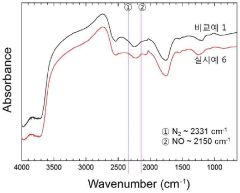
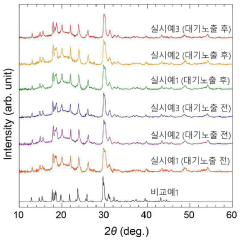
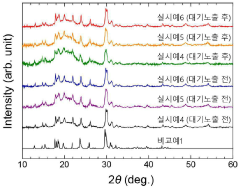
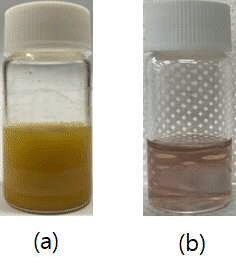
Air stability improvement in sulfide-based sodium electrolytes
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfide Electrolyte Air Stability Background and Objectives
Sulfide-based solid electrolytes have emerged as promising candidates for next-generation sodium-ion batteries due to their high ionic conductivity and potential to enable high-energy-density solid-state batteries. The development of these materials traces back to the early 2000s, when researchers began exploring alternatives to liquid electrolytes to address safety concerns and enhance energy density. Over the past decade, significant advancements have been made in improving the ionic conductivity of sulfide-based sodium electrolytes, with some materials now approaching or exceeding 10^-3 S/cm at room temperature, comparable to liquid electrolytes.
The technological evolution in this field has been characterized by a shift from glass-ceramic compositions to crystalline structures with optimized sodium ion transport pathways. Notable milestones include the discovery of Na3PS4 as a promising sodium superionic conductor in 2012, followed by various compositional and structural modifications to enhance its properties. Recent trends indicate growing interest in argyrodite-type Na-containing sulfides and mixed-anion systems that combine sulfide with other anion species to balance conductivity and stability.
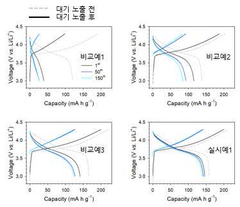
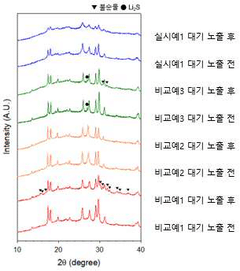
Despite these advances, the extreme sensitivity of sulfide-based electrolytes to air remains a critical challenge. When exposed to moisture and oxygen, these materials rapidly degrade, forming hydrogen sulfide gas and various oxysulfide compounds. This degradation not only compromises the electrolyte's performance but also poses safety and manufacturing challenges, limiting the commercial viability of sulfide-based sodium batteries.
The primary technical objective of current research is to develop sulfide-based sodium electrolytes with significantly improved air stability while maintaining their superior ionic conductivity. Specific goals include extending the air exposure tolerance from seconds to hours or days, eliminating or substantially reducing H2S gas formation, and developing practical protection strategies that can be implemented in industrial manufacturing settings.
Secondary objectives focus on understanding the fundamental degradation mechanisms at the molecular level, establishing standardized protocols for evaluating air stability, and exploring the correlation between chemical composition, crystal structure, and air stability. Additionally, researchers aim to develop cost-effective and scalable synthesis methods that can be conducted in ambient conditions rather than requiring inert atmospheres, which would significantly reduce manufacturing costs and complexity.
The achievement of these objectives would represent a paradigm shift in solid-state battery technology, potentially enabling the mass production of high-performance, safe sodium-ion batteries that could complement or even replace current lithium-ion technology in various applications, particularly in grid-scale energy storage where cost considerations are paramount.
The technological evolution in this field has been characterized by a shift from glass-ceramic compositions to crystalline structures with optimized sodium ion transport pathways. Notable milestones include the discovery of Na3PS4 as a promising sodium superionic conductor in 2012, followed by various compositional and structural modifications to enhance its properties. Recent trends indicate growing interest in argyrodite-type Na-containing sulfides and mixed-anion systems that combine sulfide with other anion species to balance conductivity and stability.
