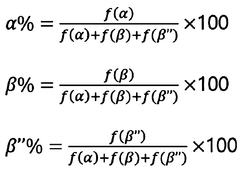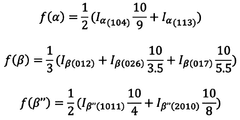Fabrication methods for dense sodium solid electrolyte ceramics
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Solid Electrolyte Development Background and Objectives
Sodium-based solid-state batteries have emerged as a promising alternative to conventional lithium-ion batteries due to the abundance and low cost of sodium resources. The development of sodium solid electrolytes (SSEs) began in the 1970s with the discovery of Na-β-alumina, which demonstrated significant sodium ion conductivity. However, early research was limited by fabrication challenges and the dominance of lithium-ion technology in the energy storage market.
Over the past decade, renewed interest in sodium-based energy storage systems has been driven by concerns about lithium resource limitations and increasing demand for large-scale energy storage solutions. This revival has catalyzed extensive research into various sodium solid electrolyte systems, including NASICON-type materials, sodium beta-alumina, and sodium halide-based electrolytes, each offering unique advantages for specific applications.
The technical evolution of sodium solid electrolytes has focused on addressing key performance metrics: ionic conductivity, mechanical stability, and electrochemical stability against electrode materials. Early sodium solid electrolytes exhibited conductivities in the range of 10^-5 to 10^-4 S/cm at room temperature, whereas recent advancements have pushed this boundary to 10^-3 S/cm, approaching the performance of liquid electrolytes.
Fabrication methods for dense sodium solid electrolyte ceramics represent a critical technological challenge. Traditional ceramic processing techniques often result in materials with high porosity, grain boundary resistance, and poor mechanical properties. The sensitivity of sodium compounds to moisture and air further complicates processing conditions, requiring specialized handling protocols and equipment.
The primary technical objectives in this field include developing scalable fabrication methods that can produce dense (>95% relative density) sodium solid electrolyte ceramics with minimal grain boundary resistance, optimizing sintering protocols to achieve desired microstructures while preventing sodium volatilization, and establishing processing techniques compatible with large-scale manufacturing requirements.
Current research trends indicate a shift toward advanced fabrication approaches such as spark plasma sintering, cold sintering, and solution-based processing methods. These techniques offer potential advantages in terms of lower processing temperatures, shorter fabrication times, and improved control over microstructure development.
The ultimate goal of sodium solid electrolyte development is to enable the commercialization of safe, high-performance, and cost-effective sodium-based solid-state batteries for applications ranging from grid-scale energy storage to electric vehicles. This requires not only advances in fundamental materials science but also innovations in manufacturing processes that can bridge the gap between laboratory-scale demonstrations and industrial production.
Over the past decade, renewed interest in sodium-based energy storage systems has been driven by concerns about lithium resource limitations and increasing demand for large-scale energy storage solutions. This revival has catalyzed extensive research into various sodium solid electrolyte systems, including NASICON-type materials, sodium beta-alumina, and sodium halide-based electrolytes, each offering unique advantages for specific applications.
The technical evolution of sodium solid electrolytes has focused on addressing key performance metrics: ionic conductivity, mechanical stability, and electrochemical stability against electrode materials. Early sodium solid electrolytes exhibited conductivities in the range of 10^-5 to 10^-4 S/cm at room temperature, whereas recent advancements have pushed this boundary to 10^-3 S/cm, approaching the performance of liquid electrolytes.
Fabrication methods for dense sodium solid electrolyte ceramics represent a critical technological challenge. Traditional ceramic processing techniques often result in materials with high porosity, grain boundary resistance, and poor mechanical properties. The sensitivity of sodium compounds to moisture and air further complicates processing conditions, requiring specialized handling protocols and equipment.
The primary technical objectives in this field include developing scalable fabrication methods that can produce dense (>95% relative density) sodium solid electrolyte ceramics with minimal grain boundary resistance, optimizing sintering protocols to achieve desired microstructures while preventing sodium volatilization, and establishing processing techniques compatible with large-scale manufacturing requirements.
Current research trends indicate a shift toward advanced fabrication approaches such as spark plasma sintering, cold sintering, and solution-based processing methods. These techniques offer potential advantages in terms of lower processing temperatures, shorter fabrication times, and improved control over microstructure development.
