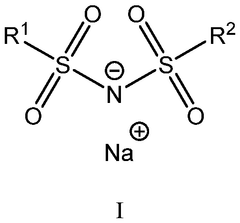
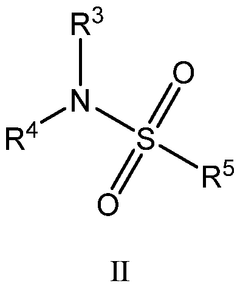
Electrochemical performance of oxide–sulfide hybrid Na electrolytes
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Oxide-Sulfide Hybrid Na Electrolytes Background and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The development of efficient solid-state electrolytes for SIBs represents a critical frontier in energy storage technology, with oxide-sulfide hybrid Na electrolytes gaining significant attention in recent years. These hybrid electrolytes combine the advantages of both oxide and sulfide components, potentially addressing the limitations of single-component systems.
The evolution of solid-state electrolytes for sodium batteries has progressed through several distinct phases. Initially, research focused on single-component systems, with oxide-based electrolytes offering high stability but suffering from low ionic conductivity at room temperature. Conversely, sulfide-based electrolytes demonstrated superior ionic conductivity but presented challenges related to air stability and interfacial resistance with electrode materials.
The concept of hybrid oxide-sulfide electrolytes emerged as researchers sought to synergistically combine the beneficial properties of both material classes. This approach aims to achieve enhanced electrochemical performance while mitigating the inherent limitations of each component. The technological trajectory has been driven by the need for safer, more efficient, and cost-effective energy storage solutions for grid-scale applications and electric vehicles.
Current research objectives in this field focus on optimizing the electrochemical performance of oxide-sulfide hybrid Na electrolytes across several key parameters. Primary goals include achieving room-temperature ionic conductivity exceeding 10^-3 S/cm, enhancing electrochemical stability windows to over 4V, improving interfacial compatibility with electrode materials, and ensuring long-term cycling stability under various operating conditions.
Additionally, researchers aim to develop scalable and cost-effective synthesis methods that can facilitate the transition from laboratory-scale production to industrial manufacturing. This includes exploring novel composite architectures, interface engineering strategies, and innovative processing techniques to optimize the microstructural characteristics of these hybrid electrolytes.
The technological landscape is further shaped by the broader transition toward sustainable energy systems and the growing demand for stationary energy storage solutions. As renewable energy integration increases, the need for efficient, safe, and economical energy storage technologies becomes more pressing, positioning advanced sodium electrolytes as a key enabling technology for the future energy infrastructure.
Understanding the fundamental mechanisms governing ion transport in these hybrid systems represents another critical research objective, as this knowledge will inform rational design principles for next-generation electrolyte materials with optimized performance characteristics.
The evolution of solid-state electrolytes for sodium batteries has progressed through several distinct phases. Initially, research focused on single-component systems, with oxide-based electrolytes offering high stability but suffering from low ionic conductivity at room temperature. Conversely, sulfide-based electrolytes demonstrated superior ionic conductivity but presented challenges related to air stability and interfacial resistance with electrode materials.
The concept of hybrid oxide-sulfide electrolytes emerged as researchers sought to synergistically combine the beneficial properties of both material classes. This approach aims to achieve enhanced electrochemical performance while mitigating the inherent limitations of each component. The technological trajectory has been driven by the need for safer, more efficient, and cost-effective energy storage solutions for grid-scale applications and electric vehicles.
Current research objectives in this field focus on optimizing the electrochemical performance of oxide-sulfide hybrid Na electrolytes across several key parameters. Primary goals include achieving room-temperature ionic conductivity exceeding 10^-3 S/cm, enhancing electrochemical stability windows to over 4V, improving interfacial compatibility with electrode materials, and ensuring long-term cycling stability under various operating conditions.
