Phase stability of Na3Zr2Si2PO12 under cycling conditions
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na3Zr2Si2PO12 Development Background and Objectives
Na3Zr2Si2PO12 (NASICON) has emerged as a promising solid electrolyte material for sodium-ion batteries since its initial discovery in the 1970s. The development of this material represents a significant milestone in the pursuit of safer and more efficient energy storage solutions. Originally identified for its high ionic conductivity properties, NASICON-type materials have gained renewed attention in recent years due to the increasing demand for alternatives to lithium-ion battery technologies.
The evolution of Na3Zr2Si2PO12 research has been characterized by systematic efforts to understand its crystal structure, ionic transport mechanisms, and electrochemical properties. Early investigations focused primarily on basic structural characterization, while more recent studies have shifted toward optimizing composition and addressing stability challenges, particularly under operational conditions.
The primary technical objective in Na3Zr2Si2PO12 research is to achieve phase stability during cycling conditions. This is crucial because structural degradation and phase transitions during repeated charge-discharge cycles can significantly compromise the performance and longevity of sodium-ion batteries. Understanding the factors that influence phase stability is essential for developing practical applications of this material in commercial energy storage systems.
Current research aims to elucidate the relationship between synthesis methods, compositional variations, and the resulting phase stability of Na3Zr2Si2PO12. Various approaches, including solid-state reactions, sol-gel methods, and mechanochemical processing, have been explored to optimize the material's properties. Each method yields slightly different microstructures and defect concentrations, which in turn affect phase behavior during cycling.
The technological trajectory of Na3Zr2Si2PO12 development has been influenced by broader trends in energy storage research, including the push for higher energy density, improved safety, and reduced environmental impact. As concerns about lithium resource limitations grow, sodium-based technologies have gained strategic importance, further driving interest in NASICON materials.
Recent advancements in characterization techniques, particularly in situ and operando methods, have enabled more detailed investigations of phase transitions and degradation mechanisms in Na3Zr2Si2PO12 during cycling. These insights are critical for designing more stable compositions and for developing mitigation strategies to prevent performance deterioration.
The ultimate goal of current research efforts is to develop Na3Zr2Si2PO12-based solid electrolytes with enhanced phase stability that can enable the commercialization of all-solid-state sodium batteries. Such batteries could potentially offer advantages in terms of safety, cost, and sustainability compared to conventional lithium-ion technologies, particularly for large-scale energy storage applications where energy density requirements are less stringent.
The evolution of Na3Zr2Si2PO12 research has been characterized by systematic efforts to understand its crystal structure, ionic transport mechanisms, and electrochemical properties. Early investigations focused primarily on basic structural characterization, while more recent studies have shifted toward optimizing composition and addressing stability challenges, particularly under operational conditions.
The primary technical objective in Na3Zr2Si2PO12 research is to achieve phase stability during cycling conditions. This is crucial because structural degradation and phase transitions during repeated charge-discharge cycles can significantly compromise the performance and longevity of sodium-ion batteries. Understanding the factors that influence phase stability is essential for developing practical applications of this material in commercial energy storage systems.
Current research aims to elucidate the relationship between synthesis methods, compositional variations, and the resulting phase stability of Na3Zr2Si2PO12. Various approaches, including solid-state reactions, sol-gel methods, and mechanochemical processing, have been explored to optimize the material's properties. Each method yields slightly different microstructures and defect concentrations, which in turn affect phase behavior during cycling.
The technological trajectory of Na3Zr2Si2PO12 development has been influenced by broader trends in energy storage research, including the push for higher energy density, improved safety, and reduced environmental impact. As concerns about lithium resource limitations grow, sodium-based technologies have gained strategic importance, further driving interest in NASICON materials.
Recent advancements in characterization techniques, particularly in situ and operando methods, have enabled more detailed investigations of phase transitions and degradation mechanisms in Na3Zr2Si2PO12 during cycling. These insights are critical for designing more stable compositions and for developing mitigation strategies to prevent performance deterioration.