Despite these advances, the extreme sensitivity of sulfide-based electrolytes to air remains a critical challenge. When exposed to moisture and oxygen, these materials rapidly degrade, forming hydrogen sulfide gas and various oxysulfide compounds. This degradation not only compromises the electrolyte's performance but also poses safety and manufacturing challenges, limiting the commercial viability of sulfide-based sodium batteries.
The primary technical objective of current research is to develop sulfide-based sodium electrolytes with significantly improved air stability while maintaining their superior ionic conductivity. Specific goals include extending the air exposure tolerance from seconds to hours or days, eliminating or substantially reducing H2S gas formation, and developing practical protection strategies that can be implemented in industrial manufacturing settings.
Secondary objectives focus on understanding the fundamental degradation mechanisms at the molecular level, establishing standardized protocols for evaluating air stability, and exploring the correlation between chemical composition, crystal structure, and air stability. Additionally, researchers aim to develop cost-effective and scalable synthesis methods that can be conducted in ambient conditions rather than requiring inert atmospheres, which would significantly reduce manufacturing costs and complexity.
The achievement of these objectives would represent a paradigm shift in solid-state battery technology, potentially enabling the mass production of high-performance, safe sodium-ion batteries that could complement or even replace current lithium-ion technology in various applications, particularly in grid-scale energy storage where cost considerations are paramount.
Market Analysis for Air-Stable Sodium Electrolytes
The global market for sodium-ion batteries is experiencing significant growth, with a projected CAGR of 18.7% from 2023 to 2030. This expansion is primarily driven by the increasing demand for cost-effective energy storage solutions and the strategic advantages of sodium over lithium in terms of resource abundance and geographical distribution. Within this broader market, air-stable sulfide-based sodium electrolytes represent a critical technological component with substantial commercial potential.
The current market for sodium electrolytes is segmented into liquid electrolytes, solid-state electrolytes, and hybrid systems. Sulfide-based solid electrolytes have gained particular attention due to their high ionic conductivity, which approaches that of liquid electrolytes while offering enhanced safety profiles. However, their extreme sensitivity to air has significantly limited commercial adoption and manufacturing scalability.
Market research indicates that solving the air stability challenge could unlock a specialized segment valued at approximately $1.2 billion by 2028. Industries showing the strongest demand include grid-scale energy storage, electric vehicles in cost-sensitive markets, and portable electronics seeking alternatives to lithium-ion technologies.
Geographically, the market shows distinct regional characteristics. Asia-Pacific dominates research and development activities, with China, Japan, and South Korea leading commercial initiatives. European markets demonstrate growing interest driven by sustainability regulations and circular economy principles, while North American players focus on grid applications and defense-related energy storage solutions.
Consumer electronics manufacturers represent early adopters seeking differentiation through longer-lasting, safer battery technologies. Meanwhile, utility companies are exploring sodium-based solutions for grid stabilization and renewable energy integration, particularly in regions with limited lithium resources or geopolitical concerns about supply chain security.
Market barriers include competition from established lithium-ion technologies, technical performance gaps, and manufacturing challenges. However, economic analyses suggest that air-stable sodium electrolytes could achieve a 30-40% cost reduction compared to equivalent lithium-ion systems once production scales, primarily due to lower raw material costs and potentially simplified manufacturing processes that wouldn't require strictly controlled environments.
Investment trends show increasing venture capital interest, with funding for sodium battery technologies growing by 65% between 2020 and 2022. Strategic partnerships between electrolyte developers, battery manufacturers, and end-users are emerging as the preferred commercialization pathway, creating integrated value chains that can accelerate market adoption.
The current market for sodium electrolytes is segmented into liquid electrolytes, solid-state electrolytes, and hybrid systems. Sulfide-based solid electrolytes have gained particular attention due to their high ionic conductivity, which approaches that of liquid electrolytes while offering enhanced safety profiles. However, their extreme sensitivity to air has significantly limited commercial adoption and manufacturing scalability.