The ultimate goal of sodium solid electrolyte development is to enable the commercialization of safe, high-performance, and cost-effective sodium-based solid-state batteries for applications ranging from grid-scale energy storage to electric vehicles. This requires not only advances in fundamental materials science but also innovations in manufacturing processes that can bridge the gap between laboratory-scale demonstrations and industrial production.
Market Analysis for Sodium-based Battery Technologies
The sodium-ion battery market is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions and concerns about lithium resource limitations. Current market projections indicate that the global sodium-ion battery market is expected to grow at a compound annual growth rate of 18% between 2023 and 2030, reaching a market value of approximately 1.2 billion USD by 2030. This growth trajectory is supported by substantial investments in research and development from both private companies and government entities worldwide.
The market for sodium-based battery technologies is segmented across various applications, with grid energy storage emerging as the dominant sector due to the cost advantages of sodium-ion batteries compared to lithium-ion alternatives. Electric vehicles represent another promising market segment, particularly in regions where cost sensitivity outweighs energy density requirements. Consumer electronics constitutes a smaller but growing segment, especially for applications where battery cost is a critical factor.
Geographically, China leads the sodium-ion battery market, accounting for nearly 40% of global production capacity. This dominance is attributed to strategic government initiatives and substantial investments in manufacturing infrastructure. Europe follows with approximately 25% market share, driven by stringent environmental regulations and ambitious renewable energy targets. North America represents about 20% of the market, with growth accelerating due to recent policy shifts favoring domestic battery production.
From a supply chain perspective, sodium-based batteries offer significant advantages over lithium-ion technologies. Sodium is approximately 1,000 times more abundant than lithium in the Earth's crust, resulting in lower raw material costs and reduced geopolitical supply risks. This abundance translates to a potential 30-40% cost reduction compared to lithium-ion batteries at scale, making sodium-based technologies particularly attractive for price-sensitive applications.
Market adoption barriers include the lower energy density of sodium-ion batteries compared to lithium-ion counterparts, which currently limits their application in premium electric vehicles and high-performance portable electronics. Additionally, the relative immaturity of manufacturing processes for dense sodium solid electrolyte ceramics contributes to higher production costs in the short term, though these are expected to decrease as production scales.
Consumer awareness and acceptance represent another challenge, as sodium-based battery technologies are less familiar to end-users compared to established lithium-ion solutions. However, increasing concerns about the environmental impact and ethical sourcing of lithium and cobalt are creating market opportunities for alternative battery chemistries like sodium-ion.
The market for sodium-based battery technologies is segmented across various applications, with grid energy storage emerging as the dominant sector due to the cost advantages of sodium-ion batteries compared to lithium-ion alternatives. Electric vehicles represent another promising market segment, particularly in regions where cost sensitivity outweighs energy density requirements. Consumer electronics constitutes a smaller but growing segment, especially for applications where battery cost is a critical factor.
Geographically, China leads the sodium-ion battery market, accounting for nearly 40% of global production capacity. This dominance is attributed to strategic government initiatives and substantial investments in manufacturing infrastructure. Europe follows with approximately 25% market share, driven by stringent environmental regulations and ambitious renewable energy targets. North America represents about 20% of the market, with growth accelerating due to recent policy shifts favoring domestic battery production.
From a supply chain perspective, sodium-based batteries offer significant advantages over lithium-ion technologies. Sodium is approximately 1,000 times more abundant than lithium in the Earth's crust, resulting in lower raw material costs and reduced geopolitical supply risks. This abundance translates to a potential 30-40% cost reduction compared to lithium-ion batteries at scale, making sodium-based technologies particularly attractive for price-sensitive applications.
Market adoption barriers include the lower energy density of sodium-ion batteries compared to lithium-ion counterparts, which currently limits their application in premium electric vehicles and high-performance portable electronics. Additionally, the relative immaturity of manufacturing processes for dense sodium solid electrolyte ceramics contributes to higher production costs in the short term, though these are expected to decrease as production scales.
Consumer awareness and acceptance represent another challenge, as sodium-based battery technologies are less familiar to end-users compared to established lithium-ion solutions. However, increasing concerns about the environmental impact and ethical sourcing of lithium and cobalt are creating market opportunities for alternative battery chemistries like sodium-ion.