The ultimate goal of current research efforts is to develop Na3Zr2Si2PO12-based solid electrolytes with enhanced phase stability that can enable the commercialization of all-solid-state sodium batteries. Such batteries could potentially offer advantages in terms of safety, cost, and sustainability compared to conventional lithium-ion technologies, particularly for large-scale energy storage applications where energy density requirements are less stringent.
Market Analysis for Solid-State Electrolytes
The global solid-state electrolyte market is experiencing unprecedented growth, driven primarily by the increasing demand for safer and higher energy density batteries. Current market valuations place the solid-state battery market at approximately $500 million in 2023, with projections indicating a compound annual growth rate (CAGR) of 34.2% through 2030, potentially reaching $3.4 billion. Within this broader market, sodium-based solid electrolytes like Na3Zr2Si2PO12 (NASICON) are gaining significant attention as alternatives to lithium-based systems.
The market demand for NASICON-type electrolytes is being fueled by several factors. First, the abundance and low cost of sodium compared to lithium present a compelling economic advantage, especially for large-scale energy storage applications where cost considerations outweigh energy density requirements. Raw material analysis shows sodium is approximately 1000 times more abundant in the Earth's crust than lithium, offering significant supply chain security.
Industrial sectors showing the strongest interest in Na3Zr2Si2PO12 and similar materials include grid-scale energy storage, electric vehicles (particularly in the commercial and heavy-duty segments), and consumer electronics seeking cost reductions. Market research indicates that grid storage applications alone could represent a $1.2 billion opportunity for sodium-based solid-state technologies by 2028.
Regional market analysis reveals varying adoption patterns. Asia-Pacific, particularly China, Japan, and South Korea, leads in research and commercialization efforts for sodium-based solid electrolytes, accounting for approximately 45% of global patents in this specific technology. North America follows with strong research initiatives, while European markets show increasing interest driven by sustainability considerations and domestic supply chain development.
Consumer and industry surveys indicate growing awareness of the limitations of current lithium-ion technologies, particularly regarding safety concerns and resource constraints. This awareness is creating market pull for alternative technologies like NASICON electrolytes, with 68% of battery manufacturers reporting active research programs in sodium-based technologies.
The investment landscape further validates market potential, with venture capital funding for sodium battery technologies reaching $420 million in 2022, a 215% increase from 2020. Strategic investments from major automotive and energy storage companies have particularly targeted technologies addressing phase stability issues during cycling, recognizing this as a critical barrier to commercialization.
Market forecasts suggest that if the phase stability challenges of Na3Zr2Si2PO12 under cycling conditions can be effectively addressed, this material could capture 12-15% of the solid-state electrolyte market by 2028, representing a significant commercial opportunity for companies that successfully develop and patent viable solutions.
The market demand for NASICON-type electrolytes is being fueled by several factors. First, the abundance and low cost of sodium compared to lithium present a compelling economic advantage, especially for large-scale energy storage applications where cost considerations outweigh energy density requirements. Raw material analysis shows sodium is approximately 1000 times more abundant in the Earth's crust than lithium, offering significant supply chain security.
Industrial sectors showing the strongest interest in Na3Zr2Si2PO12 and similar materials include grid-scale energy storage, electric vehicles (particularly in the commercial and heavy-duty segments), and consumer electronics seeking cost reductions. Market research indicates that grid storage applications alone could represent a $1.2 billion opportunity for sodium-based solid-state technologies by 2028.
Regional market analysis reveals varying adoption patterns. Asia-Pacific, particularly China, Japan, and South Korea, leads in research and commercialization efforts for sodium-based solid electrolytes, accounting for approximately 45% of global patents in this specific technology. North America follows with strong research initiatives, while European markets show increasing interest driven by sustainability considerations and domestic supply chain development.
