Evolving Standards for Plasmonic Biosensor Integration in Medicine
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Plasmonic Biosensor Evolution and Medical Integration Goals
Plasmonic biosensors represent a revolutionary advancement in medical diagnostics, combining the principles of plasmonics with biosensing capabilities to create highly sensitive detection platforms. The evolution of these technologies has been marked by significant milestones over the past two decades, transitioning from laboratory curiosities to clinically relevant tools. Initially developed as fundamental research in surface plasmon resonance (SPR), these sensors have progressively incorporated nanomaterials and advanced fabrication techniques to enhance sensitivity and specificity.
The technological trajectory has been characterized by miniaturization, increased multiplexing capabilities, and integration with complementary technologies such as microfluidics and artificial intelligence. Early plasmonic biosensors relied primarily on gold films, while contemporary designs leverage sophisticated nanostructures including nanorods, nanoholes, and core-shell nanoparticles to manipulate light-matter interactions at unprecedented scales.
Current trends indicate a convergence toward standardized platforms that can be readily adopted in clinical settings. This standardization encompasses not only the physical components and fabrication processes but also data interpretation protocols and quality control measures. The International Organization for Standardization (ISO) has begun developing frameworks specifically addressing nanomaterial-based biosensors, while regulatory bodies like the FDA are establishing guidelines for validating these technologies in medical applications.
The integration goals for plasmonic biosensors in medicine are multifaceted and ambitious. Primary objectives include achieving point-of-care capabilities with sensitivity comparable to laboratory-based tests, developing wearable formats for continuous monitoring of biomarkers, and creating implantable sensors for real-time health monitoring. These goals necessitate overcoming challenges in biocompatibility, long-term stability, and signal consistency in complex biological environments.
Another critical aim is the development of multiplexed sensing platforms capable of simultaneously detecting multiple biomarkers from a single sample, thereby providing comprehensive diagnostic information. This requires sophisticated surface chemistry strategies and advanced signal processing algorithms to differentiate between multiple analyte responses.
The ultimate technological vision encompasses fully integrated diagnostic systems that combine sample preparation, sensing, data analysis, and result interpretation in autonomous platforms accessible to healthcare providers across diverse settings. This integration must address interoperability with existing medical infrastructure and electronic health record systems while maintaining stringent standards for accuracy, reliability, and security.
Achieving these ambitious goals requires coordinated efforts across disciplines, including physics, materials science, biochemistry, electronics, and clinical medicine, highlighting the inherently interdisciplinary nature of this technological frontier.
The technological trajectory has been characterized by miniaturization, increased multiplexing capabilities, and integration with complementary technologies such as microfluidics and artificial intelligence. Early plasmonic biosensors relied primarily on gold films, while contemporary designs leverage sophisticated nanostructures including nanorods, nanoholes, and core-shell nanoparticles to manipulate light-matter interactions at unprecedented scales.
Current trends indicate a convergence toward standardized platforms that can be readily adopted in clinical settings. This standardization encompasses not only the physical components and fabrication processes but also data interpretation protocols and quality control measures. The International Organization for Standardization (ISO) has begun developing frameworks specifically addressing nanomaterial-based biosensors, while regulatory bodies like the FDA are establishing guidelines for validating these technologies in medical applications.
The integration goals for plasmonic biosensors in medicine are multifaceted and ambitious. Primary objectives include achieving point-of-care capabilities with sensitivity comparable to laboratory-based tests, developing wearable formats for continuous monitoring of biomarkers, and creating implantable sensors for real-time health monitoring. These goals necessitate overcoming challenges in biocompatibility, long-term stability, and signal consistency in complex biological environments.
Another critical aim is the development of multiplexed sensing platforms capable of simultaneously detecting multiple biomarkers from a single sample, thereby providing comprehensive diagnostic information. This requires sophisticated surface chemistry strategies and advanced signal processing algorithms to differentiate between multiple analyte responses.
The ultimate technological vision encompasses fully integrated diagnostic systems that combine sample preparation, sensing, data analysis, and result interpretation in autonomous platforms accessible to healthcare providers across diverse settings. This integration must address interoperability with existing medical infrastructure and electronic health record systems while maintaining stringent standards for accuracy, reliability, and security.
Achieving these ambitious goals requires coordinated efforts across disciplines, including physics, materials science, biochemistry, electronics, and clinical medicine, highlighting the inherently interdisciplinary nature of this technological frontier.
Market Analysis of Plasmonic Biosensors in Healthcare
The global market for plasmonic biosensors in healthcare is experiencing robust growth, driven by increasing demand for rapid, sensitive, and portable diagnostic solutions. Current market valuations place the plasmonic biosensor segment at approximately 1.2 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 8.7% through 2030. This growth trajectory significantly outpaces traditional biosensing technologies, reflecting the superior performance characteristics of plasmonic platforms.
North America currently dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 23%. The remaining regions account for 7% of the global market. Within these regions, the United States, Germany, China, and Japan represent the largest individual markets, with India and Brazil emerging as high-growth potential areas due to expanding healthcare infrastructure and increasing adoption of advanced diagnostic technologies.