Current Challenges in Dense Ceramic Electrolyte Fabrication
Despite significant advancements in sodium solid electrolyte ceramics, fabrication of dense ceramic structures remains a formidable challenge in the field. The primary obstacle lies in achieving high relative density (>95%) while maintaining phase purity and minimizing grain boundary resistance. Conventional sintering methods often require temperatures exceeding 1200°C, which frequently triggers undesirable phase transitions and sodium volatilization, compromising ionic conductivity.
Mechanical processing challenges present another significant hurdle. The inherent brittleness of sodium ceramic electrolytes makes them susceptible to microcrack formation during grinding, polishing, and handling processes. These microcracks create non-conductive pathways that dramatically reduce overall ionic conductivity and compromise the mechanical integrity of the electrolyte.
Compositional homogeneity represents a persistent fabrication challenge. Many sodium solid electrolytes contain multiple elements that must be uniformly distributed at the atomic level. Current mixing and processing techniques often result in compositional gradients and secondary phase formation, particularly at grain boundaries, which act as barriers to sodium ion transport.
Scalability issues further complicate industrial adoption. Laboratory-scale fabrication methods that produce high-quality samples often fail to translate effectively to mass production environments. Techniques like spark plasma sintering (SPS) and hot pressing yield excellent results but face significant barriers to scaling due to equipment limitations and high operational costs.
Environmental sensitivity poses additional complications during fabrication. Many sodium-based ceramic precursors are hygroscopic, readily reacting with atmospheric moisture and CO2. This necessitates stringent handling protocols in controlled environments, adding complexity and cost to the manufacturing process.
Interface engineering between the electrolyte and electrodes remains problematic. Current fabrication methods struggle to create clean, intimate contacts at these critical interfaces. Poor interfacial contact leads to high resistance and accelerated degradation during cycling, significantly limiting device performance and longevity.
Reproducibility challenges persist across different production batches. Minor variations in raw materials, processing parameters, and environmental conditions can lead to significant differences in the final electrolyte properties. This inconsistency hampers quality control efforts and complicates the establishment of standardized fabrication protocols necessary for commercial viability.
Addressing these challenges requires interdisciplinary approaches combining materials science, chemical engineering, and advanced manufacturing techniques. Recent research has begun exploring novel fabrication routes including solution-based processing, cold sintering, and additive manufacturing technologies, which show promise for overcoming these persistent obstacles.
Mechanical processing challenges present another significant hurdle. The inherent brittleness of sodium ceramic electrolytes makes them susceptible to microcrack formation during grinding, polishing, and handling processes. These microcracks create non-conductive pathways that dramatically reduce overall ionic conductivity and compromise the mechanical integrity of the electrolyte.
Compositional homogeneity represents a persistent fabrication challenge. Many sodium solid electrolytes contain multiple elements that must be uniformly distributed at the atomic level. Current mixing and processing techniques often result in compositional gradients and secondary phase formation, particularly at grain boundaries, which act as barriers to sodium ion transport.
Scalability issues further complicate industrial adoption. Laboratory-scale fabrication methods that produce high-quality samples often fail to translate effectively to mass production environments. Techniques like spark plasma sintering (SPS) and hot pressing yield excellent results but face significant barriers to scaling due to equipment limitations and high operational costs.
Environmental sensitivity poses additional complications during fabrication. Many sodium-based ceramic precursors are hygroscopic, readily reacting with atmospheric moisture and CO2. This necessitates stringent handling protocols in controlled environments, adding complexity and cost to the manufacturing process.
Interface engineering between the electrolyte and electrodes remains problematic. Current fabrication methods struggle to create clean, intimate contacts at these critical interfaces. Poor interfacial contact leads to high resistance and accelerated degradation during cycling, significantly limiting device performance and longevity.
Reproducibility challenges persist across different production batches. Minor variations in raw materials, processing parameters, and environmental conditions can lead to significant differences in the final electrolyte properties. This inconsistency hampers quality control efforts and complicates the establishment of standardized fabrication protocols necessary for commercial viability.
Addressing these challenges requires interdisciplinary approaches combining materials science, chemical engineering, and advanced manufacturing techniques. Recent research has begun exploring novel fabrication routes including solution-based processing, cold sintering, and additive manufacturing technologies, which show promise for overcoming these persistent obstacles.