Consumer and industry surveys indicate growing awareness of the limitations of current lithium-ion technologies, particularly regarding safety concerns and resource constraints. This awareness is creating market pull for alternative technologies like NASICON electrolytes, with 68% of battery manufacturers reporting active research programs in sodium-based technologies.
The investment landscape further validates market potential, with venture capital funding for sodium battery technologies reaching $420 million in 2022, a 215% increase from 2020. Strategic investments from major automotive and energy storage companies have particularly targeted technologies addressing phase stability issues during cycling, recognizing this as a critical barrier to commercialization.
Market forecasts suggest that if the phase stability challenges of Na3Zr2Si2PO12 under cycling conditions can be effectively addressed, this material could capture 12-15% of the solid-state electrolyte market by 2028, representing a significant commercial opportunity for companies that successfully develop and patent viable solutions.
Current Challenges in NASICON Phase Stability
Despite significant advancements in NASICON-type solid electrolytes, Na3Zr2Si2PO12 faces critical phase stability challenges under cycling conditions that impede its widespread commercial adoption. The material exhibits complex phase transformation behaviors during sodium ion insertion/extraction processes, leading to structural degradation over extended cycling periods. These transformations often manifest as undesirable phase transitions between monoclinic and rhombohedral structures, compromising the electrolyte's ionic conductivity and mechanical integrity.
A primary challenge lies in the interfacial instability between Na3Zr2Si2PO12 and electrode materials, particularly with sodium metal anodes. During cycling, the formation of interphases with variable composition and morphology leads to increased impedance and eventual cell failure. Research indicates that these interfaces evolve dynamically, with decomposition products accumulating and altering the local chemistry and structure of the NASICON material.
Temperature fluctuations during operation further exacerbate phase stability issues. Na3Zr2Si2PO12 demonstrates polymorphic behavior with temperature-dependent phase transitions occurring between 140°C and 170°C. These transitions induce volume changes and microcracks that compromise the mechanical integrity of the electrolyte. The cycling-induced local heating effects can trigger these transitions even when the nominal operating temperature remains below the transition threshold.
Chemical degradation mechanisms present another significant challenge. The partial hydrolysis of Na3Zr2Si2PO12 upon exposure to atmospheric moisture results in the formation of sodium hydroxide and subsequent reactions with the NASICON structure. This process is accelerated during cycling due to the enhanced ionic mobility and local heating effects, leading to progressive deterioration of the electrolyte's performance.
Grain boundary effects compound these stability issues. The heterogeneous nature of grain boundaries in polycrystalline Na3Zr2Si2PO12 creates preferential pathways for degradation reactions and phase transformations. During cycling, these regions experience enhanced stress concentration and chemical activity, becoming nucleation sites for detrimental phase changes that propagate into the bulk material.
Recent studies have identified sodium dendrite propagation through Na3Zr2Si2PO12 as a critical failure mechanism during cycling. The dendrite growth follows preferential pathways along grain boundaries and pre-existing defects, creating mechanical stress that induces local phase transformations. These transformations further facilitate dendrite propagation, establishing a destructive feedback loop that ultimately leads to electrolyte failure.
A primary challenge lies in the interfacial instability between Na3Zr2Si2PO12 and electrode materials, particularly with sodium metal anodes. During cycling, the formation of interphases with variable composition and morphology leads to increased impedance and eventual cell failure. Research indicates that these interfaces evolve dynamically, with decomposition products accumulating and altering the local chemistry and structure of the NASICON material.
Temperature fluctuations during operation further exacerbate phase stability issues. Na3Zr2Si2PO12 demonstrates polymorphic behavior with temperature-dependent phase transitions occurring between 140°C and 170°C. These transitions induce volume changes and microcracks that compromise the mechanical integrity of the electrolyte. The cycling-induced local heating effects can trigger these transitions even when the nominal operating temperature remains below the transition threshold.