Additionally, researchers aim to develop scalable and cost-effective synthesis methods that can facilitate the transition from laboratory-scale production to industrial manufacturing. This includes exploring novel composite architectures, interface engineering strategies, and innovative processing techniques to optimize the microstructural characteristics of these hybrid electrolytes.
The technological landscape is further shaped by the broader transition toward sustainable energy systems and the growing demand for stationary energy storage solutions. As renewable energy integration increases, the need for efficient, safe, and economical energy storage technologies becomes more pressing, positioning advanced sodium electrolytes as a key enabling technology for the future energy infrastructure.
Understanding the fundamental mechanisms governing ion transport in these hybrid systems represents another critical research objective, as this knowledge will inform rational design principles for next-generation electrolyte materials with optimized performance characteristics.
Market Analysis for Sodium-Based Battery Technologies
The sodium-ion battery market is experiencing significant growth, driven by the increasing demand for sustainable and cost-effective energy storage solutions. Current market projections indicate that the global sodium-ion battery market is expected to grow at a compound annual growth rate of 18% between 2023 and 2030, reaching a market value of approximately 1.2 billion USD by 2030. This growth is primarily fueled by the rising need for grid-scale energy storage systems and the automotive industry's shift towards electrification.
The market for oxide-sulfide hybrid Na electrolytes specifically represents a promising segment within the broader sodium-based battery technologies. These hybrid electrolytes address key limitations of traditional sodium-ion batteries, particularly in terms of ionic conductivity and electrochemical stability. Market research suggests that hybrid electrolyte technologies could capture up to 25% of the sodium-ion battery market by 2028, particularly in applications requiring enhanced performance characteristics.
Regional analysis reveals that Asia-Pacific dominates the sodium-based battery market, with China leading in both research and commercial production. European markets are showing accelerated adoption rates, driven by stringent environmental regulations and substantial investments in renewable energy infrastructure. North America follows with growing interest, particularly in grid storage applications.
End-user segmentation indicates that utility-scale energy storage represents the largest market share for sodium-based batteries at 45%, followed by electric vehicles at 30% and consumer electronics at 15%. The remaining 10% encompasses various industrial applications. The oxide-sulfide hybrid electrolytes are particularly gaining traction in the utility-scale segment due to their enhanced safety profile and performance stability at varying temperatures.
Price sensitivity analysis reveals that sodium-based batteries currently offer a 30-40% cost advantage over lithium-ion alternatives, primarily due to the abundance and accessibility of sodium resources. This cost differential is expected to remain a key market driver, especially as raw material constraints continue to affect lithium supply chains.
Market barriers include technological maturity concerns, with sodium-ion technologies still perceived as less developed compared to lithium-ion counterparts. Additionally, manufacturing scale-up challenges and limited commercial deployment examples create adoption hesitancy among potential end-users. However, these barriers are gradually diminishing as more demonstration projects come online and manufacturing processes become optimized.
Investment trends show increasing venture capital interest, with funding for sodium battery startups growing by 65% in 2022 compared to the previous year. Strategic partnerships between electrolyte developers, battery manufacturers, and end-users are becoming more common, accelerating the commercialization timeline for advanced sodium battery technologies including hybrid electrolyte systems.
The market for oxide-sulfide hybrid Na electrolytes specifically represents a promising segment within the broader sodium-based battery technologies. These hybrid electrolytes address key limitations of traditional sodium-ion batteries, particularly in terms of ionic conductivity and electrochemical stability. Market research suggests that hybrid electrolyte technologies could capture up to 25% of the sodium-ion battery market by 2028, particularly in applications requiring enhanced performance characteristics.
Regional analysis reveals that Asia-Pacific dominates the sodium-based battery market, with China leading in both research and commercial production. European markets are showing accelerated adoption rates, driven by stringent environmental regulations and substantial investments in renewable energy infrastructure. North America follows with growing interest, particularly in grid storage applications.