State-of-the-Art Fabrication Methods for Dense Ceramic Electrolytes
01 Density optimization of sodium solid electrolyte ceramics
The density of sodium solid electrolyte ceramics can be optimized through various processing techniques such as sintering conditions, pressure application, and temperature control. Higher density ceramics typically exhibit improved ionic conductivity and mechanical strength, which are crucial for battery applications. Optimization methods include hot pressing, spark plasma sintering, and controlled cooling rates to achieve densities approaching theoretical limits.- Density optimization in sodium solid electrolyte ceramics: Optimizing the density of sodium solid electrolyte ceramics is crucial for enhancing ionic conductivity and mechanical strength. Various processing techniques such as sintering parameters adjustment, pressure application during fabrication, and particle size control can be employed to achieve higher density ceramics. Higher density typically results in reduced porosity, which minimizes resistance to ion transport and improves overall battery performance.
- Composition effects on density of sodium solid electrolytes: The chemical composition of sodium solid electrolytes significantly impacts their density properties. Incorporating specific dopants, adjusting sodium content, and using various ceramic formulations can tailor the density characteristics. Certain additives can promote densification during sintering while maintaining or enhancing ionic conductivity. The ratio of different components in multi-phase ceramics also plays a crucial role in determining the final density of the electrolyte material.
- Manufacturing processes for high-density sodium ceramic electrolytes: Advanced manufacturing processes have been developed to achieve high-density sodium ceramic electrolytes. These include cold isostatic pressing, hot pressing, spark plasma sintering, and solution-based synthesis methods. Each technique offers different advantages in terms of achieving uniform density, controlling grain growth, and minimizing defects. The selection of appropriate manufacturing processes is essential for producing sodium solid electrolytes with optimal density characteristics for specific applications.
- Relationship between density and electrochemical performance: The density of sodium solid electrolyte ceramics directly influences their electrochemical performance. Higher density materials generally exhibit superior ionic conductivity, reduced interfacial resistance, and improved cycling stability. However, extremely high densities may sometimes limit sodium ion mobility. Finding the optimal density range is crucial for balancing mechanical integrity with efficient ion transport properties, ultimately affecting battery performance metrics such as capacity, rate capability, and cycle life.
- Characterization and measurement of density in sodium solid electrolytes: Various techniques are employed to characterize and measure the density of sodium solid electrolyte ceramics. These include Archimedes method, helium pycnometry, X-ray diffraction analysis, and microscopy techniques. Accurate density measurement is essential for quality control and performance prediction. The relative density (compared to theoretical maximum) is often used as a key parameter to evaluate the effectiveness of fabrication processes and to correlate with electrochemical properties of the solid electrolyte materials.
02 Composition effects on density of sodium solid electrolytes
The chemical composition of sodium solid electrolytes significantly impacts their density. Various dopants and additives can be incorporated to modify the crystal structure and enhance densification. Elements such as zirconium, aluminum, and rare earth metals can be added to sodium-based ceramics to control grain growth and pore formation during sintering, resulting in higher density materials with improved electrochemical performance.Expand Specific Solutions03 Microstructural control for density enhancement
Controlling the microstructure of sodium solid electrolyte ceramics is essential for achieving optimal density. Techniques such as grain size regulation, pore elimination, and interface engineering can lead to denser materials. Methods include using nanostructured precursors, applying mechanical activation, and implementing two-step sintering processes to minimize porosity and maximize density, which directly correlates with improved ionic conductivity.Expand Specific Solutions04 Relationship between density and ionic conductivity
The density of sodium solid electrolyte ceramics has a direct impact on their ionic conductivity. Higher density materials typically exhibit fewer grain boundaries and reduced porosity, leading to enhanced sodium ion transport. Research shows that achieving relative densities above 95% of theoretical density can significantly improve conductivity performance, making density optimization a critical factor in developing efficient solid-state sodium batteries.Expand Specific Solutions05 Manufacturing techniques for high-density sodium ceramics
Various manufacturing techniques have been developed to produce high-density sodium solid electrolyte ceramics. These include advanced sintering methods like microwave sintering, cold isostatic pressing followed by conventional sintering, and solution-based synthesis approaches. Novel fabrication routes such as 3D printing combined with post-processing treatments can also yield ceramics with controlled density profiles tailored for specific electrochemical applications.Expand Specific Solutions
Leading Companies and Research Institutions in Solid Electrolyte Field
The fabrication of dense sodium solid electrolyte ceramics is currently in a transitional phase from research to early commercialization, with a global market expected to grow significantly as solid-state battery technologies mature. The competitive landscape features established materials companies like Murata Manufacturing and Toda Kogyo developing advanced ceramic processing techniques, while energy sector players including Idemitsu Kosan, EDF, and KEPCO are investing in this technology for grid storage applications. Academic institutions such as Harbin Institute of Technology, Wuhan University of Technology, and Vanderbilt University are driving fundamental research in sintering methods and composition optimization. Emerging companies like Xinjie Energy Technology and eJoule are focusing on scalable manufacturing processes to bridge the gap between laboratory success and commercial viability of these critical battery components.