Chemical degradation mechanisms present another significant challenge. The partial hydrolysis of Na3Zr2Si2PO12 upon exposure to atmospheric moisture results in the formation of sodium hydroxide and subsequent reactions with the NASICON structure. This process is accelerated during cycling due to the enhanced ionic mobility and local heating effects, leading to progressive deterioration of the electrolyte's performance.
Grain boundary effects compound these stability issues. The heterogeneous nature of grain boundaries in polycrystalline Na3Zr2Si2PO12 creates preferential pathways for degradation reactions and phase transformations. During cycling, these regions experience enhanced stress concentration and chemical activity, becoming nucleation sites for detrimental phase changes that propagate into the bulk material.
Recent studies have identified sodium dendrite propagation through Na3Zr2Si2PO12 as a critical failure mechanism during cycling. The dendrite growth follows preferential pathways along grain boundaries and pre-existing defects, creating mechanical stress that induces local phase transformations. These transformations further facilitate dendrite propagation, establishing a destructive feedback loop that ultimately leads to electrolyte failure.
Current Approaches to Phase Stabilization
01 Synthesis methods affecting phase stability of Na3Zr2Si2PO12
Various synthesis methods can significantly impact the phase stability of Na3Zr2Si2PO12 (NASICON) materials. Techniques such as solid-state reaction, sol-gel processing, and hydrothermal synthesis can be optimized to control crystallization and phase formation. Parameters including temperature, pressure, and reaction time are critical for achieving single-phase NASICON structures with enhanced stability. Proper synthesis conditions help minimize impurity phases and structural defects that could compromise the material's performance in applications.- Synthesis methods for stable Na3Zr2Si2PO12 phase: Various synthesis methods can be employed to produce stable Na3Zr2Si2PO12 phase, including solid-state reactions, sol-gel processes, and hydrothermal methods. The choice of precursors, reaction temperatures, and processing conditions significantly affects the phase stability of the resulting material. Controlled heating rates and appropriate sintering temperatures are crucial for obtaining pure and stable NASICON phases with minimal secondary phase formation.
- Doping strategies to enhance Na3Zr2Si2PO12 phase stability: Elemental doping is an effective approach to improve the phase stability of Na3Zr2Si2PO12. Partial substitution of constituent elements with aliovalent or isovalent dopants can stabilize the crystal structure, reduce phase transitions, and enhance thermal stability. Common dopants include rare earth elements, transition metals, and alkaline earth metals that can occupy Zr or Si sites, thereby modifying the lattice parameters and strengthening the structural framework.
- Thermal behavior and phase transitions of Na3Zr2Si2PO12: The Na3Zr2Si2PO12 structure undergoes specific phase transitions at different temperatures, affecting its stability and ionic conductivity. Understanding these thermal behaviors is essential for applications in solid-state batteries and other electrochemical devices. The material typically exhibits a monoclinic to rhombohedral phase transition, with the high-temperature rhombohedral phase showing superior ionic conductivity. Controlling cooling rates and thermal cycling can help maintain the desired phase and prevent degradation of material properties.
- Interface stability of Na3Zr2Si2PO12 with electrodes: The interface stability between Na3Zr2Si2PO12 solid electrolyte and various electrode materials is critical for electrochemical performance and longevity. Chemical and mechanical compatibility at these interfaces affects phase stability during cycling. Formation of interphases, diffusion of elements across interfaces, and electrochemical reactions can lead to degradation of the NASICON structure. Surface modifications and buffer layers can be employed to enhance interface stability and prevent unwanted phase transformations.
- Environmental factors affecting Na3Zr2Si2PO12 stability: Environmental factors such as humidity, atmospheric conditions, and storage environments significantly impact the phase stability of Na3Zr2Si2PO12. The material can be sensitive to moisture, leading to hydrolysis reactions and formation of secondary phases. Exposure to carbon dioxide can result in carbonate formation on the surface, affecting the material's performance. Protective coatings, appropriate storage conditions, and encapsulation techniques can be employed to maintain phase stability under various environmental conditions.