By application segment, clinical diagnostics represents the largest market share at 38%, followed by point-of-care testing (27%), pharmaceutical research (18%), and environmental monitoring (12%). The remaining 5% encompasses various niche applications. The point-of-care segment is experiencing the fastest growth rate at 11.2% annually, driven by increasing demand for decentralized healthcare solutions and remote patient monitoring capabilities.
Key market drivers include the rising prevalence of chronic and infectious diseases, growing demand for minimally invasive diagnostic procedures, increasing healthcare expenditure, and technological advancements in nanofabrication techniques. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostic capabilities and creating unprecedented demand for testing solutions.
Significant market restraints include high development and manufacturing costs, technical challenges in standardization, regulatory hurdles, and limited awareness among healthcare professionals about plasmonic technology benefits. The average development timeline for bringing a new plasmonic biosensor to market remains between 3-5 years, with regulatory approval processes accounting for approximately 40% of this timeframe.
Customer segmentation reveals that hospital laboratories constitute 45% of end-users, followed by diagnostic centers (25%), research institutions (20%), and pharmaceutical companies (10%). The pricing structure varies significantly based on application complexity, with high-end research systems commanding premium prices while point-of-care solutions are increasingly becoming cost-competitive with traditional diagnostic methods.
North America currently dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 23%. The remaining regions account for 7% of the global market. Within these regions, the United States, Germany, China, and Japan represent the largest individual markets, with India and Brazil emerging as high-growth potential areas due to expanding healthcare infrastructure and increasing adoption of advanced diagnostic technologies.
By application segment, clinical diagnostics represents the largest market share at 38%, followed by point-of-care testing (27%), pharmaceutical research (18%), and environmental monitoring (12%). The remaining 5% encompasses various niche applications. The point-of-care segment is experiencing the fastest growth rate at 11.2% annually, driven by increasing demand for decentralized healthcare solutions and remote patient monitoring capabilities.
Key market drivers include the rising prevalence of chronic and infectious diseases, growing demand for minimally invasive diagnostic procedures, increasing healthcare expenditure, and technological advancements in nanofabrication techniques. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid diagnostic capabilities and creating unprecedented demand for testing solutions.
Significant market restraints include high development and manufacturing costs, technical challenges in standardization, regulatory hurdles, and limited awareness among healthcare professionals about plasmonic technology benefits. The average development timeline for bringing a new plasmonic biosensor to market remains between 3-5 years, with regulatory approval processes accounting for approximately 40% of this timeframe.
Customer segmentation reveals that hospital laboratories constitute 45% of end-users, followed by diagnostic centers (25%), research institutions (20%), and pharmaceutical companies (10%). The pricing structure varies significantly based on application complexity, with high-end research systems commanding premium prices while point-of-care solutions are increasingly becoming cost-competitive with traditional diagnostic methods.
Current Challenges in Plasmonic Biosensor Medical Applications
Despite significant advancements in plasmonic biosensor technology, several critical challenges impede their widespread integration into medical applications. The primary obstacle remains standardization across manufacturing processes, which leads to inconsistent sensor performance between different production batches and manufacturers. This variability undermines clinical reliability and complicates regulatory approval pathways, particularly for point-of-care diagnostic applications where reproducibility is paramount.
Miniaturization presents another significant hurdle, as current plasmonic biosensor systems often require bulky optical components and precise alignment mechanisms. While laboratory prototypes demonstrate impressive sensitivity, translating these capabilities into compact, user-friendly medical devices suitable for clinical settings remains problematic. The integration of microfluidics with plasmonic sensing elements introduces additional complexity in terms of fabrication consistency and operational stability.
Biological sample complexity poses substantial challenges for plasmonic biosensors in real-world medical applications. Clinical samples such as blood, saliva, or urine contain numerous interfering substances that can cause non-specific binding, signal drift, and reduced sensitivity. Current surface functionalization strategies often fail to provide sufficient selectivity in complex biological matrices, limiting diagnostic accuracy in clinical environments.
Long-term stability issues further complicate medical implementation. Many plasmonic biosensors exhibit performance degradation over time due to surface fouling, biomolecule denaturation, or changes in optical properties. This instability necessitates frequent recalibration or replacement, which is impractical for implantable devices or continuous monitoring applications in healthcare settings.
Cost-effectiveness remains a significant barrier, particularly for disposable diagnostic applications. Current fabrication methods for high-performance plasmonic structures often involve expensive nanolithography techniques or specialized materials that are prohibitively costly for widespread clinical adoption. Balancing performance requirements with economic viability continues to challenge researchers and manufacturers alike.
Regulatory hurdles present formidable obstacles for clinical translation. The novel nature of plasmonic biosensing technology means that standardized validation protocols and performance benchmarks are still evolving. Regulatory bodies like the FDA require extensive validation data demonstrating not only analytical performance but also clinical utility, which necessitates large-scale clinical trials that are both time-consuming and expensive.