Market research indicates that solving the air stability challenge could unlock a specialized segment valued at approximately $1.2 billion by 2028. Industries showing the strongest demand include grid-scale energy storage, electric vehicles in cost-sensitive markets, and portable electronics seeking alternatives to lithium-ion technologies.
Geographically, the market shows distinct regional characteristics. Asia-Pacific dominates research and development activities, with China, Japan, and South Korea leading commercial initiatives. European markets demonstrate growing interest driven by sustainability regulations and circular economy principles, while North American players focus on grid applications and defense-related energy storage solutions.
Consumer electronics manufacturers represent early adopters seeking differentiation through longer-lasting, safer battery technologies. Meanwhile, utility companies are exploring sodium-based solutions for grid stabilization and renewable energy integration, particularly in regions with limited lithium resources or geopolitical concerns about supply chain security.
Market barriers include competition from established lithium-ion technologies, technical performance gaps, and manufacturing challenges. However, economic analyses suggest that air-stable sodium electrolytes could achieve a 30-40% cost reduction compared to equivalent lithium-ion systems once production scales, primarily due to lower raw material costs and potentially simplified manufacturing processes that wouldn't require strictly controlled environments.
Investment trends show increasing venture capital interest, with funding for sodium battery technologies growing by 65% between 2020 and 2022. Strategic partnerships between electrolyte developers, battery manufacturers, and end-users are emerging as the preferred commercialization pathway, creating integrated value chains that can accelerate market adoption.
Current Challenges in Sulfide-Based Electrolyte Stability
Sulfide-based solid electrolytes have emerged as promising candidates for next-generation sodium batteries due to their high ionic conductivity and favorable mechanical properties. However, their extreme sensitivity to moisture and oxygen presents significant challenges for practical implementation. When exposed to ambient air, these materials rapidly degrade through hydrolysis reactions, producing toxic hydrogen sulfide gas and forming insulating oxide/hydroxide layers that severely compromise ionic conductivity.
The reaction kinetics of sulfide electrolytes with moisture are particularly aggressive, occurring almost instantaneously upon air exposure. Studies have shown that even brief contact with ambient atmosphere (relative humidity >30%) can reduce ionic conductivity by several orders of magnitude within minutes. This sensitivity necessitates stringent handling protocols, including inert atmosphere gloveboxes for all processing steps, which substantially increases manufacturing complexity and costs.
Material composition significantly influences air stability, with lithium-containing sulfides generally showing higher reactivity than their sodium counterparts. However, even sodium-based sulfides like Na3PS4 and Na3SbS4 exhibit critical degradation upon air exposure. Structural factors also play a role, with glass-ceramic phases often demonstrating marginally better stability than fully crystalline counterparts, though still insufficient for practical applications.
Interface degradation represents another critical challenge. The formation of high-resistance interphases at electrode-electrolyte boundaries accelerates upon air exposure, further compromising electrochemical performance. These degradation layers not only increase internal resistance but can also trigger cascading failure mechanisms throughout the cell architecture.
Current mitigation strategies include surface coating technologies, compositional modifications, and controlled partial oxidation approaches. Alumina, lithium phosphorus oxynitride (LiPON), and hydrophobic polymer coatings have shown some promise in laboratory settings, but scalable solutions remain elusive. Partial substitution of sulfur with oxygen or nitrogen has demonstrated improved stability in some systems, though often at the expense of ionic conductivity.
The economic implications of these stability issues are substantial. The requirement for dry-room or inert-atmosphere manufacturing significantly increases production costs compared to conventional liquid electrolyte systems. Industry estimates suggest that air-stable sulfide electrolytes could reduce manufacturing costs by 30-40%, representing a critical threshold for commercial viability.
Regulatory and safety concerns further complicate development efforts. The potential for H2S generation during accidental air exposure creates significant handling and transportation challenges, necessitating robust encapsulation strategies and safety protocols that add additional layers of complexity to product development.