End-user segmentation indicates that utility-scale energy storage represents the largest market share for sodium-based batteries at 45%, followed by electric vehicles at 30% and consumer electronics at 15%. The remaining 10% encompasses various industrial applications. The oxide-sulfide hybrid electrolytes are particularly gaining traction in the utility-scale segment due to their enhanced safety profile and performance stability at varying temperatures.
Price sensitivity analysis reveals that sodium-based batteries currently offer a 30-40% cost advantage over lithium-ion alternatives, primarily due to the abundance and accessibility of sodium resources. This cost differential is expected to remain a key market driver, especially as raw material constraints continue to affect lithium supply chains.
Market barriers include technological maturity concerns, with sodium-ion technologies still perceived as less developed compared to lithium-ion counterparts. Additionally, manufacturing scale-up challenges and limited commercial deployment examples create adoption hesitancy among potential end-users. However, these barriers are gradually diminishing as more demonstration projects come online and manufacturing processes become optimized.
Investment trends show increasing venture capital interest, with funding for sodium battery startups growing by 65% in 2022 compared to the previous year. Strategic partnerships between electrolyte developers, battery manufacturers, and end-users are becoming more common, accelerating the commercialization timeline for advanced sodium battery technologies including hybrid electrolyte systems.
Current Status and Technical Barriers in Hybrid Electrolytes
Hybrid electrolytes combining oxide and sulfide components for sodium-ion batteries represent a significant advancement in solid-state electrolyte technology. Currently, these hybrid systems are being extensively investigated due to their potential to overcome the limitations of single-component electrolytes. Oxide-based electrolytes typically offer excellent chemical and electrochemical stability but suffer from low ionic conductivity at room temperature. Conversely, sulfide-based electrolytes demonstrate superior ionic conductivity but are often hampered by poor chemical stability and challenging processing requirements.
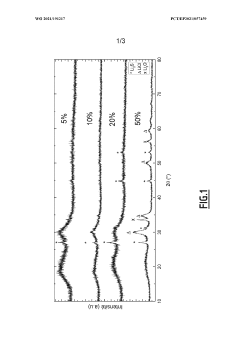
The state-of-the-art hybrid Na electrolytes have achieved ionic conductivities ranging from 10^-5 to 10^-3 S/cm at room temperature, representing a substantial improvement over traditional oxide electrolytes. Recent research has successfully demonstrated the integration of NASICON-type oxides with various sulfide components, creating composite structures that leverage the advantages of both materials. These hybrid systems have shown promising cycling performance in laboratory settings, with some prototypes achieving over 500 cycles with minimal capacity degradation.
Despite these advancements, significant technical barriers persist in the development of oxide-sulfide hybrid Na electrolytes. The interfacial resistance between oxide and sulfide components remains a critical challenge, often resulting in diminished overall performance. Chemical incompatibility at these interfaces can lead to the formation of resistive interlayers that impede Na-ion transport. Additionally, the mechanical mismatch between the rigid oxide and relatively soft sulfide components creates stress concentrations during cycling, potentially leading to microcrack formation and performance deterioration.
Manufacturing scalability presents another substantial hurdle. Current laboratory-scale synthesis methods for hybrid electrolytes often involve complex processes that are difficult to scale for industrial production. The sensitivity of sulfide components to moisture and air necessitates stringent handling protocols, significantly increasing production complexity and cost. Furthermore, the long-term stability of these hybrid systems under realistic operating conditions remains inadequately characterized, with concerns about gradual interface degradation during extended cycling.
The electrochemical window of hybrid electrolytes also requires optimization. While the oxide components typically offer wide electrochemical stability, the sulfide elements may undergo oxidation at high potentials, limiting compatibility with high-voltage cathode materials. This constraint restricts the energy density achievable in full cells utilizing these hybrid electrolytes. Additionally, the formation of dendrites at the anode interface during high-rate cycling continues to challenge the safety and reliability of these systems.