Data interpretation and integration with existing clinical workflows represent additional challenges. Converting complex plasmonic sensor signals into actionable medical information requires sophisticated algorithms and reference standards that are still under development. Furthermore, integration with electronic health records and clinical decision support systems remains limited, hindering adoption by healthcare professionals.
Miniaturization presents another significant hurdle, as current plasmonic biosensor systems often require bulky optical components and precise alignment mechanisms. While laboratory prototypes demonstrate impressive sensitivity, translating these capabilities into compact, user-friendly medical devices suitable for clinical settings remains problematic. The integration of microfluidics with plasmonic sensing elements introduces additional complexity in terms of fabrication consistency and operational stability.
Biological sample complexity poses substantial challenges for plasmonic biosensors in real-world medical applications. Clinical samples such as blood, saliva, or urine contain numerous interfering substances that can cause non-specific binding, signal drift, and reduced sensitivity. Current surface functionalization strategies often fail to provide sufficient selectivity in complex biological matrices, limiting diagnostic accuracy in clinical environments.
Long-term stability issues further complicate medical implementation. Many plasmonic biosensors exhibit performance degradation over time due to surface fouling, biomolecule denaturation, or changes in optical properties. This instability necessitates frequent recalibration or replacement, which is impractical for implantable devices or continuous monitoring applications in healthcare settings.
Cost-effectiveness remains a significant barrier, particularly for disposable diagnostic applications. Current fabrication methods for high-performance plasmonic structures often involve expensive nanolithography techniques or specialized materials that are prohibitively costly for widespread clinical adoption. Balancing performance requirements with economic viability continues to challenge researchers and manufacturers alike.
Regulatory hurdles present formidable obstacles for clinical translation. The novel nature of plasmonic biosensing technology means that standardized validation protocols and performance benchmarks are still evolving. Regulatory bodies like the FDA require extensive validation data demonstrating not only analytical performance but also clinical utility, which necessitates large-scale clinical trials that are both time-consuming and expensive.
Data interpretation and integration with existing clinical workflows represent additional challenges. Converting complex plasmonic sensor signals into actionable medical information requires sophisticated algorithms and reference standards that are still under development. Furthermore, integration with electronic health records and clinical decision support systems remains limited, hindering adoption by healthcare professionals.
Current Integration Standards and Protocols
01 Standardization of plasmonic biosensor fabrication
Standardized methods for fabricating plasmonic biosensors ensure consistency in performance and reliability. These standards cover the deposition of metallic nanostructures, surface functionalization protocols, and quality control measures during manufacturing. Standardization helps in achieving reproducible sensor responses across different batches and laboratories, which is crucial for commercial applications and regulatory approval.- Standardization of plasmonic biosensor fabrication: Standardized methods for fabricating plasmonic biosensors ensure consistency in performance and reliability. These standards cover the deposition of metallic nanostructures, surface functionalization protocols, and quality control measures during manufacturing. Standardization helps in achieving reproducible sensor responses across different batches and laboratories, which is crucial for commercial applications and regulatory approval.
- Calibration and performance metrics for plasmonic biosensors: Standardized calibration procedures and performance metrics are essential for evaluating plasmonic biosensors. These include sensitivity measurements, detection limits, dynamic range, and response time. Reference materials and control samples are used to validate sensor performance against established benchmarks. These standards enable comparison between different sensor designs and ensure reliable detection of target analytes in various applications.
- Integration standards for plasmonic biosensor systems: Standards for integrating plasmonic biosensors with other components such as microfluidics, optical systems, and electronic readouts ensure proper system functionality. These standards define interface specifications, signal processing protocols, and data formats. Standardized integration approaches facilitate the development of complete biosensing platforms that can be deployed in clinical, environmental, or industrial settings.
- Validation protocols for plasmonic biosensor applications: Standardized validation protocols ensure that plasmonic biosensors perform reliably in specific applications such as medical diagnostics, environmental monitoring, or food safety testing. These protocols define sample preparation methods, testing procedures, and acceptance criteria. Validation standards help establish the clinical or analytical validity of biosensor-based tests and are often required for regulatory approval.
- Advanced materials standards for plasmonic biosensors: Standards for advanced materials used in plasmonic biosensors ensure consistent optical and chemical properties. These include specifications for noble metal nanoparticles, nanofabricated structures, and surface functionalization chemistries. Material standards cover purity levels, size distributions, and stability requirements. Adherence to these standards results in biosensors with predictable plasmonic responses and improved shelf life.