The reaction kinetics of sulfide electrolytes with moisture are particularly aggressive, occurring almost instantaneously upon air exposure. Studies have shown that even brief contact with ambient atmosphere (relative humidity >30%) can reduce ionic conductivity by several orders of magnitude within minutes. This sensitivity necessitates stringent handling protocols, including inert atmosphere gloveboxes for all processing steps, which substantially increases manufacturing complexity and costs.
Material composition significantly influences air stability, with lithium-containing sulfides generally showing higher reactivity than their sodium counterparts. However, even sodium-based sulfides like Na3PS4 and Na3SbS4 exhibit critical degradation upon air exposure. Structural factors also play a role, with glass-ceramic phases often demonstrating marginally better stability than fully crystalline counterparts, though still insufficient for practical applications.
Interface degradation represents another critical challenge. The formation of high-resistance interphases at electrode-electrolyte boundaries accelerates upon air exposure, further compromising electrochemical performance. These degradation layers not only increase internal resistance but can also trigger cascading failure mechanisms throughout the cell architecture.
Current mitigation strategies include surface coating technologies, compositional modifications, and controlled partial oxidation approaches. Alumina, lithium phosphorus oxynitride (LiPON), and hydrophobic polymer coatings have shown some promise in laboratory settings, but scalable solutions remain elusive. Partial substitution of sulfur with oxygen or nitrogen has demonstrated improved stability in some systems, though often at the expense of ionic conductivity.
The economic implications of these stability issues are substantial. The requirement for dry-room or inert-atmosphere manufacturing significantly increases production costs compared to conventional liquid electrolyte systems. Industry estimates suggest that air-stable sulfide electrolytes could reduce manufacturing costs by 30-40%, representing a critical threshold for commercial viability.
Regulatory and safety concerns further complicate development efforts. The potential for H2S generation during accidental air exposure creates significant handling and transportation challenges, necessitating robust encapsulation strategies and safety protocols that add additional layers of complexity to product development.
Current Approaches to Air Stability Enhancement
01 Protective coatings for sulfide-based sodium electrolytes
Various protective coating materials can be applied to sulfide-based sodium electrolytes to enhance their air stability. These coatings act as barriers against moisture and oxygen, preventing degradation of the electrolyte when exposed to air. Materials such as metal oxides, polymers, and carbon-based compounds can form effective protective layers while maintaining ionic conductivity. These coatings significantly extend the shelf life and handling capabilities of sulfide electrolytes under ambient conditions.- Protective coatings for sulfide-based sodium electrolytes: Applying protective coatings on sulfide-based sodium electrolytes can significantly improve their air stability. These coatings act as barriers against moisture and oxygen, preventing degradation when exposed to air. Various coating materials such as metal oxides, polymers, and carbon-based materials can be used to encapsulate the electrolyte particles, effectively isolating them from atmospheric contaminants while maintaining ionic conductivity.
- Chemical modification of sulfide electrolyte composition: Modifying the chemical composition of sulfide-based sodium electrolytes by incorporating stabilizing elements or compounds can enhance their air stability. Substitution or doping with elements like germanium, tin, or halides can create more stable chemical bonds within the electrolyte structure. These modifications reduce reactivity with moisture and oxygen while maintaining or even improving ionic conductivity properties essential for battery performance.
- Composite electrolyte systems with enhanced stability: Developing composite electrolyte systems by combining sulfide-based sodium electrolytes with other materials can improve air stability. These composites often incorporate oxide-based components, polymer matrices, or ceramic additives that provide protection against atmospheric degradation. The composite structure creates synergistic effects where the secondary components shield the sulfide electrolytes from air exposure while maintaining efficient sodium ion transport pathways.
