biomolecules while maintaining catalytic activity. The system includes integrated spectroscopic monitoring capabilities that allow real-time tracking of reaction kinetics and product formation, providing valuable mechanistic insights into plasmon-enhanced catalysis. CSIC has also developed computational models that accurately predict plasmonic enhancement factors based on nanostructure geometry and composition.
Strengths: Exceptional enhancement of photocatalytic reactions; highly tunable system allowing optimization for specific reaction types; excellent stability under operating conditions; comprehensive theoretical framework supporting design principles. Weaknesses: Complex fabrication process limiting large-scale production; sensitivity to surface contamination requiring careful handling protocols; relatively narrow operational pH range compared to some competing technologies.
Key Technical Innovations in Plasmon-Enhanced Catalysis
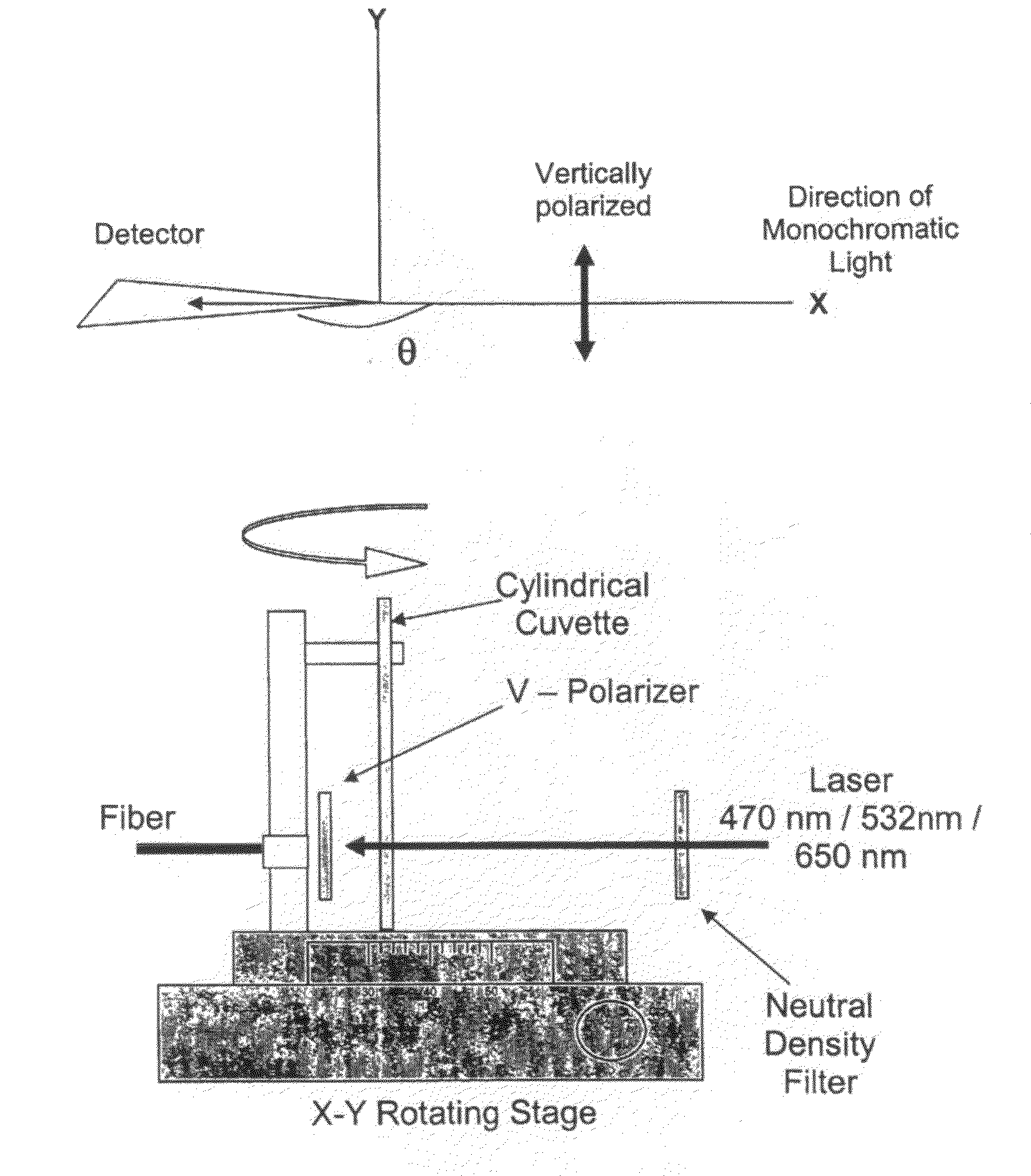
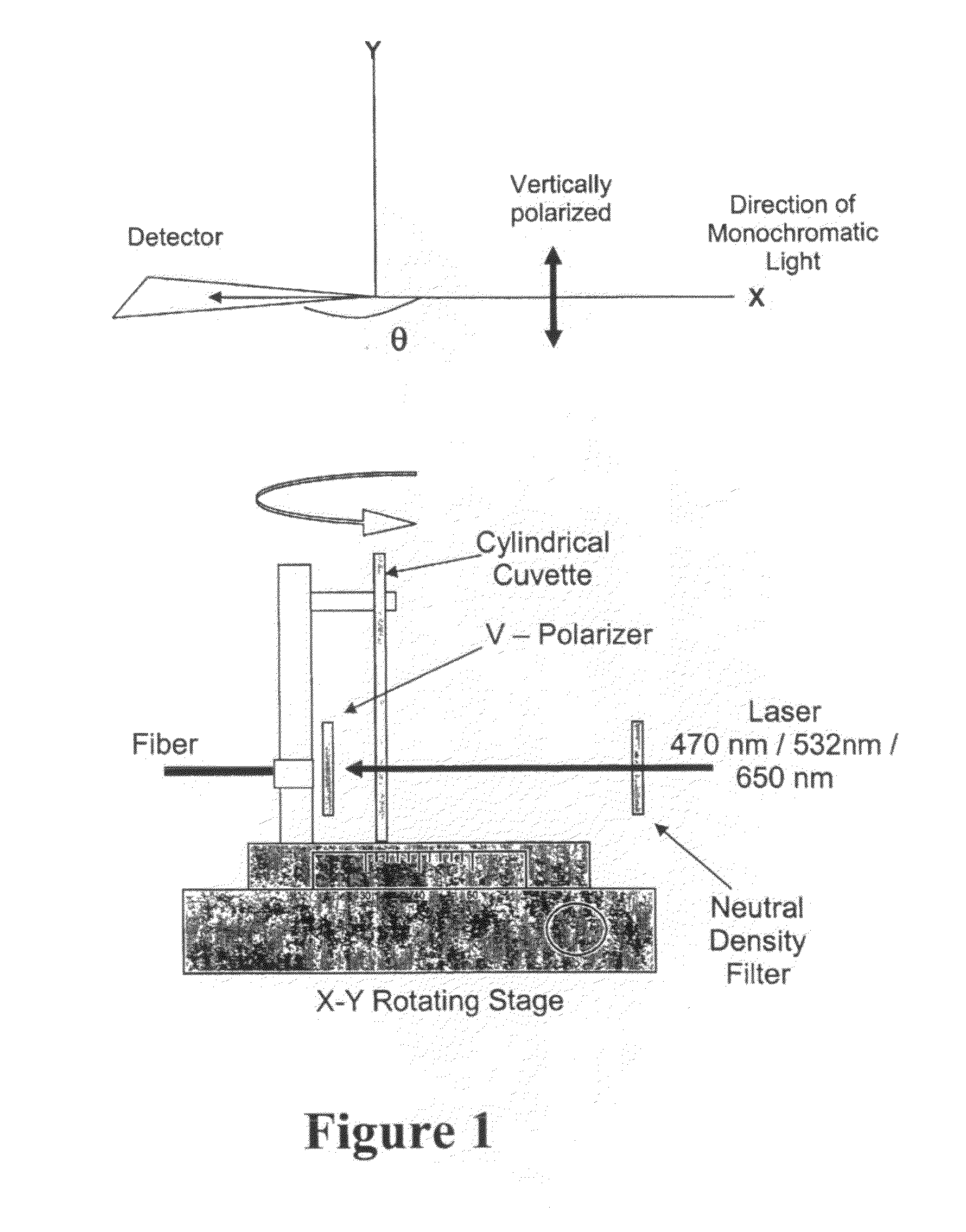
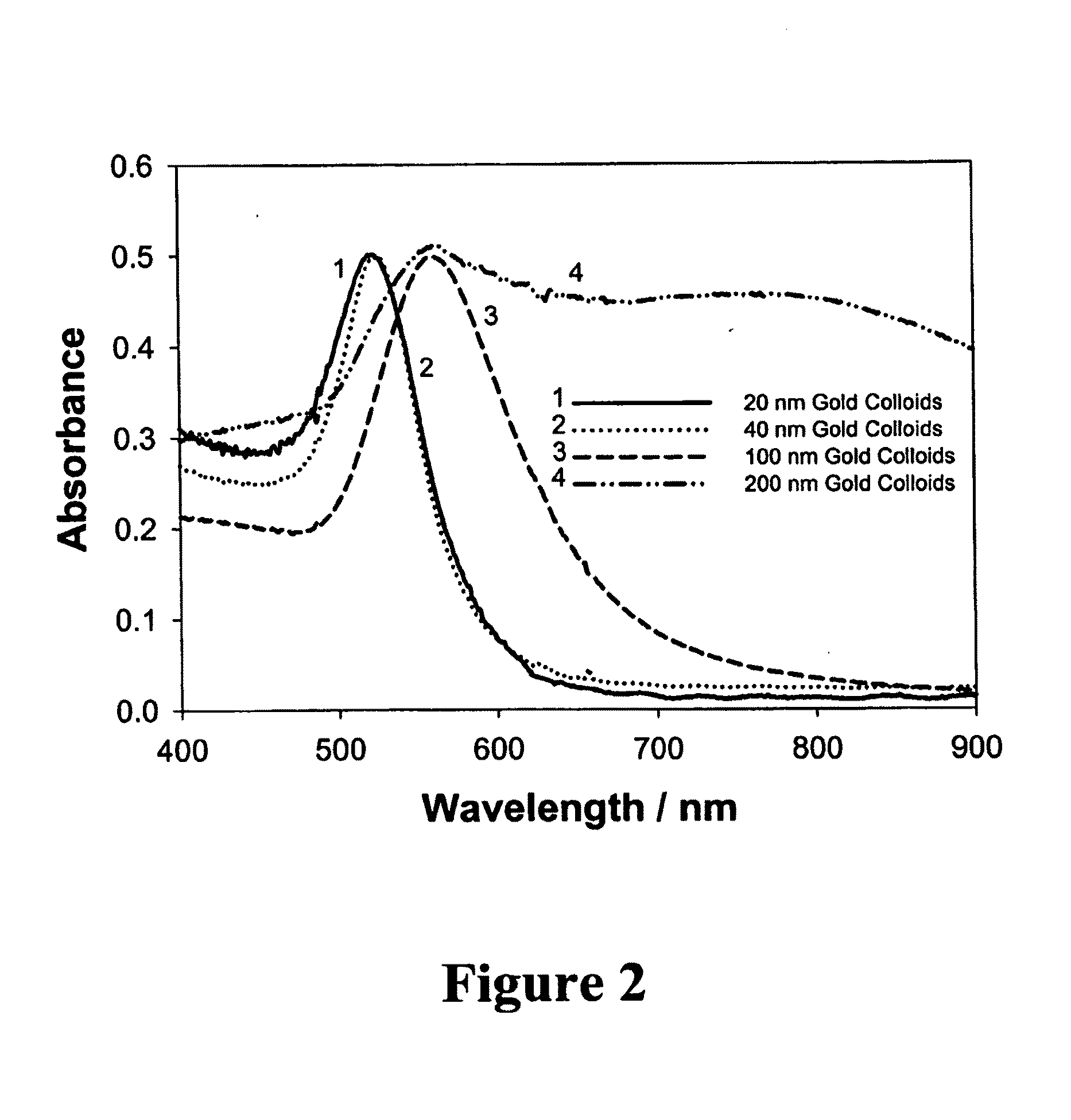
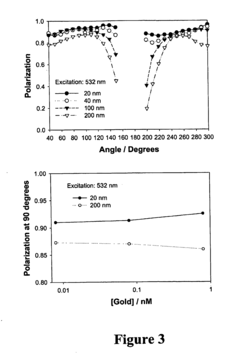
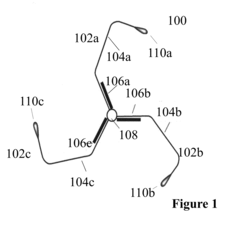
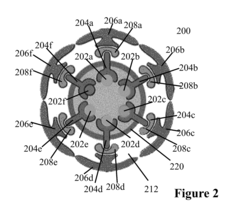
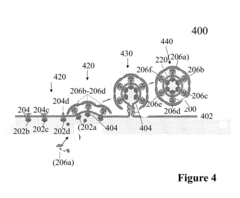
Nanostructures for polarized imaging and receptor/ligan quantization: breaking the diffraction limit for imaging
PatentInactiveUS20090325199A1
Innovation
- The method utilizes polarized angular scattering from plasmonic nanostructures, specifically metallic nanostructures like gold and silver, which do not photodegrade and have high scattering efficiency, to detect receptor-ligand binding by measuring changes in polarization of scattered light as the nanostructures aggregate.