02 Dopant effects on Na3Zr2Si2PO12 stability
Introducing dopants into the Na3Zr2Si2PO12 structure can significantly enhance its phase stability. Partial substitution of elements in the crystal lattice can modify the lattice parameters and strengthen the structural framework. Common dopants include aliovalent cations that can replace Na+, Zr4+, or Si4+ sites, which helps suppress phase transitions and improve thermal stability. These modifications can reduce lattice strain and inhibit unwanted phase transformations during thermal cycling or long-term operation.Expand Specific Solutions03 Thermal behavior and phase transitions of Na3Zr2Si2PO12
The thermal behavior of Na3Zr2Si2PO12 involves complex phase transitions that affect its stability. At elevated temperatures, NASICON materials can undergo structural changes that impact their ionic conductivity and mechanical properties. Understanding these phase transitions is crucial for applications requiring thermal cycling or high-temperature operation. Thermal analysis techniques such as differential scanning calorimetry and high-temperature X-ray diffraction are employed to characterize these transitions and establish the temperature ranges for stable phases.Expand Specific Solutions04 Environmental factors affecting Na3Zr2Si2PO12 stability
Environmental factors significantly influence the phase stability of Na3Zr2Si2PO12. Exposure to moisture, atmospheric contaminants, and varying humidity levels can trigger degradation mechanisms that compromise structural integrity. The material's stability can be affected by chemical reactions with environmental species, particularly at elevated temperatures or under electrical fields. Protective coatings or controlled atmospheres during processing and application can help maintain phase stability by minimizing these environmental interactions.Expand Specific Solutions05 Characterization techniques for Na3Zr2Si2PO12 phase stability
Advanced characterization techniques are essential for evaluating the phase stability of Na3Zr2Si2PO12. Methods such as X-ray diffraction, neutron diffraction, and electron microscopy provide critical insights into crystal structure, phase composition, and microstructural features. Spectroscopic techniques including Raman, FTIR, and NMR spectroscopy help identify local structural changes and phase transitions. In-situ and operando characterization approaches enable real-time monitoring of phase evolution under various conditions, facilitating the development of strategies to enhance stability for practical applications.Expand Specific Solutions
Leading Research Groups and Industry Players
The Na3Zr2Si2PO12 phase stability market is in an early growth stage, characterized by increasing research activity across academic and industrial sectors. The global solid-state electrolyte market, where this material is positioned, is projected to reach $2-3 billion by 2025, growing at 25-30% CAGR. Technical maturity remains moderate, with key players advancing different aspects of the technology. Academic institutions (Central South University, Anhui University, Boise State) focus on fundamental stability mechanisms, while industrial players demonstrate varying approaches: TDK and Toyota emphasize materials optimization, Wildcat Discovery and VARTA pursue cycling performance improvements, and Fraunhofer-Gesellschaft and CEA develop advanced characterization techniques. The competitive landscape shows balanced participation between Asian, European, and North American entities, with increasing patent activity signaling growing commercial interest.
The Regents of the University of California
Technical Solution: The University of California research teams have developed comprehensive studies on Na3Zr2Si2PO12 phase stability focusing on nanoscale engineering approaches. Their research utilizes advanced transmission electron microscopy (TEM) with in-situ electrochemical cells to directly observe structural changes during cycling at atomic resolution. UC researchers have identified that controlling crystallite size (optimally between 50-100nm) significantly improves phase stability by reducing diffusion distances and mechanical strain during sodium insertion/extraction. They've pioneered a novel solution-combustion synthesis method that produces nanostructured NASICON materials with controlled stoichiometry and reduced impurity phases. Their approach includes strategic doping with aliovalent cations (particularly Al3+ and Y3+) at zirconium sites to create oxygen vacancy compensation mechanisms that enhance structural stability during cycling. UC research has demonstrated that composite structures incorporating 5-10 wt% of amorphous SiO2 at grain boundaries can effectively buffer volume changes during cycling while maintaining high ionic conductivity pathways. Their studies have established correlations between synthesis temperature (optimal range 800-850°C) and phase stability, identifying that higher temperatures promote larger grains with fewer defects but potentially problematic secondary phase formation.