02 Calibration and performance metrics for plasmonic biosensors
Standardized calibration procedures and performance metrics are essential for evaluating plasmonic biosensors. These include sensitivity measurements, detection limits, dynamic range, and response time. Reference materials and control samples are used to validate sensor performance against established benchmarks. These standards enable comparison between different sensor designs and ensure reliable detection of target analytes in various applications.Expand Specific Solutions03 Integration standards for plasmonic biosensor systems
Standards for integrating plasmonic biosensors with other components such as microfluidics, optical systems, and electronic readouts ensure proper functionality of complete biosensing platforms. These standards address interface specifications, signal processing protocols, and system validation methods. Standardized integration approaches facilitate the development of portable and user-friendly biosensor devices suitable for point-of-care diagnostics and field applications.Expand Specific Solutions04 Validation protocols for plasmonic biosensor applications
Standardized validation protocols ensure that plasmonic biosensors perform reliably in specific applications such as medical diagnostics, environmental monitoring, and food safety testing. These protocols include testing with known samples, interference studies, and reproducibility assessments under various conditions. Application-specific standards help establish the clinical or analytical validity of biosensor results and support regulatory compliance.Expand Specific Solutions05 Data processing and reporting standards for plasmonic biosensors
Standards for data processing and reporting ensure consistent interpretation of plasmonic biosensor results. These include signal analysis algorithms, noise filtering methods, and formats for presenting quantitative results. Standardized data handling practices improve the reliability of biosensor measurements and facilitate data sharing between different research groups and clinical laboratories. These standards also address data security and privacy considerations for sensitive applications.Expand Specific Solutions
Leading Developers and Manufacturers in Plasmonic Biosensing
Plasmonic biosensor integration in medicine is evolving rapidly, currently transitioning from research to early commercialization phase. The market is projected to grow significantly, driven by increasing demand for point-of-care diagnostics and personalized medicine applications. Technologically, companies like FUJIFILM, Canon, Philips, and Abbott Diabetes Care are advancing sensor miniaturization and integration with existing medical platforms, while academic institutions including Washington University, Sun Yat-Sen University, and Auburn University are pioneering fundamental research. Emerging players like Bialoom and Attomarker are developing innovative point-of-care applications, while established technology companies such as Apple and Lenovo are exploring integration with consumer devices. The field is characterized by increasing cross-sector collaboration between medical device manufacturers, technology companies, and research institutions to overcome remaining challenges in sensitivity, specificity, and mass production.
Koninklijke Philips NV
Technical Solution: Philips has developed an integrated plasmonic biosensor platform that combines microfluidics, surface plasmon resonance (SPR) technology, and advanced signal processing for rapid point-of-care diagnostics. Their system utilizes a proprietary gold nanostructured film with precisely engineered geometries to enhance sensitivity through localized surface plasmon resonance effects. The platform incorporates a miniaturized optical detection system with LED light sources and CMOS detectors, making it suitable for portable medical applications. Philips' technology enables multiplexed detection of various biomarkers including proteins, nucleic acids, and small molecules with minimal sample preparation. Their recent advancements include integration with their broader healthcare informatics ecosystem, allowing for immediate data transmission to electronic health records and clinical decision support systems. The company has conducted clinical validation studies demonstrating performance comparable to laboratory-based immunoassays but with significantly reduced time-to-result (under 15 minutes) and sample volume requirements.
Strengths: Strong integration capabilities with existing healthcare IT infrastructure; extensive experience in medical device regulatory pathways; global manufacturing and distribution network; comprehensive R&D resources spanning optics, microfluidics, and clinical applications. Weaknesses: Complex technology requiring integration of multiple components may increase production costs; potential challenges in achieving ultra-low detection limits compared to specialized research-grade systems; competition from both established diagnostic companies and innovative startups.
Abbott Diabetes Care, Inc.
Technical Solution: Abbott has pioneered the FreeStyle Libre continuous glucose monitoring system, which incorporates advanced biosensing technology for diabetes management. While not purely plasmonic, their latest research integrates plasmonic nanostructures with their existing electrochemical sensors to enhance sensitivity and specificity. Their approach combines gold nanoparticle-enhanced surfaces with proprietary enzyme immobilization techniques to create more stable and sensitive glucose detection. Abbott's R&D has focused on developing plasmonic-enhanced sensors that maintain accuracy over longer periods (14+ days) while minimizing foreign body responses. Their technology roadmap includes integration of LSPR (Localized Surface Plasmon Resonance) principles to enable detection of multiple analytes beyond glucose, potentially expanding into monitoring inflammatory markers and medication levels in interstitial fluid. Abbott has established manufacturing processes that can scale these complex biosensors to millions of units annually while maintaining precision and reliability.
Strengths: Extensive experience in commercializing biosensors at scale; robust regulatory expertise and established quality systems; strong distribution network and market acceptance; advanced manufacturing capabilities. Weaknesses: Current plasmonic integration remains primarily in research phase rather than commercialized products; potential increased manufacturing costs compared to current technologies; challenges in maintaining sensor stability when incorporating plasmonic elements.