Environmental considerations and raw material availability also pose constraints on widespread adoption. Some sulfide components contain elements with limited natural abundance or geopolitical supply risks, potentially affecting the long-term sustainability of these technologies. These multifaceted challenges necessitate innovative approaches to hybrid electrolyte design and integration strategies to fully realize their potential in next-generation sodium-ion battery systems.
The state-of-the-art hybrid Na electrolytes have achieved ionic conductivities ranging from 10^-5 to 10^-3 S/cm at room temperature, representing a substantial improvement over traditional oxide electrolytes. Recent research has successfully demonstrated the integration of NASICON-type oxides with various sulfide components, creating composite structures that leverage the advantages of both materials. These hybrid systems have shown promising cycling performance in laboratory settings, with some prototypes achieving over 500 cycles with minimal capacity degradation.
Despite these advancements, significant technical barriers persist in the development of oxide-sulfide hybrid Na electrolytes. The interfacial resistance between oxide and sulfide components remains a critical challenge, often resulting in diminished overall performance. Chemical incompatibility at these interfaces can lead to the formation of resistive interlayers that impede Na-ion transport. Additionally, the mechanical mismatch between the rigid oxide and relatively soft sulfide components creates stress concentrations during cycling, potentially leading to microcrack formation and performance deterioration.
Manufacturing scalability presents another substantial hurdle. Current laboratory-scale synthesis methods for hybrid electrolytes often involve complex processes that are difficult to scale for industrial production. The sensitivity of sulfide components to moisture and air necessitates stringent handling protocols, significantly increasing production complexity and cost. Furthermore, the long-term stability of these hybrid systems under realistic operating conditions remains inadequately characterized, with concerns about gradual interface degradation during extended cycling.
The electrochemical window of hybrid electrolytes also requires optimization. While the oxide components typically offer wide electrochemical stability, the sulfide elements may undergo oxidation at high potentials, limiting compatibility with high-voltage cathode materials. This constraint restricts the energy density achievable in full cells utilizing these hybrid electrolytes. Additionally, the formation of dendrites at the anode interface during high-rate cycling continues to challenge the safety and reliability of these systems.
Environmental considerations and raw material availability also pose constraints on widespread adoption. Some sulfide components contain elements with limited natural abundance or geopolitical supply risks, potentially affecting the long-term sustainability of these technologies. These multifaceted challenges necessitate innovative approaches to hybrid electrolyte design and integration strategies to fully realize their potential in next-generation sodium-ion battery systems.
Contemporary Approaches to Oxide-Sulfide Hybrid Electrolytes
01 Oxide-sulfide hybrid electrolyte compositions
Hybrid electrolytes combining oxide and sulfide materials can offer improved ionic conductivity and electrochemical stability for sodium-ion batteries. These compositions typically include a sodium-containing oxide component and a sulfide component that work synergistically to enhance Na+ ion transport while maintaining structural integrity. The hybrid approach addresses limitations of single-material electrolytes by combining the mechanical strength of oxides with the high ionic conductivity of sulfides.- Oxide-sulfide hybrid electrolyte compositions: Hybrid electrolytes combining oxide and sulfide materials offer improved ionic conductivity and electrochemical stability for sodium-ion batteries. These compositions typically include sodium-containing oxides mixed with sulfide materials to create a composite structure that leverages the advantages of both material types. The hybrid approach helps overcome the limitations of pure oxide electrolytes (low conductivity) and pure sulfide electrolytes (poor stability), resulting in enhanced overall performance for sodium-ion battery applications.
- Interface engineering for oxide-sulfide electrolytes: Interface engineering techniques are employed to improve the contact between oxide and sulfide components in hybrid electrolytes, as well as between the electrolyte and electrodes. These methods include surface modifications, buffer layers, and specialized coating processes that reduce interfacial resistance and enhance ion transport across boundaries. Proper interface design is crucial for maintaining electrochemical stability during cycling and preventing unwanted side reactions that could degrade battery performance.