Murata Manufacturing Co. Ltd.
Technical Solution: Murata has developed sophisticated fabrication methods for dense sodium solid electrolyte ceramics, focusing primarily on NASICON-type materials for energy storage applications. Their approach combines advanced powder synthesis techniques with specialized sintering protocols to achieve high-density ceramics (>97% theoretical density). Murata employs a proprietary spray pyrolysis method to produce highly homogeneous precursor powders with controlled stoichiometry and nanoscale particle size (typically 50-100 nm). This is followed by a carefully optimized sintering process using atmosphere-controlled furnaces with precise temperature profiles (typically ramping at 1-2°C/min) and controlled oxygen partial pressure to minimize sodium volatilization. They've developed specialized sintering aids based on lithium and copper compounds that promote densification while maintaining high ionic conductivity. Murata has also pioneered thin-film fabrication techniques using aerosol deposition, achieving dense electrolyte layers as thin as 10-20 μm with excellent mechanical integrity and ionic conductivities of 1-3 mS/cm at room temperature.
Strengths: Extensive experience in ceramic component manufacturing at scale; sophisticated quality control systems; strong integration capabilities with other battery components. Weaknesses: Traditionally focused on electronic components rather than energy storage; faces competition from specialized battery material manufacturers; relatively high production costs.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced fabrication methods for dense sodium solid electrolyte ceramics focusing on NASICON-type materials. Their approach combines conventional solid-state reaction methods with specialized sintering techniques, achieving relative densities exceeding 95%. Toyota employs a two-step sintering process where materials are first calcined at 900-1000°C followed by high-temperature sintering at 1200-1300°C under controlled atmospheres to minimize sodium volatilization. They've pioneered the use of sintering aids such as lithium salts to promote densification at lower temperatures, reducing processing costs while maintaining ionic conductivity of 10^-3 S/cm at room temperature. Toyota has also developed scalable tape-casting methods for thin electrolyte fabrication (50-100 μm), enabling higher energy density in their solid-state sodium battery prototypes.
Strengths: Extensive manufacturing infrastructure and experience in battery production; ability to integrate research directly into automotive applications; strong IP portfolio in solid electrolyte fabrication. Weaknesses: Primary focus on lithium systems may limit resources dedicated to sodium technologies; higher production costs compared to liquid electrolyte systems.
Critical Patents and Research on Sodium Solid Electrolyte Densification
Method for producing solid sodium electrolyte through gas-phase conversion, solid sodium electrolyte, and laminate for producing solid sodium electrolyte
PatentWO2024177341A1
Innovation
- The method involves preparing atmospheric powder pellets or packs containing a sodium source and alternately stacking them with a sintered body containing alpha alumina and yttria-stabilized zirconia, followed by heat-treating to convert the sintered body into beta alumina, allowing for the reuse of atmospheric powder and reducing process steps and production time.
solid ELECTROLYTE BASED ON FULLY STABILIZED ZIRCONIUM DIOXIDE AND METHOD FOR ITS PRODUCTION
PatentActiveRU2014125250A
Innovation
- Utilization of a specific composition of slip (5-40 weight parts of stabilized zirconium dioxide powder, 10-20 weight parts of solvent, up to 10 weight parts binder, 0.4-4 weight part of plasticizer) to produce dense ceramic solid electrolytes.
- Novel air bubble removal technique using rotation of ball mill without grinding bodies at a rate of less than 25 rpm, which helps achieve low porosity in the final product.
- Specific composition of zirconium dioxide stabilized with 0.5-3 mol% CeO2 and 7-11 mol% Sc2O3 to achieve fine-grained structure with high density and conductivity.