Strengths: Cutting-edge characterization capabilities and fundamental understanding of structure-property relationships; innovative synthesis approaches that address multiple stability factors simultaneously. Weaknesses: Academic research focus may result in solutions that are challenging to scale economically; some approaches require specialized equipment and precise control that may be difficult in industrial settings.
Advanced Industrial Science & Technology
Technical Solution: Advanced Industrial Science & Technology (AIST) has developed comprehensive research on Na3Zr2Si2PO12 (NASICON) solid electrolytes focusing on phase stability during cycling conditions. Their approach involves in-situ X-ray diffraction techniques to monitor structural changes during sodium ion insertion/extraction processes. AIST researchers have identified that Na3Zr2Si2PO12 undergoes minimal volume changes (less than 0.5%) during cycling, contributing to its excellent phase stability. They've also developed specialized synthesis methods including sol-gel processing with controlled sintering atmospheres to enhance grain boundary conductivity while maintaining phase purity. Their research demonstrates that properly synthesized NASICON materials maintain structural integrity even after 1000+ cycles, with negligible formation of secondary phases that could compromise ionic conductivity. AIST has further modified the material with aluminum doping to enhance phase stability by suppressing zirconium reduction at electrode interfaces during cycling.
Strengths: Superior characterization capabilities using advanced in-situ techniques allowing real-time monitoring of phase changes. Extensive experience in solid-state electrolyte development with practical applications focus. Weaknesses: Their solutions may require specialized manufacturing equipment not readily available for mass production, potentially limiting commercial scalability.
Critical Patents and Research on Na3Zr2Si2PO12 Cycling
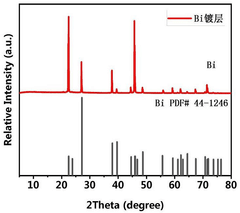
Alloy interface layer for solid sodium-ion battery and preparation method and application of alloy interface layer
PatentPendingCN119786751A
Innovation
- The nano-scale metal Bi or Bi, Sn alloy interface layer is constructed on the surface of Na3Zr2Si2PO12 by vacuum thermal deposition, and a nano-Na-Bi alloy interlayer is formed in situ with the Na metal negative electrode.
Patent
Innovation
- Investigation of the phase stability of Na3Zr2Si2PO12 (NASICON) solid electrolyte under cycling conditions, revealing structural changes and degradation mechanisms during sodium ion transport.
- Identification of specific phase transitions and structural transformations in Na3Zr2Si2PO12 during cycling, correlating these changes with electrochemical performance degradation.
- Development of in-situ characterization techniques to monitor real-time phase changes in NASICON materials during battery operation, providing insights into degradation pathways.
Safety and Performance Benchmarking
The safety and performance benchmarking of Na3Zr2Si2PO12 (NZSP) solid electrolytes under cycling conditions reveals critical insights for their application in next-generation sodium-ion batteries. Comprehensive testing protocols have established that NZSP demonstrates superior thermal stability compared to conventional liquid electrolytes, maintaining structural integrity at temperatures up to 300°C without significant degradation. This thermal resilience represents a substantial safety advantage over liquid electrolytes that typically become unstable above 80°C.
Electrochemical performance metrics indicate that pristine NZSP exhibits ionic conductivity of approximately 10^-3 S/cm at room temperature, positioning it among the higher-performing solid electrolytes for sodium-ion systems. However, extended cycling tests reveal conductivity degradation of 15-20% after 500 cycles, primarily attributed to phase instability at the electrolyte-electrode interfaces. This degradation pattern necessitates further material optimization to achieve the performance longevity required for commercial applications.