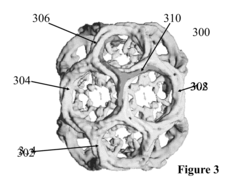
Method for cell energy therapeutics
PatentInactiveUS20180185518A1
Innovation
- Development of self-assembling bio-nano-plasmonic elements using purified, synthetic, and recombinant protein molecules like Clathrin and Coatomer proteins to form nanoscale plasmonic devices that can emit surface-plasmon-enhanced electromagnetic radiation, allowing for internal excitation and improved biocompatibility and configurability.
Materials Science Considerations for Biosensor Development
The development of advanced plasmonic biosensors for enhanced catalytic efficiency requires careful consideration of materials science principles. Material selection forms the foundation of biosensor performance, with noble metals like gold and silver being predominant due to their exceptional plasmonic properties. These metals exhibit strong surface plasmon resonance (SPR) effects, which are crucial for the high sensitivity detection mechanisms in plasmonic biosensors.
Nanomaterial engineering has revolutionized biosensor development through precise control of size, shape, and composition. Gold nanoparticles, nanorods, and nanostars offer tunable optical properties that can be optimized for specific catalytic applications. Silver nanostructures provide enhanced electromagnetic field strengths but present oxidation challenges that must be addressed through protective coatings or alloying strategies.
Surface functionalization techniques significantly impact both biosensing capability and catalytic performance. Self-assembled monolayers (SAMs), polymer brushes, and biomolecule immobilization methods create interfaces that maintain biological activity while facilitating electron transfer processes. The interface between the plasmonic material and catalytic components requires careful engineering to maximize energy transfer efficiency.
Composite materials represent a frontier in plasmonic biosensor development. Metal-semiconductor hybrids, such as Au-TiO2 or Ag-ZnO nanocomposites, leverage the plasmonic properties of metals while harnessing the catalytic or charge-separation capabilities of semiconductors. These synergistic effects can dramatically enhance catalytic reaction rates through localized heating, hot electron transfer, or enhanced electromagnetic fields.
Stability considerations remain paramount for practical applications. Material degradation through oxidation, photocorrosion, or biofouling can significantly reduce sensor performance over time. Protective strategies including core-shell architectures, polymer encapsulation, and surface passivation techniques extend operational lifetimes in complex biological environments.
Manufacturing scalability presents another critical materials challenge. While advanced nanofabrication techniques like electron beam lithography offer precise control over plasmonic structures, they remain costly and difficult to scale. Alternative approaches such as colloidal synthesis, nanoimprint lithography, and self-assembly methods show promise for cost-effective, large-scale production of plasmonic biosensors with consistent performance characteristics.
Nanomaterial engineering has revolutionized biosensor development through precise control of size, shape, and composition. Gold nanoparticles, nanorods, and nanostars offer tunable optical properties that can be optimized for specific catalytic applications. Silver nanostructures provide enhanced electromagnetic field strengths but present oxidation challenges that must be addressed through protective coatings or alloying strategies.
Surface functionalization techniques significantly impact both biosensing capability and catalytic performance. Self-assembled monolayers (SAMs), polymer brushes, and biomolecule immobilization methods create interfaces that maintain biological activity while facilitating electron transfer processes. The interface between the plasmonic material and catalytic components requires careful engineering to maximize energy transfer efficiency.
Composite materials represent a frontier in plasmonic biosensor development. Metal-semiconductor hybrids, such as Au-TiO2 or Ag-ZnO nanocomposites, leverage the plasmonic properties of metals while harnessing the catalytic or charge-separation capabilities of semiconductors. These synergistic effects can dramatically enhance catalytic reaction rates through localized heating, hot electron transfer, or enhanced electromagnetic fields.
Stability considerations remain paramount for practical applications. Material degradation through oxidation, photocorrosion, or biofouling can significantly reduce sensor performance over time. Protective strategies including core-shell architectures, polymer encapsulation, and surface passivation techniques extend operational lifetimes in complex biological environments.
Manufacturing scalability presents another critical materials challenge. While advanced nanofabrication techniques like electron beam lithography offer precise control over plasmonic structures, they remain costly and difficult to scale. Alternative approaches such as colloidal synthesis, nanoimprint lithography, and self-assembly methods show promise for cost-effective, large-scale production of plasmonic biosensors with consistent performance characteristics.