Material Supply Chain Analysis for Sodium Solid Electrolytes
The global supply chain for sodium solid electrolytes represents a complex network spanning raw material extraction to final component manufacturing. Sodium-based solid electrolytes primarily require sodium carbonate (Na₂CO₃), various metal oxides (Al₂O₃, ZrO₂), and specialized additives. Unlike lithium-based alternatives, sodium resources are abundantly available worldwide, with significant deposits in China, the United States, India, and several African nations, reducing geopolitical supply risks.
The processing chain begins with sodium carbonate extraction, which benefits from established industrial processes due to its widespread use in glass, detergent, and chemical industries. This established infrastructure provides stability to the upstream supply chain. However, high-purity grades required for electrolyte fabrication necessitate additional refining steps, creating potential bottlenecks.
Critical challenges emerge in the midstream supply chain, particularly regarding specialized ceramic precursors and dopants. These materials often require sophisticated processing techniques and stringent quality control measures. The limited number of suppliers capable of meeting these specifications creates vulnerability points in the supply network, especially for zirconium-based compounds and rare earth dopants used in NASICON-type electrolytes.
Manufacturing equipment represents another crucial element in the supply chain. Specialized high-temperature furnaces, isostatic presses, and advanced ceramic processing equipment are predominantly produced in Germany, Japan, and the United States. This geographic concentration creates potential risks during global disruptions, as evidenced during recent pandemic-related supply chain challenges.
The fabrication of dense sodium solid electrolyte ceramics requires precise control of sintering atmospheres and temperatures, necessitating specialized gas supplies and monitoring equipment. These technical requirements further narrow the supplier base, creating additional supply chain vulnerabilities that manufacturers must address through strategic partnerships or vertical integration.
Labor and expertise constitute a frequently overlooked component of the supply chain. The specialized knowledge required for sodium solid electrolyte fabrication is concentrated in academic institutions and established industrial players, creating potential workforce shortages as production scales. This human resource constraint may become increasingly significant as the technology transitions from laboratory to commercial production.
Regulatory frameworks governing material transportation, handling, and waste management vary significantly across regions, adding complexity to international supply chain management. Environmental considerations, particularly regarding energy-intensive ceramic processing steps, will likely influence future supply chain development as sustainability metrics gain importance in industrial evaluation frameworks.
The processing chain begins with sodium carbonate extraction, which benefits from established industrial processes due to its widespread use in glass, detergent, and chemical industries. This established infrastructure provides stability to the upstream supply chain. However, high-purity grades required for electrolyte fabrication necessitate additional refining steps, creating potential bottlenecks.
Critical challenges emerge in the midstream supply chain, particularly regarding specialized ceramic precursors and dopants. These materials often require sophisticated processing techniques and stringent quality control measures. The limited number of suppliers capable of meeting these specifications creates vulnerability points in the supply network, especially for zirconium-based compounds and rare earth dopants used in NASICON-type electrolytes.
Manufacturing equipment represents another crucial element in the supply chain. Specialized high-temperature furnaces, isostatic presses, and advanced ceramic processing equipment are predominantly produced in Germany, Japan, and the United States. This geographic concentration creates potential risks during global disruptions, as evidenced during recent pandemic-related supply chain challenges.
The fabrication of dense sodium solid electrolyte ceramics requires precise control of sintering atmospheres and temperatures, necessitating specialized gas supplies and monitoring equipment. These technical requirements further narrow the supplier base, creating additional supply chain vulnerabilities that manufacturers must address through strategic partnerships or vertical integration.
Labor and expertise constitute a frequently overlooked component of the supply chain. The specialized knowledge required for sodium solid electrolyte fabrication is concentrated in academic institutions and established industrial players, creating potential workforce shortages as production scales. This human resource constraint may become increasingly significant as the technology transitions from laboratory to commercial production.
Regulatory frameworks governing material transportation, handling, and waste management vary significantly across regions, adding complexity to international supply chain management. Environmental considerations, particularly regarding energy-intensive ceramic processing steps, will likely influence future supply chain development as sustainability metrics gain importance in industrial evaluation frameworks.