- Processing techniques for air-stable sulfide electrolytes: Specialized processing techniques can significantly improve the air stability of sulfide-based sodium electrolytes. These include controlled atmosphere synthesis, surface passivation treatments, and particle engineering approaches. Techniques such as dry processing, mechanical alloying under inert conditions, and rapid solidification methods can produce electrolytes with reduced surface area or modified surface chemistry that are less reactive with air components.
- Encapsulation and packaging solutions: Innovative encapsulation and packaging solutions can protect sulfide-based sodium electrolytes from air exposure during handling, storage, and battery assembly. These include hermetic sealing technologies, specialized packaging materials with low moisture permeability, and in-situ formation approaches where the electrolyte is generated within the protected battery environment. Such solutions enable practical application of these highly conductive but air-sensitive materials in commercial sodium batteries.
02 Chemical modification of sulfide electrolyte composition
Chemical modification strategies involve altering the composition of sulfide-based sodium electrolytes to inherently improve their air stability. This includes partial substitution of sulfur with more stable elements, incorporation of stabilizing additives, or creation of composite structures. These modifications can reduce reactivity with moisture and oxygen while maintaining or even enhancing ionic conductivity. The modified electrolyte compositions demonstrate significantly improved tolerance to air exposure during processing and application in sodium batteries.Expand Specific Solutions03 Encapsulation techniques for air-sensitive electrolytes
Encapsulation methods involve completely sealing sulfide-based sodium electrolytes within protective matrices or structures to prevent air contact. These techniques include core-shell structures, polymer encapsulation, or integration within specialized composite frameworks. The encapsulation materials are designed to be impermeable to moisture and oxygen while allowing sodium ion transport. This approach enables handling of highly air-sensitive sulfide electrolytes under normal atmospheric conditions without significant performance degradation.Expand Specific Solutions04 Surface passivation treatments
Surface passivation involves treating the surface of sulfide-based sodium electrolytes to create a thin, stable layer that prevents further reaction with air components. These treatments can include controlled pre-oxidation, chemical surface reactions, or plasma treatments that convert the reactive surface to a more stable form. The passivation layer acts as a protective barrier while maintaining good interfacial contact and ion transport properties. This approach significantly improves the air handling tolerance of sulfide electrolytes without compromising their electrochemical performance.Expand Specific Solutions05 Solid-state interface engineering
Interface engineering focuses on designing and controlling the interfaces between sulfide-based sodium electrolytes and electrodes to enhance overall stability. This includes development of buffer layers, gradient compositions, or specialized interface materials that prevent degradation reactions. By creating stable interfaces, these approaches minimize the formation of high-resistance layers that typically form when sulfide electrolytes react with air components. These engineered interfaces maintain good ionic conductivity while providing protection against atmospheric exposure.Expand Specific Solutions
Leading Research Groups and Industrial Players
The sodium-ion battery market is in an early growth phase, with significant research momentum in sulfide-based electrolyte stability. The global market is projected to expand rapidly as companies seek alternatives to lithium-ion technologies. Leading players like LG Energy Solution, Samsung SDI, and SVOLT Energy are investing heavily in R&D to overcome air stability challenges in sulfide electrolytes. Research institutions including KIST, Chinese Academy of Sciences, and Beijing Institute of Technology are making breakthrough contributions. Japanese firms such as Mitsui Kinzoku and GS Yuasa are leveraging their materials expertise, while Korean companies like Ecopro BM focus on commercialization pathways. The technology remains pre-commercial but is advancing rapidly through collaborative industry-academic partnerships.
LG Chem Ltd.
Technical Solution: LG Chem has developed a multi-layered protection strategy for sulfide-based sodium electrolytes that addresses air stability issues. Their approach involves surface modification of Na3PS4 particles with sodium phosphate compounds, creating a protective barrier that prevents moisture and oxygen penetration. The company has implemented a core-shell structure where the sulfide electrolyte core is encapsulated by an air-stable phosphate shell, effectively reducing hydrolysis reactions that produce toxic H2S gas. Additionally, LG Chem utilizes specialized polymer coatings with hydrophobic properties to further enhance moisture resistance while maintaining ionic conductivity. Their manufacturing process incorporates strict environmental controls, including dry room facilities with <0.1% relative humidity to prevent degradation during production. Recent advancements include the development of composite electrolytes that combine the high ionic conductivity of sulfides with the stability of oxide materials.