- Electrochemical performance enhancement strategies: Various strategies are employed to enhance the electrochemical performance of oxide-sulfide hybrid Na electrolytes, including doping with additional elements, controlling particle size distribution, and optimizing the ratio between oxide and sulfide components. These approaches aim to increase ionic conductivity, improve cycling stability, and enhance rate capability. Advanced synthesis methods such as mechanochemical processing and sol-gel techniques are utilized to create homogeneous hybrid structures with optimized performance characteristics.
- Structural design of oxide-sulfide hybrid electrolytes: The structural design of oxide-sulfide hybrid electrolytes focuses on creating optimized architectures that facilitate sodium ion transport while maintaining mechanical integrity. These designs include core-shell structures, layered configurations, and three-dimensional networks that maximize the contact area between components. Advanced characterization techniques are used to understand the relationship between structural features and electrochemical performance, enabling the development of electrolytes with enhanced properties for sodium-ion battery applications.
- Stability and safety improvements: Improving the stability and safety of oxide-sulfide hybrid Na electrolytes involves addressing challenges related to moisture sensitivity, thermal stability, and long-term cycling performance. Protective coatings, stabilizing additives, and encapsulation techniques are employed to shield sensitive components from environmental factors. These improvements are essential for practical applications, as they extend battery lifespan, enhance operational safety, and enable reliable performance under various operating conditions.
02 Interface engineering in hybrid electrolytes
Interface engineering between oxide and sulfide components is crucial for optimizing electrochemical performance in hybrid Na electrolytes. Techniques include creating gradient interfaces, surface modifications, and incorporating buffer layers to reduce interfacial resistance. These approaches minimize chemical and electrochemical reactions at interfaces, enhance ion transport across boundaries, and improve the overall stability and cycling performance of sodium batteries using hybrid electrolytes.Expand Specific Solutions03 Manufacturing methods for hybrid Na electrolytes
Various manufacturing techniques are employed to produce oxide-sulfide hybrid Na electrolytes with optimal electrochemical performance. These include mechanical milling, sol-gel processing, co-precipitation, and solid-state reactions. Advanced processing methods like cold sintering and spark plasma sintering help maintain the integrity of both oxide and sulfide components while creating effective interfaces between them. The manufacturing approach significantly impacts the microstructure, density, and ultimately the ionic conductivity of the hybrid electrolytes.Expand Specific Solutions04 Electrochemical performance enhancement strategies
Various strategies are employed to enhance the electrochemical performance of oxide-sulfide hybrid Na electrolytes. These include doping with elements like Al, Ga, or Zr to improve ionic conductivity, incorporating nanostructured materials to increase active surface area, and using polymer additives to improve mechanical properties. Optimization of the oxide-to-sulfide ratio is also critical for balancing conductivity, stability, and mechanical strength to achieve superior battery performance with extended cycle life.Expand Specific Solutions05 Applications in advanced sodium battery systems
Oxide-sulfide hybrid Na electrolytes are being applied in various advanced sodium battery systems. These include all-solid-state sodium batteries, sodium-sulfur batteries, and sodium-air batteries. The hybrid electrolytes enable higher energy density, improved safety, and better rate capability compared to conventional liquid electrolyte systems. They are particularly promising for grid-scale energy storage applications where cost, safety, and long-term stability are critical factors.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrochemical performance of oxide-sulfide hybrid Na electrolytes represents an emerging field in solid-state battery technology, currently in its early growth phase. The market is expanding rapidly with projections suggesting significant growth as sodium-ion technology offers a cost-effective alternative to lithium-ion batteries. Technical maturity remains moderate, with key players advancing at different rates. Research institutions like CNRS, University of Maryland, and Chinese Academy of Sciences are driving fundamental research, while commercial entities including LG Energy Solution, SVOLT, and Faradion are focusing on practical applications. TDK, Idemitsu Kosan, and Saft Groupe bring manufacturing expertise, creating a competitive landscape where academic-industrial partnerships are accelerating development toward commercial viability.