Key Patents and Scientific Breakthroughs
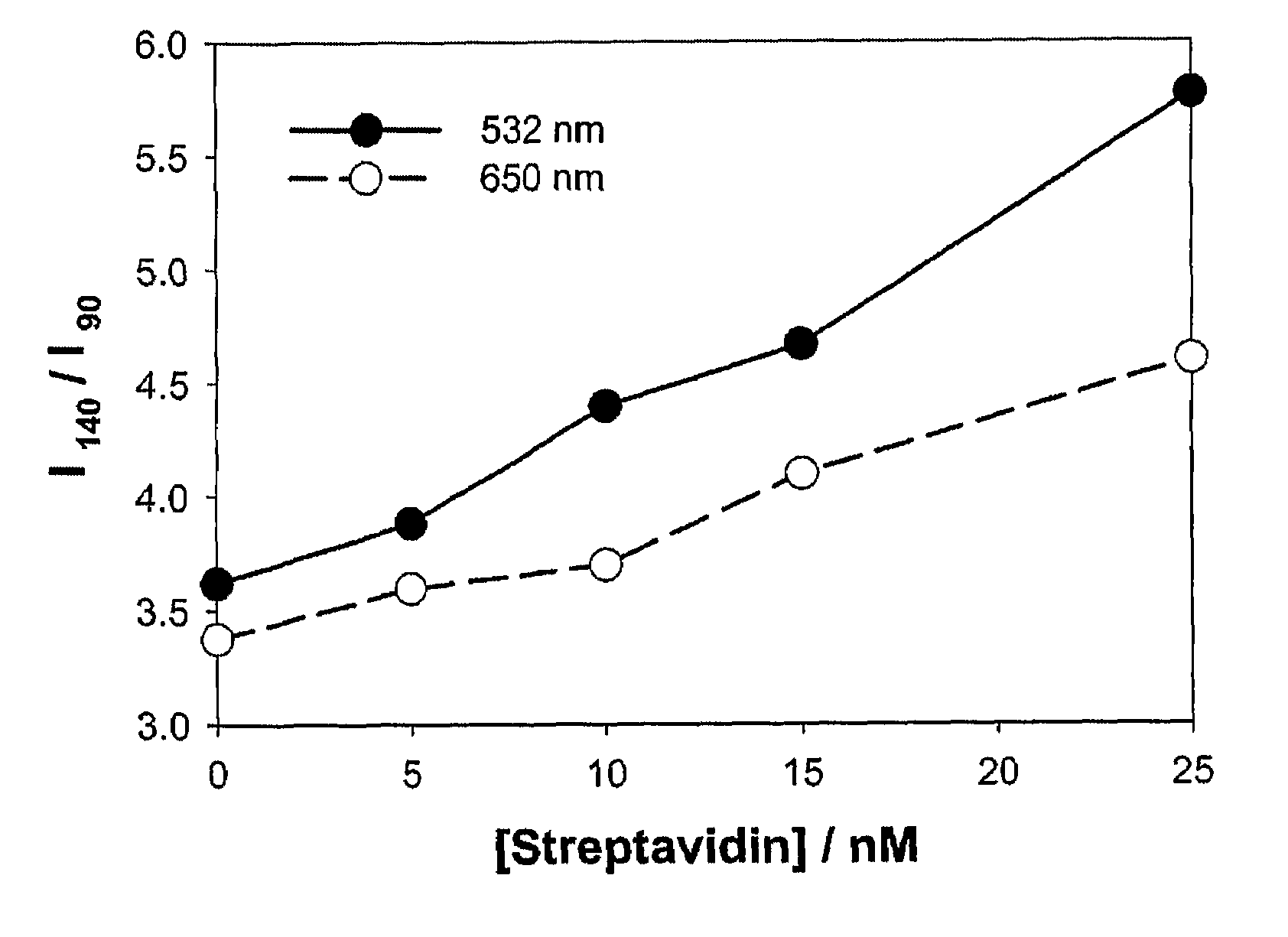
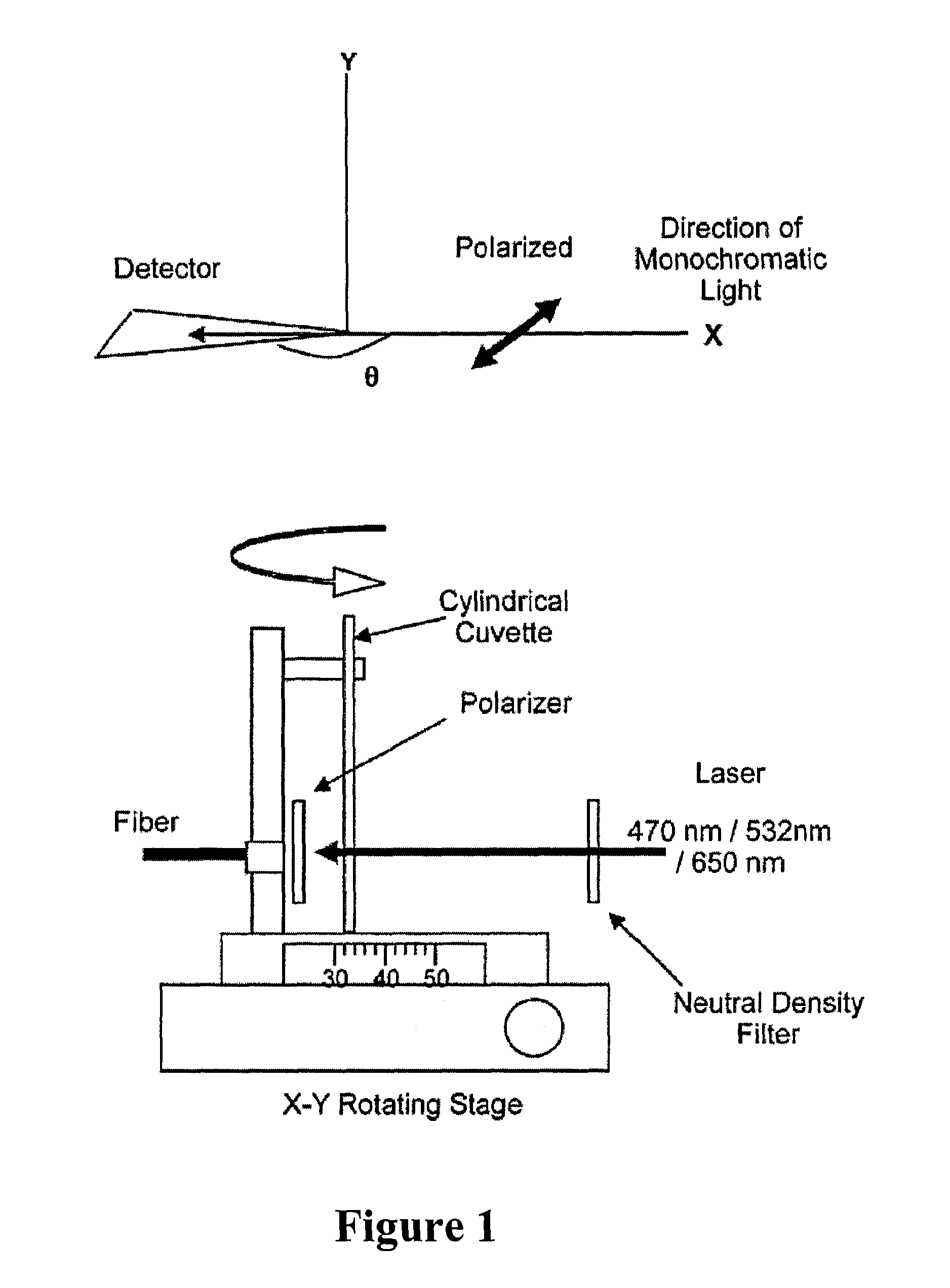
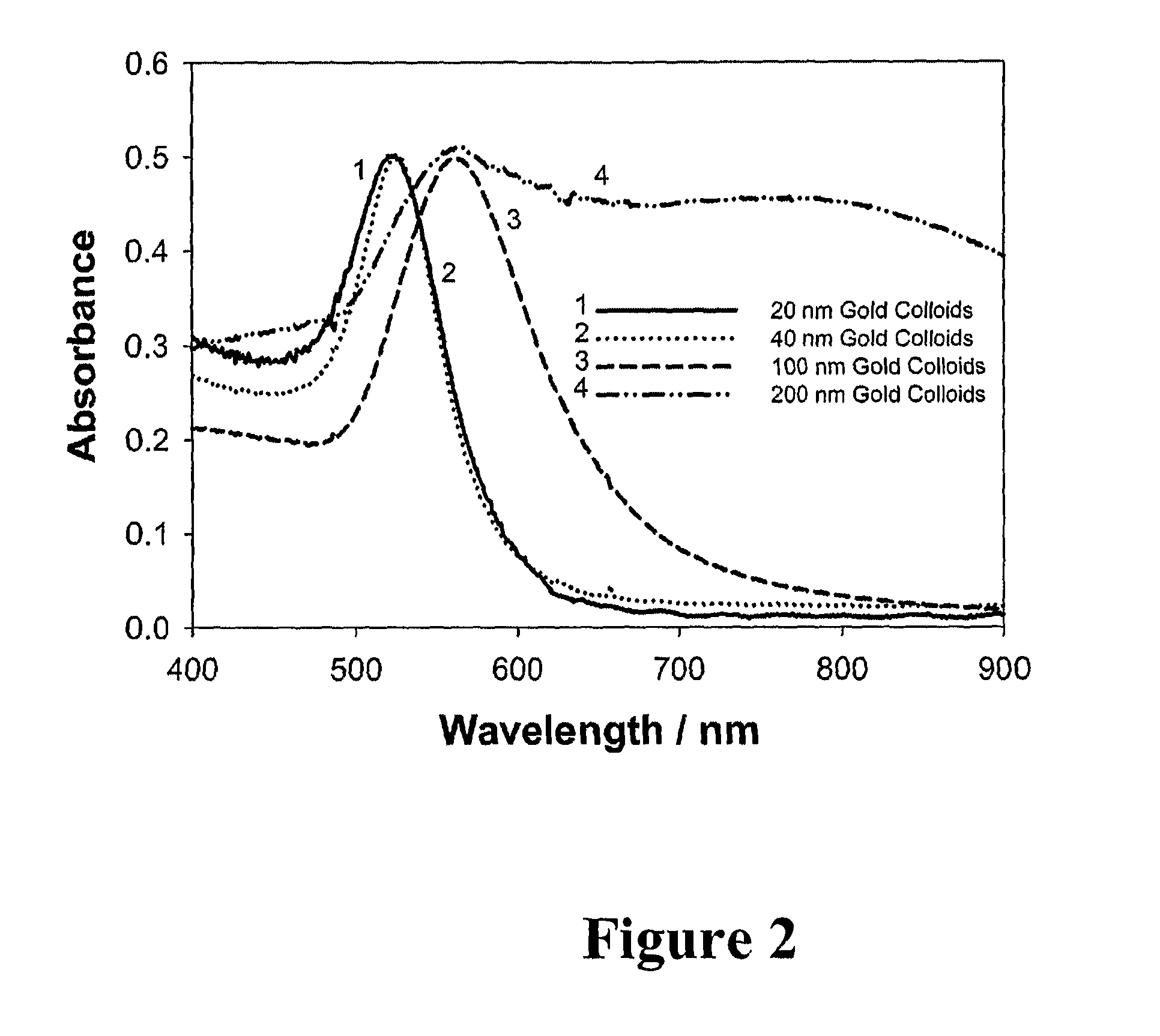
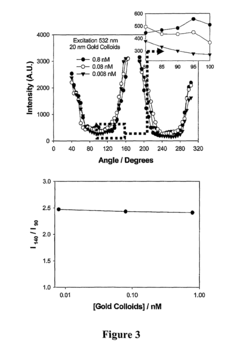
Bioassays using plasmonic scattering from noble metal nanostructures
PatentInactiveUS8101424B2
Innovation
- The use of surface plasmons from metallic nanoparticles to measure scattering effects at different angles and wavelengths, allowing for the detection of analyte concentration through changes in light intensity and polarization, which is more stable and sensitive than traditional fluorescence methods.
Device and method for detecting biomarkers
PatentInactiveEP3121587A1
Innovation
- A biosensor system utilizing localized surface plasmon resonance (LSPR) with nanostructured surfaces that change refractive index in response to biomarkers, allowing for sensitive and specific detection of CTCs and miRs without the need for specialized personnel, using biofunctionalized nanostructures and a microfluidic cell for sample interaction.
Regulatory Framework for Medical Biosensor Approval
The regulatory landscape for plasmonic biosensor integration in medicine presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA categorizes these advanced diagnostic tools primarily under Class II medical devices, requiring premarket notification (510(k)) or, in cases of novel technology applications, premarket approval (PMA). The regulatory pathway depends largely on the intended clinical application, risk profile, and degree of innovation compared to predicate devices already on the market.