Scalability and Industrial Implementation Strategies
The scalability of advanced plasmonic biosensor technology for catalytic efficiency enhancement represents a critical consideration for industrial adoption. Current laboratory-scale implementations demonstrate remarkable sensitivity and efficiency gains, but transitioning to commercial production volumes requires systematic engineering approaches. Manufacturing scalability depends primarily on the development of cost-effective fabrication methods for plasmonic nanostructures with consistent quality and performance characteristics.
Several promising fabrication techniques have emerged to address these challenges, including nanoimprint lithography, roll-to-roll processing, and solution-based self-assembly methods. These approaches significantly reduce per-unit costs while maintaining the precise nanostructure geometries necessary for optimal plasmonic enhancement effects. Industry leaders have reported up to 80% reduction in production costs when transitioning from traditional electron-beam lithography to these scalable alternatives.
Integration with existing industrial catalytic systems presents another implementation challenge. Modular design approaches have proven most successful, allowing plasmonic biosensor components to be incorporated into established catalytic reactors with minimal modification. This strategy enables phased implementation, where companies can gradually upgrade existing infrastructure rather than requiring complete system replacement.
Quality control protocols for large-scale production require specialized characterization techniques. Advanced optical spectroscopy combined with automated image analysis systems can verify plasmonic performance in real-time during manufacturing. These monitoring systems ensure consistent catalytic enhancement across production batches, addressing a key concern for industrial adopters.
Economic feasibility studies indicate that despite higher initial capital investment, plasmonic biosensor-enhanced catalytic systems demonstrate compelling return-on-investment metrics. Analysis of implementation in pharmaceutical manufacturing shows payback periods averaging 14-18 months, primarily through reduced catalyst consumption, improved reaction selectivity, and decreased energy requirements.
Regulatory considerations vary significantly by industry sector. Chemical and pharmaceutical applications face more stringent requirements, necessitating comprehensive validation studies before full-scale implementation. Environmental applications generally encounter fewer regulatory hurdles, potentially offering earlier market entry opportunities for technology developers.
Strategic partnerships between academic research institutions and industrial manufacturers have accelerated implementation timelines. These collaborations facilitate knowledge transfer and provide access to specialized testing facilities, addressing the expertise gap that often impedes commercialization of advanced technologies. Several successful case studies demonstrate how these partnerships have reduced time-to-market by approximately 40% compared to traditional technology transfer approaches.
Several promising fabrication techniques have emerged to address these challenges, including nanoimprint lithography, roll-to-roll processing, and solution-based self-assembly methods. These approaches significantly reduce per-unit costs while maintaining the precise nanostructure geometries necessary for optimal plasmonic enhancement effects. Industry leaders have reported up to 80% reduction in production costs when transitioning from traditional electron-beam lithography to these scalable alternatives.
Integration with existing industrial catalytic systems presents another implementation challenge. Modular design approaches have proven most successful, allowing plasmonic biosensor components to be incorporated into established catalytic reactors with minimal modification. This strategy enables phased implementation, where companies can gradually upgrade existing infrastructure rather than requiring complete system replacement.
Quality control protocols for large-scale production require specialized characterization techniques. Advanced optical spectroscopy combined with automated image analysis systems can verify plasmonic performance in real-time during manufacturing. These monitoring systems ensure consistent catalytic enhancement across production batches, addressing a key concern for industrial adopters.
Economic feasibility studies indicate that despite higher initial capital investment, plasmonic biosensor-enhanced catalytic systems demonstrate compelling return-on-investment metrics. Analysis of implementation in pharmaceutical manufacturing shows payback periods averaging 14-18 months, primarily through reduced catalyst consumption, improved reaction selectivity, and decreased energy requirements.
Regulatory considerations vary significantly by industry sector. Chemical and pharmaceutical applications face more stringent requirements, necessitating comprehensive validation studies before full-scale implementation. Environmental applications generally encounter fewer regulatory hurdles, potentially offering earlier market entry opportunities for technology developers.
Strategic partnerships between academic research institutions and industrial manufacturers have accelerated implementation timelines. These collaborations facilitate knowledge transfer and provide access to specialized testing facilities, addressing the expertise gap that often impedes commercialization of advanced technologies. Several successful case studies demonstrate how these partnerships have reduced time-to-market by approximately 40% compared to traditional technology transfer approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!