Chinese Academy of Sciences Institute of Physics
Technical Solution: The Chinese Academy of Sciences Institute of Physics has developed a sophisticated "dual-phase interpenetrating network" approach to oxide-sulfide hybrid Na electrolytes. Their technology creates a three-dimensional bicontinuous structure where both oxide (Na3Zr2Si2PO12) and sulfide (Na3PS4) phases form continuous networks throughout the material, maximizing ionic conductivity while maintaining mechanical integrity[1]. The manufacturing process involves a specialized freeze-casting technique followed by controlled sintering, creating aligned porous oxide structures that are subsequently infiltrated with sulfide electrolytes. This approach achieves room temperature ionic conductivities of 0.7-1.3 mS/cm with exceptional mechanical properties. A key innovation in their technology is the incorporation of small amounts (2-5 mol%) of Nb or Ta dopants into the oxide phase, which significantly improves the interfacial compatibility with the sulfide phase by modifying the local charge distribution and reducing interfacial resistance[2]. Their hybrid electrolytes demonstrate excellent electrochemical stability when tested against high-voltage cathodes (up to 4.4V vs. Na/Na+) and show minimal capacity degradation during extended cycling (>1000 cycles at 1C rate). The Institute has also developed specialized surface treatments using thin Al2O3 or Li3PO4 buffer layers to further enhance the oxide-sulfide interface properties.
Strengths: Superior long-term cycling stability compared to conventional electrolytes; excellent mechanical properties preventing dendrite penetration; high ionic conductivity enabling fast-charging capabilities. Weaknesses: Complex manufacturing process requiring precise control of multiple parameters; higher production costs compared to single-phase systems; potential challenges in quality control during large-scale production.
University of Maryland
Technical Solution: The University of Maryland has developed a groundbreaking approach to oxide-sulfide hybrid Na electrolytes through their "gradient interface engineering" technology. Their system creates a functionally graded structure where the composition gradually transitions from oxide-rich to sulfide-rich regions, eliminating abrupt interfaces that typically cause high resistance[1]. The core technology employs Na3Zr2Si2PO12 (NASICON) oxide and Na3PS4 sulfide materials with specially designed intermediate phases containing both oxygen and sulfur anions (Na3PO4-xSx) to facilitate smooth ion transport. Their manufacturing process involves controlled co-sintering under moderate pressure (200-300 MPa) and temperatures (150-250°C), creating dense electrolyte layers with minimal interfacial resistance. The University of Maryland researchers have demonstrated room temperature ionic conductivities of 0.8-1.2 mS/cm in these hybrid systems, with exceptional electrochemical stability windows exceeding 4.2V vs. Na/Na+[2]. Their technology also incorporates nanoscale ceramic fillers (Al2O3, SiO2) at specific concentrations to further enhance mechanical properties and suppress dendrite formation during cycling.
Strengths: Exceptionally low interfacial resistance due to gradient composition design; superior electrochemical stability window compared to pure sulfide systems; excellent mechanical properties preventing dendrite penetration. Weaknesses: Complex composition control requirements during manufacturing; potential challenges in large-scale production consistency; higher material costs compared to single-phase electrolytes.
Key Patents and Scientific Breakthroughs in Hybrid Electrolytes
Lithium mixed inorganic electrolytes
PatentWO2021191217A1
Innovation
- Development of mixed inorganic compounds with a specific formula ((A(t-v)Bv/2)[(PS4)(1-x)(OIX)x](1-y)(LinX2)y that combine the stability of oxides with the conductivity of sulphides, reducing toxic gas release and maintaining electrochemical performance.
Fluorinated sulfonamide-based electrolytes for non-lithium batteries thereof
PatentWO2025019663A2
Innovation
- The development of hybrid solvating electrolytes (HSEs) that combine strongly and weakly solvating solvents, specifically using halogenated sodium salts, halogenated sulfonates, and co-solvents like THF, to optimize key electrochemical parameters such as Coulombic efficiency and overpotential.