Strengths: Superior ionic conductivity retention after air exposure compared to unmodified sulfides; scalable manufacturing process compatible with existing battery production lines; reduced H2S gas generation improves safety profile. Weaknesses: The protective coatings may slightly reduce initial ionic conductivity; additional processing steps increase production costs; long-term stability under real-world operating conditions still requires further validation.
Korea Electrotechnology Research Institute
Technical Solution: Korea Electrotechnology Research Institute (KERI) has developed a comprehensive solution for air stability in sulfide-based sodium electrolytes through their "StableSulfide" technology platform. Their approach combines materials science innovations with advanced processing techniques to create inherently more stable electrolyte systems. KERI's primary strategy involves atomic-level substitution within the sulfide structure, where selected sulfur atoms are replaced with more electronegativity-matched elements like selenium or tellurium, creating stronger bonds that resist hydrolysis. Additionally, they've pioneered a surface passivation technique using lithium phosphorus oxynitride (LiPON) analogs specifically adapted for sodium systems, which forms a nanometer-thick protective layer that blocks moisture penetration while allowing sodium ion transport. Their manufacturing protocol incorporates specialized dry processing equipment with integrated glove box systems that maintain inert atmospheres throughout production. KERI has also developed composite electrolyte formulations that strategically combine sulfide materials with more stable components like sodium-beta-alumina, creating synergistic structures with enhanced stability. Recent testing demonstrates their protected Na3PS4-based electrolytes can maintain over 75% of original conductivity after 36 hours of exposure to ambient air conditions.
Strengths: Balanced approach that maintains high ionic conductivity while significantly improving air stability; reduced sensitivity to environmental conditions simplifies handling requirements; minimized H2S gas generation improves safety profile. Weaknesses: The atomic substitution approach may alter the intrinsic properties of the electrolyte; protection systems add complexity to synthesis processes; slightly higher material costs compared to unmodified sulfide electrolytes; optimization required for different cell chemistries.
Key Patents and Innovations in Protective Strategies
Preparation method of sulfide-based solid electrolyte having excellent air-stable proprety
PatentActiveKR1020180021616A
Innovation
- A method involving the preparation of sulfide-based solid electrolyte particles followed by treatment in a reactive gas atmosphere to form a stable layer, enhancing atmospheric stability through heat or plasma treatment, and optimizing conditions like pressure and temperature.
A method of manufacturing a sulfide based solid electrolyte with improved air-stability, a sulfide based solid electrolyte manufactured thereby, and an all-solid-state battery comprising the sulfide based solid electrolyte
PatentActiveKR1020240002109A
Innovation
- A method involving the use of a co-solvent comprising an amine-based and thiol-based solvent to disperse lithium and sulfide precursors, followed by heat-treatment under vacuum, to form sulfide-based solid electrolytes represented by specific chemical formulas, enhancing solubility and replacing part of the composition with metal chalcogenides for improved stability and conductivity.
Environmental Impact and Sustainability Assessment
The environmental impact of developing and implementing air-stable sulfide-based sodium electrolytes extends beyond their immediate technological benefits. These advanced materials offer significant sustainability advantages compared to traditional lithium-ion battery technologies, particularly in terms of resource availability and environmental footprint.
Sodium resources are approximately 1000 times more abundant than lithium in the Earth's crust, making sodium-based energy storage systems inherently more sustainable from a resource perspective. The widespread geographical distribution of sodium resources also reduces geopolitical supply risks and potentially lowers the environmental impact associated with resource extraction and transportation.