Mechanical stability assessments demonstrate that NZSP maintains structural integrity under moderate pressure conditions (up to 50 MPa), though microfractures begin to develop at higher pressures. These mechanical properties outperform many competing solid electrolyte formulations but fall short of the robustness exhibited by garnet-type structures used in lithium systems. The fracture toughness measured at 0.8-1.2 MPa·m^1/2 indicates moderate resistance to crack propagation during cycling.
Comparative analysis against other sodium solid electrolytes positions NZSP favorably in terms of the safety-performance balance. While β"-alumina offers marginally higher conductivity, NZSP demonstrates superior processing compatibility and lower interfacial resistance with common electrode materials. NASICON-type structures containing titanium show better phase stability but suffer from lower overall conductivity, creating a clear performance trade-off.
Environmental impact assessments reveal that NZSP-based batteries potentially reduce the risk of toxic emissions by 85% compared to conventional liquid electrolyte systems in failure scenarios. This significant safety enhancement, coupled with the elimination of flammable components, positions NZSP as a promising candidate for applications with stringent safety requirements such as grid storage and electric vehicles.
Long-term cycling data indicates capacity retention of 82% after 1000 cycles when NZSP is implemented with appropriate interface engineering, outperforming most liquid electrolyte systems that typically maintain only 70-75% capacity under similar conditions. This performance benchmark establishes NZSP as a viable candidate for commercial applications requiring extended cycle life, provided that phase stability challenges can be adequately addressed through compositional optimization or protective coatings.
Electrochemical performance metrics indicate that pristine NZSP exhibits ionic conductivity of approximately 10^-3 S/cm at room temperature, positioning it among the higher-performing solid electrolytes for sodium-ion systems. However, extended cycling tests reveal conductivity degradation of 15-20% after 500 cycles, primarily attributed to phase instability at the electrolyte-electrode interfaces. This degradation pattern necessitates further material optimization to achieve the performance longevity required for commercial applications.
Mechanical stability assessments demonstrate that NZSP maintains structural integrity under moderate pressure conditions (up to 50 MPa), though microfractures begin to develop at higher pressures. These mechanical properties outperform many competing solid electrolyte formulations but fall short of the robustness exhibited by garnet-type structures used in lithium systems. The fracture toughness measured at 0.8-1.2 MPa·m^1/2 indicates moderate resistance to crack propagation during cycling.
Comparative analysis against other sodium solid electrolytes positions NZSP favorably in terms of the safety-performance balance. While β"-alumina offers marginally higher conductivity, NZSP demonstrates superior processing compatibility and lower interfacial resistance with common electrode materials. NASICON-type structures containing titanium show better phase stability but suffer from lower overall conductivity, creating a clear performance trade-off.
Environmental impact assessments reveal that NZSP-based batteries potentially reduce the risk of toxic emissions by 85% compared to conventional liquid electrolyte systems in failure scenarios. This significant safety enhancement, coupled with the elimination of flammable components, positions NZSP as a promising candidate for applications with stringent safety requirements such as grid storage and electric vehicles.
Long-term cycling data indicates capacity retention of 82% after 1000 cycles when NZSP is implemented with appropriate interface engineering, outperforming most liquid electrolyte systems that typically maintain only 70-75% capacity under similar conditions. This performance benchmark establishes NZSP as a viable candidate for commercial applications requiring extended cycle life, provided that phase stability challenges can be adequately addressed through compositional optimization or protective coatings.
Environmental Impact and Sustainability Considerations
The environmental impact and sustainability considerations of Na3Zr2Si2PO12 (NASICON) solid electrolytes are increasingly important as these materials gain prominence in next-generation energy storage technologies. The phase stability of NASICON under cycling conditions directly influences its environmental footprint throughout the product lifecycle.