Materials Sustainability and Resource Availability
The sustainability of materials used in oxide-sulfide hybrid Na electrolytes represents a critical consideration for their large-scale implementation in energy storage technologies. Sodium-based batteries offer a promising alternative to lithium-ion systems due to the abundant nature of sodium resources. Sodium is the sixth most abundant element in the Earth's crust, with reserves estimated at 23,000 times greater than lithium, making it significantly more sustainable from a resource availability perspective.
When examining oxide-sulfide hybrid electrolytes specifically, the material composition typically includes sodium compounds combined with oxide materials (such as Na₃PS₄, Na₃PO₄) and sulfide components (like Na₃PS₄, Na₂S-P₂S₅). The oxide components often contain elements like silicon, aluminum, and phosphorus, which are generally abundant and accessible. However, some high-performance formulations may incorporate less common elements that could present supply chain challenges.
The sulfide components present more complex sustainability considerations. While sulfur itself is abundant as a byproduct of petroleum refining, some specialized sulfide compounds may require energy-intensive synthesis processes. The environmental impact of sulfide material production must be carefully evaluated, particularly regarding potential sulfur emissions during manufacturing and end-of-life recycling.
Manufacturing processes for hybrid electrolytes often require controlled environments due to the moisture sensitivity of sulfide materials, resulting in increased energy consumption. This energy requirement affects the overall carbon footprint and sustainability profile of these electrolyte systems. Implementing more efficient production methods and renewable energy sources in manufacturing facilities could significantly improve the environmental performance of these materials.
Recycling potential represents another crucial aspect of material sustainability. Current recycling infrastructure for sodium-based battery systems remains underdeveloped compared to lithium-ion technologies. The complex nature of hybrid electrolytes, combining both oxide and sulfide components, presents unique challenges for material recovery and separation. Developing effective recycling protocols specifically designed for these hybrid systems will be essential for closing the material loop and enhancing long-term sustainability.
The geographical distribution of raw materials for oxide-sulfide hybrid electrolytes generally presents fewer geopolitical concerns than lithium-based systems. Sodium resources are widely distributed globally, reducing supply chain vulnerabilities and potential resource monopolies. This distribution advantage could facilitate more equitable global access to energy storage technologies based on these materials.
When examining oxide-sulfide hybrid electrolytes specifically, the material composition typically includes sodium compounds combined with oxide materials (such as Na₃PS₄, Na₃PO₄) and sulfide components (like Na₃PS₄, Na₂S-P₂S₅). The oxide components often contain elements like silicon, aluminum, and phosphorus, which are generally abundant and accessible. However, some high-performance formulations may incorporate less common elements that could present supply chain challenges.
The sulfide components present more complex sustainability considerations. While sulfur itself is abundant as a byproduct of petroleum refining, some specialized sulfide compounds may require energy-intensive synthesis processes. The environmental impact of sulfide material production must be carefully evaluated, particularly regarding potential sulfur emissions during manufacturing and end-of-life recycling.
Manufacturing processes for hybrid electrolytes often require controlled environments due to the moisture sensitivity of sulfide materials, resulting in increased energy consumption. This energy requirement affects the overall carbon footprint and sustainability profile of these electrolyte systems. Implementing more efficient production methods and renewable energy sources in manufacturing facilities could significantly improve the environmental performance of these materials.
Recycling potential represents another crucial aspect of material sustainability. Current recycling infrastructure for sodium-based battery systems remains underdeveloped compared to lithium-ion technologies. The complex nature of hybrid electrolytes, combining both oxide and sulfide components, presents unique challenges for material recovery and separation. Developing effective recycling protocols specifically designed for these hybrid systems will be essential for closing the material loop and enhancing long-term sustainability.
The geographical distribution of raw materials for oxide-sulfide hybrid electrolytes generally presents fewer geopolitical concerns than lithium-based systems. Sodium resources are widely distributed globally, reducing supply chain vulnerabilities and potential resource monopolies. This distribution advantage could facilitate more equitable global access to energy storage technologies based on these materials.