However, the production of sulfide-based electrolytes presents specific environmental challenges. The synthesis processes often involve energy-intensive steps and the use of hazardous precursors such as hydrogen sulfide. The air instability of these materials necessitates controlled manufacturing environments with inert atmospheres, increasing energy consumption and infrastructure requirements. Improving air stability would significantly reduce these environmental burdens by enabling more conventional manufacturing processes.
Life cycle assessment (LCA) studies indicate that the environmental benefits of sodium-ion batteries with improved sulfide electrolytes could outweigh their production impacts. The reduced dependency on critical materials like cobalt and nickel, which are associated with significant social and environmental concerns in mining regions, represents a major sustainability advantage. Additionally, the potential for longer cycle life through enhanced electrolyte stability would extend device lifespans, reducing waste generation and resource consumption.
Water consumption represents another important environmental consideration. Current protection methods for air-sensitive sulfide electrolytes often involve organic solvents, which can pose environmental risks if not properly managed. Developing water-resistant or water-compatible sulfide electrolytes would minimize these risks while potentially reducing manufacturing complexity and associated environmental impacts.
End-of-life management also benefits from advances in air stability. More stable materials facilitate safer recycling processes, potentially increasing recovery rates of valuable elements. This circular economy approach could further enhance the sustainability profile of sodium-based energy storage technologies, creating a more environmentally responsible alternative to current battery systems.
Carbon footprint analyses suggest that the transition to sodium-based technologies with improved air-stable sulfide electrolytes could contribute to climate change mitigation efforts, particularly when coupled with renewable energy sources for manufacturing and operation. The reduced embodied energy in these systems, compared to conventional lithium-ion technologies, offers promising pathways toward more sustainable energy storage solutions.
Sodium resources are approximately 1000 times more abundant than lithium in the Earth's crust, making sodium-based energy storage systems inherently more sustainable from a resource perspective. The widespread geographical distribution of sodium resources also reduces geopolitical supply risks and potentially lowers the environmental impact associated with resource extraction and transportation.
However, the production of sulfide-based electrolytes presents specific environmental challenges. The synthesis processes often involve energy-intensive steps and the use of hazardous precursors such as hydrogen sulfide. The air instability of these materials necessitates controlled manufacturing environments with inert atmospheres, increasing energy consumption and infrastructure requirements. Improving air stability would significantly reduce these environmental burdens by enabling more conventional manufacturing processes.
Life cycle assessment (LCA) studies indicate that the environmental benefits of sodium-ion batteries with improved sulfide electrolytes could outweigh their production impacts. The reduced dependency on critical materials like cobalt and nickel, which are associated with significant social and environmental concerns in mining regions, represents a major sustainability advantage. Additionally, the potential for longer cycle life through enhanced electrolyte stability would extend device lifespans, reducing waste generation and resource consumption.
Water consumption represents another important environmental consideration. Current protection methods for air-sensitive sulfide electrolytes often involve organic solvents, which can pose environmental risks if not properly managed. Developing water-resistant or water-compatible sulfide electrolytes would minimize these risks while potentially reducing manufacturing complexity and associated environmental impacts.
End-of-life management also benefits from advances in air stability. More stable materials facilitate safer recycling processes, potentially increasing recovery rates of valuable elements. This circular economy approach could further enhance the sustainability profile of sodium-based energy storage technologies, creating a more environmentally responsible alternative to current battery systems.
Carbon footprint analyses suggest that the transition to sodium-based technologies with improved air-stable sulfide electrolytes could contribute to climate change mitigation efforts, particularly when coupled with renewable energy sources for manufacturing and operation. The reduced embodied energy in these systems, compared to conventional lithium-ion technologies, offers promising pathways toward more sustainable energy storage solutions.
Safety Protocols and Handling Requirements
Handling sulfide-based sodium electrolytes requires stringent safety protocols due to their high reactivity with moisture and air. All operations involving these materials must be conducted in controlled environments such as glove boxes with inert atmospheres (typically argon or nitrogen) maintaining oxygen and moisture levels below 0.1 ppm. Personnel must undergo specialized training focusing on proper material handling techniques and emergency response procedures before working with these electrolytes.
