Interfacial resistance reduction strategies in solid sodium cells
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid Sodium Batteries: Background and Objectives
Solid sodium batteries represent a promising next-generation energy storage technology that has gained significant attention in recent years due to their potential advantages over conventional lithium-ion batteries. The development of these batteries can be traced back to the 1970s, when researchers began exploring solid electrolytes for sodium-ion conduction. However, substantial progress has only been achieved in the past decade, driven by increasing concerns about lithium resource limitations and the growing demand for sustainable energy storage solutions.
The evolution of solid sodium battery technology has followed a trajectory marked by incremental improvements in electrolyte materials, electrode compositions, and cell architectures. Early research focused primarily on ceramic electrolytes such as NASICON-type materials, which demonstrated good ionic conductivity but suffered from poor mechanical properties and high interfacial resistance. Recent advances have expanded the material options to include polymer electrolytes, sulfide-based systems, and composite structures that combine the advantages of different material classes.
A critical challenge in solid sodium batteries is the high interfacial resistance between the solid electrolyte and electrodes, which significantly limits battery performance. This resistance arises from chemical incompatibility, mechanical contact issues, and space charge effects at the interfaces. Addressing these interfacial challenges has become a central focus in the field, with researchers exploring various strategies including buffer layers, interface engineering, and novel electrode designs.
The primary technical objectives for solid sodium battery development include achieving room-temperature ionic conductivity comparable to liquid electrolytes (>10^-3 S/cm), minimizing interfacial resistance to enable high current density operation, improving mechanical stability to prevent dendrite formation, and ensuring long-term cycling stability under practical operating conditions. Additionally, there is a strong emphasis on developing manufacturing processes that are scalable and cost-effective to facilitate commercial adoption.
From a broader perspective, solid sodium batteries aim to offer a more sustainable alternative to lithium-ion technology by utilizing abundant sodium resources while providing comparable or superior performance metrics. The ultimate goal is to develop energy storage systems with higher energy density, enhanced safety characteristics, longer cycle life, and lower production costs than current commercial technologies, thereby enabling widespread application in electric vehicles, grid storage, and portable electronics.
The technological trajectory suggests that interfacial resistance reduction will remain a key focus area, with interdisciplinary approaches combining materials science, electrochemistry, and engineering principles being essential to overcome existing limitations and realize the full potential of solid sodium batteries.
The evolution of solid sodium battery technology has followed a trajectory marked by incremental improvements in electrolyte materials, electrode compositions, and cell architectures. Early research focused primarily on ceramic electrolytes such as NASICON-type materials, which demonstrated good ionic conductivity but suffered from poor mechanical properties and high interfacial resistance. Recent advances have expanded the material options to include polymer electrolytes, sulfide-based systems, and composite structures that combine the advantages of different material classes.
A critical challenge in solid sodium batteries is the high interfacial resistance between the solid electrolyte and electrodes, which significantly limits battery performance. This resistance arises from chemical incompatibility, mechanical contact issues, and space charge effects at the interfaces. Addressing these interfacial challenges has become a central focus in the field, with researchers exploring various strategies including buffer layers, interface engineering, and novel electrode designs.
The primary technical objectives for solid sodium battery development include achieving room-temperature ionic conductivity comparable to liquid electrolytes (>10^-3 S/cm), minimizing interfacial resistance to enable high current density operation, improving mechanical stability to prevent dendrite formation, and ensuring long-term cycling stability under practical operating conditions. Additionally, there is a strong emphasis on developing manufacturing processes that are scalable and cost-effective to facilitate commercial adoption.
From a broader perspective, solid sodium batteries aim to offer a more sustainable alternative to lithium-ion technology by utilizing abundant sodium resources while providing comparable or superior performance metrics. The ultimate goal is to develop energy storage systems with higher energy density, enhanced safety characteristics, longer cycle life, and lower production costs than current commercial technologies, thereby enabling widespread application in electric vehicles, grid storage, and portable electronics.
The technological trajectory suggests that interfacial resistance reduction will remain a key focus area, with interdisciplinary approaches combining materials science, electrochemistry, and engineering principles being essential to overcome existing limitations and realize the full potential of solid sodium batteries.
Market Analysis for Solid-State Sodium Battery Technology
The solid-state sodium battery market is experiencing significant growth driven by the increasing demand for sustainable and cost-effective energy storage solutions. Current market projections indicate that the global solid-state battery market, including sodium-based technologies, could reach $8 billion by 2030, with sodium batteries potentially capturing 15-20% of this emerging sector. This growth is primarily fueled by the inherent advantages of sodium as a more abundant and geographically distributed resource compared to lithium, offering a cost advantage of approximately 30-40% in raw material expenses.