Safety Considerations and Thermal Stability Analysis
Safety considerations and thermal stability are critical aspects when evaluating oxide-sulfide hybrid Na electrolytes for practical applications in energy storage systems. The inherent reactivity of sulfide-based components presents significant challenges that must be addressed before widespread commercial deployment. These hybrid electrolytes often exhibit thermal instability at elevated temperatures, with decomposition typically beginning around 150-200°C, considerably lower than pure oxide systems which can remain stable up to 300°C or higher.
The interface between oxide and sulfide components creates unique safety concerns due to potential chemical reactions under thermal stress. Studies have shown that these reactions can lead to the formation of volatile sulfur compounds, including hydrogen sulfide (H2S), which poses serious toxicity risks. Additionally, the release of these compounds can accelerate degradation of electrode materials and other cell components, compromising both safety and long-term performance.
Moisture sensitivity represents another critical safety consideration for hybrid electrolytes. When exposed to ambient air, sulfide components rapidly react with atmospheric moisture to produce H2S gas. This necessitates stringent handling protocols during manufacturing and assembly processes, typically requiring controlled atmosphere environments with extremely low humidity levels (<0.1 ppm H2O).
Recent thermal stability analyses using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) have revealed that the stability window of hybrid electrolytes can be significantly improved through strategic material selection and interface engineering. For instance, incorporating phosphate-based buffers between oxide and sulfide layers has demonstrated enhanced thermal stability up to 250°C in some systems, representing a substantial improvement over earlier generations.
Computational modeling approaches have emerged as valuable tools for predicting thermal behavior and potential degradation pathways. Density functional theory (DFT) calculations combined with molecular dynamics simulations now enable researchers to screen candidate materials and interface designs before experimental validation, accelerating the development of safer hybrid systems.
Fire safety testing protocols specific to oxide-sulfide hybrid electrolytes have been developed in recent years, focusing on thermal runaway behavior and combustion characteristics. These tests have shown that while hybrid systems generally present lower fire risks than liquid electrolytes, their failure modes can still produce hazardous conditions that require careful mitigation strategies, including advanced battery management systems and thermal isolation techniques.
The interface between oxide and sulfide components creates unique safety concerns due to potential chemical reactions under thermal stress. Studies have shown that these reactions can lead to the formation of volatile sulfur compounds, including hydrogen sulfide (H2S), which poses serious toxicity risks. Additionally, the release of these compounds can accelerate degradation of electrode materials and other cell components, compromising both safety and long-term performance.
Moisture sensitivity represents another critical safety consideration for hybrid electrolytes. When exposed to ambient air, sulfide components rapidly react with atmospheric moisture to produce H2S gas. This necessitates stringent handling protocols during manufacturing and assembly processes, typically requiring controlled atmosphere environments with extremely low humidity levels (<0.1 ppm H2O).
Recent thermal stability analyses using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) have revealed that the stability window of hybrid electrolytes can be significantly improved through strategic material selection and interface engineering. For instance, incorporating phosphate-based buffers between oxide and sulfide layers has demonstrated enhanced thermal stability up to 250°C in some systems, representing a substantial improvement over earlier generations.
Computational modeling approaches have emerged as valuable tools for predicting thermal behavior and potential degradation pathways. Density functional theory (DFT) calculations combined with molecular dynamics simulations now enable researchers to screen candidate materials and interface designs before experimental validation, accelerating the development of safer hybrid systems.
Fire safety testing protocols specific to oxide-sulfide hybrid electrolytes have been developed in recent years, focusing on thermal runaway behavior and combustion characteristics. These tests have shown that while hybrid systems generally present lower fire risks than liquid electrolytes, their failure modes can still produce hazardous conditions that require careful mitigation strategies, including advanced battery management systems and thermal isolation techniques.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!