Sustainability and Cost Considerations in Manufacturing Processes
The manufacturing of dense sodium solid electrolyte ceramics presents significant sustainability and cost challenges that must be addressed for commercial viability. Traditional ceramic processing methods often involve energy-intensive sintering at high temperatures (800-1200°C), contributing substantially to carbon emissions and operational costs. Recent research indicates that reducing sintering temperatures by just 100°C can decrease energy consumption by approximately 15-20%, highlighting the importance of developing lower-temperature fabrication routes.
Raw material selection significantly impacts both sustainability and cost profiles. Sodium-based precursors like Na2CO3 are relatively inexpensive compared to lithium alternatives, offering a cost advantage of 60-70%. However, the high reactivity of sodium compounds with moisture necessitates controlled processing environments, increasing manufacturing complexity and associated costs. The implementation of closed-loop material recovery systems has demonstrated potential to reclaim up to 85% of unused precursors, substantially reducing waste and raw material expenses.
Water consumption represents another critical sustainability consideration. Conventional wet chemical methods can require 15-20 liters of water per kilogram of produced electrolyte. Emerging solvent-free and mechanochemical approaches have shown promise in reducing water usage by up to 90%, while simultaneously decreasing processing time by 30-40%. These methods also minimize the generation of contaminated wastewater requiring costly treatment.
Scale-up challenges present significant economic barriers to commercialization. Laboratory-scale processes often achieve excellent results but face yield reductions of 30-50% when scaled to industrial production. Advanced process monitoring and quality control systems, while requiring initial capital investment of $50,000-200,000, can improve yield consistency by 25-35% and reduce material waste, offering return on investment within 2-3 years of operation.
Life cycle assessment (LCA) studies indicate that manufacturing phase accounts for approximately 60-70% of the total environmental impact of solid electrolyte components. Transitioning to renewable energy sources for high-temperature processes could reduce carbon footprint by 40-50%. Additionally, developing recycling protocols for end-of-life sodium solid electrolytes presents an opportunity to recover valuable materials and further enhance sustainability metrics, though current recovery technologies remain at early development stages with efficiencies below 50%.
Economic viability ultimately depends on achieving production costs below $100/kg for widespread adoption in energy storage applications. Current manufacturing costs range from $300-800/kg at pilot scale, necessitating further process optimization and economies of scale to reach commercial targets. Collaborative industry-academic partnerships focusing on sustainable manufacturing innovations represent a promising pathway toward achieving both environmental and economic objectives in this emerging technology sector.
Raw material selection significantly impacts both sustainability and cost profiles. Sodium-based precursors like Na2CO3 are relatively inexpensive compared to lithium alternatives, offering a cost advantage of 60-70%. However, the high reactivity of sodium compounds with moisture necessitates controlled processing environments, increasing manufacturing complexity and associated costs. The implementation of closed-loop material recovery systems has demonstrated potential to reclaim up to 85% of unused precursors, substantially reducing waste and raw material expenses.
Water consumption represents another critical sustainability consideration. Conventional wet chemical methods can require 15-20 liters of water per kilogram of produced electrolyte. Emerging solvent-free and mechanochemical approaches have shown promise in reducing water usage by up to 90%, while simultaneously decreasing processing time by 30-40%. These methods also minimize the generation of contaminated wastewater requiring costly treatment.
Scale-up challenges present significant economic barriers to commercialization. Laboratory-scale processes often achieve excellent results but face yield reductions of 30-50% when scaled to industrial production. Advanced process monitoring and quality control systems, while requiring initial capital investment of $50,000-200,000, can improve yield consistency by 25-35% and reduce material waste, offering return on investment within 2-3 years of operation.
Life cycle assessment (LCA) studies indicate that manufacturing phase accounts for approximately 60-70% of the total environmental impact of solid electrolyte components. Transitioning to renewable energy sources for high-temperature processes could reduce carbon footprint by 40-50%. Additionally, developing recycling protocols for end-of-life sodium solid electrolytes presents an opportunity to recover valuable materials and further enhance sustainability metrics, though current recovery technologies remain at early development stages with efficiencies below 50%.
Economic viability ultimately depends on achieving production costs below $100/kg for widespread adoption in energy storage applications. Current manufacturing costs range from $300-800/kg at pilot scale, necessitating further process optimization and economies of scale to reach commercial targets. Collaborative industry-academic partnerships focusing on sustainable manufacturing innovations represent a promising pathway toward achieving both environmental and economic objectives in this emerging technology sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!