European regulatory frameworks have undergone significant transformation with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), imposing more stringent requirements for clinical evidence and post-market surveillance. Plasmonic biosensors, particularly those utilizing nanomaterials, face additional scrutiny regarding biocompatibility, stability, and potential toxicity concerns.
Asian markets present varying approaches, with Japan's PMDA requiring substantial clinical validation while China's NMPA has recently streamlined approval processes for innovative medical technologies through special pathways, though still maintaining rigorous safety standards. These regional differences create significant challenges for global market entry strategies.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to standardize requirements across jurisdictions, though implementation remains inconsistent. The ISO 13485 quality management system serves as a foundational standard across most regulatory frameworks, with additional standards such as ISO 10993 for biocompatibility assessment being particularly relevant for plasmonic biosensors with direct or indirect patient contact.
Emerging regulatory considerations specifically addressing nanomaterial-based diagnostics are evolving rapidly. The FDA's guidance on nanotechnology applications in medical products provides a framework for evaluating these technologies, while the European Commission's recommendation on nanomaterial definition (2011/696/EU) influences regulatory approaches to plasmonic biosensors utilizing noble metal nanoparticles.
Reimbursement pathways represent another critical regulatory hurdle, with health technology assessment bodies requiring robust clinical utility evidence and cost-effectiveness data. The Centers for Medicare & Medicaid Services (CMS) in the US and similar bodies in other countries increasingly demand demonstration of clinical utility beyond analytical performance, creating additional barriers to market adoption.
Regulatory science is evolving to address the unique challenges posed by these advanced diagnostic platforms, with increasing focus on real-world performance data and patient-reported outcomes. Manufacturers pursuing plasmonic biosensor commercialization must engage early with regulatory authorities through pre-submission consultations to navigate this complex landscape effectively and establish appropriate validation protocols aligned with evolving regulatory expectations.
European regulatory frameworks have undergone significant transformation with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), imposing more stringent requirements for clinical evidence and post-market surveillance. Plasmonic biosensors, particularly those utilizing nanomaterials, face additional scrutiny regarding biocompatibility, stability, and potential toxicity concerns.
Asian markets present varying approaches, with Japan's PMDA requiring substantial clinical validation while China's NMPA has recently streamlined approval processes for innovative medical technologies through special pathways, though still maintaining rigorous safety standards. These regional differences create significant challenges for global market entry strategies.
Harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to standardize requirements across jurisdictions, though implementation remains inconsistent. The ISO 13485 quality management system serves as a foundational standard across most regulatory frameworks, with additional standards such as ISO 10993 for biocompatibility assessment being particularly relevant for plasmonic biosensors with direct or indirect patient contact.
Emerging regulatory considerations specifically addressing nanomaterial-based diagnostics are evolving rapidly. The FDA's guidance on nanotechnology applications in medical products provides a framework for evaluating these technologies, while the European Commission's recommendation on nanomaterial definition (2011/696/EU) influences regulatory approaches to plasmonic biosensors utilizing noble metal nanoparticles.
Reimbursement pathways represent another critical regulatory hurdle, with health technology assessment bodies requiring robust clinical utility evidence and cost-effectiveness data. The Centers for Medicare & Medicaid Services (CMS) in the US and similar bodies in other countries increasingly demand demonstration of clinical utility beyond analytical performance, creating additional barriers to market adoption.
Regulatory science is evolving to address the unique challenges posed by these advanced diagnostic platforms, with increasing focus on real-world performance data and patient-reported outcomes. Manufacturers pursuing plasmonic biosensor commercialization must engage early with regulatory authorities through pre-submission consultations to navigate this complex landscape effectively and establish appropriate validation protocols aligned with evolving regulatory expectations.
Bioethical Considerations in Plasmonic Biosensor Implementation
The integration of plasmonic biosensors into medical practice raises significant bioethical considerations that must be addressed proactively. Patient autonomy stands as a paramount concern, requiring robust informed consent protocols that clearly communicate the capabilities, limitations, and potential implications of plasmonic biosensor data. Healthcare providers must ensure patients understand how these advanced sensing technologies function and how their biological data will be utilized in diagnostic and treatment decisions.
Privacy and data security present another critical dimension, as plasmonic biosensors generate highly sensitive biological information that could potentially reveal genetic predispositions, disease states, or other intimate health details. The miniaturization and potential for continuous monitoring capabilities of these sensors amplify these concerns, necessitating stringent data protection frameworks and clear policies regarding data ownership, storage duration, and permissible uses.
Equity in healthcare access represents a significant ethical challenge, as advanced plasmonic biosensor technologies may initially be available only to privileged populations or healthcare systems. This technological disparity could exacerbate existing healthcare inequalities unless deliberate efforts are made to ensure equitable distribution and accessibility across diverse socioeconomic and geographic contexts.
The potential for incidental findings presents unique ethical dilemmas. Plasmonic biosensors may detect unexpected biological markers or health conditions beyond their intended diagnostic targets. Medical institutions must develop clear protocols for managing such discoveries, including determining when and how to communicate these findings to patients, particularly when actionable interventions may not exist.
Regulatory frameworks must evolve in parallel with these technologies, balancing innovation with appropriate safeguards. Current medical device regulations may prove inadequate for addressing the unique capabilities of plasmonic biosensors, particularly regarding their potential for continuous monitoring and the generation of massive biological datasets. International harmonization of these regulations will be essential as these technologies cross borders.
Professional ethics for healthcare providers must also adapt, with new guidelines needed for interpreting and acting upon plasmonic biosensor data. This includes addressing questions of clinical responsibility, appropriate intervention thresholds, and the integration of sensor data with traditional diagnostic approaches. Medical education will need to incorporate training on these ethical dimensions alongside technical instruction on biosensor implementation.
Privacy and data security present another critical dimension, as plasmonic biosensors generate highly sensitive biological information that could potentially reveal genetic predispositions, disease states, or other intimate health details. The miniaturization and potential for continuous monitoring capabilities of these sensors amplify these concerns, necessitating stringent data protection frameworks and clear policies regarding data ownership, storage duration, and permissible uses.
Equity in healthcare access represents a significant ethical challenge, as advanced plasmonic biosensor technologies may initially be available only to privileged populations or healthcare systems. This technological disparity could exacerbate existing healthcare inequalities unless deliberate efforts are made to ensure equitable distribution and accessibility across diverse socioeconomic and geographic contexts.
The potential for incidental findings presents unique ethical dilemmas. Plasmonic biosensors may detect unexpected biological markers or health conditions beyond their intended diagnostic targets. Medical institutions must develop clear protocols for managing such discoveries, including determining when and how to communicate these findings to patients, particularly when actionable interventions may not exist.
Regulatory frameworks must evolve in parallel with these technologies, balancing innovation with appropriate safeguards. Current medical device regulations may prove inadequate for addressing the unique capabilities of plasmonic biosensors, particularly regarding their potential for continuous monitoring and the generation of massive biological datasets. International harmonization of these regulations will be essential as these technologies cross borders.
Professional ethics for healthcare providers must also adapt, with new guidelines needed for interpreting and acting upon plasmonic biosensor data. This includes addressing questions of clinical responsibility, appropriate intervention thresholds, and the integration of sensor data with traditional diagnostic approaches. Medical education will need to incorporate training on these ethical dimensions alongside technical instruction on biosensor implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!