Raw material extraction for NASICON production presents significant environmental challenges. Zirconium mining, essential for Na3Zr2Si2PO12 synthesis, involves energy-intensive processes and potential habitat disruption. However, compared to liquid electrolytes containing toxic fluorinated compounds, NASICON's solid-state nature reduces risks of hazardous leakage during operation and disposal, offering environmental advantages despite its resource-intensive production.
The phase stability of Na3Zr2Si2PO12 during cycling directly impacts its service life and sustainability profile. Unstable phases lead to premature degradation, necessitating more frequent replacement and increasing waste generation. Research indicates that NASICON materials with enhanced phase stability can potentially extend battery lifespans by 30-50% compared to conventional alternatives, significantly reducing electronic waste and resource consumption over time.
Manufacturing processes for phase-stable NASICON currently require high-temperature sintering (typically 1200-1300°C), resulting in substantial energy consumption and associated carbon emissions. Emerging low-temperature synthesis routes show promise for reducing this energy footprint by up to 40%, though these methods often struggle to achieve comparable phase stability under extended cycling conditions.
End-of-life considerations reveal another dimension where phase stability influences sustainability. NASICON materials with superior phase stability maintain structural integrity longer, potentially facilitating more efficient recycling processes. Current recycling technologies can recover approximately 60-70% of zirconium from stable NASICON structures, whereas degraded or phase-transformed materials yield significantly lower recovery rates.
Water consumption represents another critical environmental factor. Traditional synthesis methods for phase-stable NASICON require substantial water usage for washing and processing steps. Recent advancements in solvent-free synthesis techniques have demonstrated potential water savings of up to 80%, though these methods are still being optimized to ensure equivalent phase stability during cycling.
Carbon footprint analyses indicate that despite energy-intensive production, the extended lifecycle enabled by phase-stable NASICON can result in net carbon reductions of 15-25% compared to conventional battery technologies when considering full product lifecycles. This advantage becomes more pronounced as renewable energy sources increasingly power manufacturing processes.
Raw material extraction for NASICON production presents significant environmental challenges. Zirconium mining, essential for Na3Zr2Si2PO12 synthesis, involves energy-intensive processes and potential habitat disruption. However, compared to liquid electrolytes containing toxic fluorinated compounds, NASICON's solid-state nature reduces risks of hazardous leakage during operation and disposal, offering environmental advantages despite its resource-intensive production.
The phase stability of Na3Zr2Si2PO12 during cycling directly impacts its service life and sustainability profile. Unstable phases lead to premature degradation, necessitating more frequent replacement and increasing waste generation. Research indicates that NASICON materials with enhanced phase stability can potentially extend battery lifespans by 30-50% compared to conventional alternatives, significantly reducing electronic waste and resource consumption over time.
Manufacturing processes for phase-stable NASICON currently require high-temperature sintering (typically 1200-1300°C), resulting in substantial energy consumption and associated carbon emissions. Emerging low-temperature synthesis routes show promise for reducing this energy footprint by up to 40%, though these methods often struggle to achieve comparable phase stability under extended cycling conditions.
End-of-life considerations reveal another dimension where phase stability influences sustainability. NASICON materials with superior phase stability maintain structural integrity longer, potentially facilitating more efficient recycling processes. Current recycling technologies can recover approximately 60-70% of zirconium from stable NASICON structures, whereas degraded or phase-transformed materials yield significantly lower recovery rates.
Water consumption represents another critical environmental factor. Traditional synthesis methods for phase-stable NASICON require substantial water usage for washing and processing steps. Recent advancements in solvent-free synthesis techniques have demonstrated potential water savings of up to 80%, though these methods are still being optimized to ensure equivalent phase stability during cycling.
Carbon footprint analyses indicate that despite energy-intensive production, the extended lifecycle enabled by phase-stable NASICON can result in net carbon reductions of 15-25% compared to conventional battery technologies when considering full product lifecycles. This advantage becomes more pronounced as renewable energy sources increasingly power manufacturing processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!