Personal protective equipment (PPE) requirements include chemical-resistant gloves (nitrile or butyl rubber), lab coats, safety goggles, and in some cases, face shields. When transferring materials between controlled environments, sealed containers with multiple protection layers should be utilized to minimize exposure risks. Vacuum-sealed packaging systems have proven particularly effective for maintaining material integrity during transportation.
Storage protocols dictate that sulfide-based electrolytes must be kept in hermetically sealed containers within inert atmosphere environments. Temperature-controlled storage (typically 15-25°C) is recommended to prevent degradation. All containers must be clearly labeled with hazard information, handling instructions, and expiration dates. Regular integrity checks of storage containers should be conducted to identify potential compromises in the sealing systems.
Laboratory design considerations include dedicated workspaces for sulfide electrolyte handling, equipped with appropriate ventilation systems and hydrogen sulfide gas detectors. Emergency equipment such as eyewash stations, safety showers, and spill containment kits must be readily accessible. Waste disposal procedures must comply with local regulations for reactive materials, typically requiring neutralization treatments before disposal.
Documentation requirements include detailed standard operating procedures (SOPs), material safety data sheets (MSDS), risk assessments, and incident reporting protocols. All handling activities should be logged in laboratory notebooks with particular attention to exposure times and environmental conditions. Regular safety audits should be conducted to ensure compliance with established protocols.
Cross-contamination prevention measures include dedicated tools and equipment for sulfide electrolyte handling, clear workspace segregation, and decontamination procedures for equipment that may be used across different material systems. Implementation of airlock systems between handling areas can further reduce contamination risks while maintaining material integrity.
Emergency response protocols must address potential hazards including accidental exposure to moisture resulting in hydrogen sulfide generation. Staff should be trained to recognize symptoms of H₂S exposure and implement appropriate first aid measures while awaiting medical assistance. Evacuation procedures and communication systems must be established to ensure rapid response to safety incidents.
Personal protective equipment (PPE) requirements include chemical-resistant gloves (nitrile or butyl rubber), lab coats, safety goggles, and in some cases, face shields. When transferring materials between controlled environments, sealed containers with multiple protection layers should be utilized to minimize exposure risks. Vacuum-sealed packaging systems have proven particularly effective for maintaining material integrity during transportation.
Storage protocols dictate that sulfide-based electrolytes must be kept in hermetically sealed containers within inert atmosphere environments. Temperature-controlled storage (typically 15-25°C) is recommended to prevent degradation. All containers must be clearly labeled with hazard information, handling instructions, and expiration dates. Regular integrity checks of storage containers should be conducted to identify potential compromises in the sealing systems.
Laboratory design considerations include dedicated workspaces for sulfide electrolyte handling, equipped with appropriate ventilation systems and hydrogen sulfide gas detectors. Emergency equipment such as eyewash stations, safety showers, and spill containment kits must be readily accessible. Waste disposal procedures must comply with local regulations for reactive materials, typically requiring neutralization treatments before disposal.
Documentation requirements include detailed standard operating procedures (SOPs), material safety data sheets (MSDS), risk assessments, and incident reporting protocols. All handling activities should be logged in laboratory notebooks with particular attention to exposure times and environmental conditions. Regular safety audits should be conducted to ensure compliance with established protocols.
Cross-contamination prevention measures include dedicated tools and equipment for sulfide electrolyte handling, clear workspace segregation, and decontamination procedures for equipment that may be used across different material systems. Implementation of airlock systems between handling areas can further reduce contamination risks while maintaining material integrity.
Emergency response protocols must address potential hazards including accidental exposure to moisture resulting in hydrogen sulfide generation. Staff should be trained to recognize symptoms of H₂S exposure and implement appropriate first aid measures while awaiting medical assistance. Evacuation procedures and communication systems must be established to ensure rapid response to safety incidents.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!