Market demand for solid sodium batteries is particularly strong in stationary energy storage applications, where cost considerations often outweigh energy density requirements. Grid-scale storage represents the largest potential market segment, with an estimated compound annual growth rate of 25% through 2028. Electric vehicles constitute another promising market, especially in regions focusing on developing affordable electric mobility solutions, where the lower cost profile of sodium batteries presents a compelling value proposition.
Consumer electronics manufacturers are also showing increased interest in solid sodium battery technology, particularly for applications where safety is paramount. The elimination of flammable liquid electrolytes addresses significant safety concerns that have plagued conventional lithium-ion batteries.
Regional market analysis reveals that Asia-Pacific, particularly China, South Korea, and Japan, leads in solid sodium battery research and commercialization efforts. European markets show strong interest driven by sustainability initiatives and strategic autonomy concerns regarding battery supply chains. North American markets are gradually increasing investments, with particular focus on grid storage applications.
Key market drivers include the rising costs and supply constraints of lithium, cobalt, and nickel, which have pushed battery manufacturers to explore alternative chemistries. Environmental regulations and sustainability goals are also accelerating market adoption, as sodium batteries offer reduced environmental impact throughout their lifecycle compared to conventional lithium-ion technologies.
Market barriers primarily center around the technical challenges of interfacial resistance in solid sodium cells, which directly impacts performance metrics valued by consumers. Until these technical hurdles are overcome, widespread commercial adoption remains limited. Additionally, the established manufacturing infrastructure for lithium-ion batteries creates significant market inertia that sodium technologies must overcome.
Market demand for solid sodium batteries is particularly strong in stationary energy storage applications, where cost considerations often outweigh energy density requirements. Grid-scale storage represents the largest potential market segment, with an estimated compound annual growth rate of 25% through 2028. Electric vehicles constitute another promising market, especially in regions focusing on developing affordable electric mobility solutions, where the lower cost profile of sodium batteries presents a compelling value proposition.
Consumer electronics manufacturers are also showing increased interest in solid sodium battery technology, particularly for applications where safety is paramount. The elimination of flammable liquid electrolytes addresses significant safety concerns that have plagued conventional lithium-ion batteries.
Regional market analysis reveals that Asia-Pacific, particularly China, South Korea, and Japan, leads in solid sodium battery research and commercialization efforts. European markets show strong interest driven by sustainability initiatives and strategic autonomy concerns regarding battery supply chains. North American markets are gradually increasing investments, with particular focus on grid storage applications.
Key market drivers include the rising costs and supply constraints of lithium, cobalt, and nickel, which have pushed battery manufacturers to explore alternative chemistries. Environmental regulations and sustainability goals are also accelerating market adoption, as sodium batteries offer reduced environmental impact throughout their lifecycle compared to conventional lithium-ion technologies.
Market barriers primarily center around the technical challenges of interfacial resistance in solid sodium cells, which directly impacts performance metrics valued by consumers. Until these technical hurdles are overcome, widespread commercial adoption remains limited. Additionally, the established manufacturing infrastructure for lithium-ion batteries creates significant market inertia that sodium technologies must overcome.
Interfacial Resistance Challenges in Solid Sodium Cells
Solid-state sodium batteries represent a promising alternative to lithium-ion technology due to sodium's abundance and cost-effectiveness. However, interfacial resistance remains one of the most significant challenges hindering their commercial viability. This resistance occurs at multiple interfaces within the cell, primarily between the solid electrolyte and electrodes, creating barriers to efficient ion transport and limiting overall battery performance.
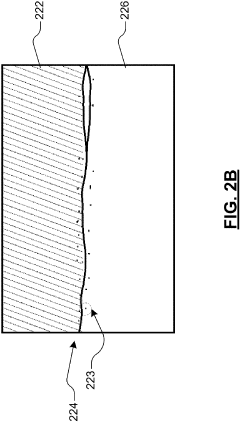
The interfacial resistance in solid sodium cells stems from several interconnected factors. Chemical incompatibility between cell components leads to the formation of resistive interphases that impede sodium ion migration. Physical contact issues, including voids and gaps at interfaces due to volume changes during cycling, further exacerbate the problem by reducing the effective contact area for ion transfer.
Mechanical stress during battery operation contributes significantly to interfacial degradation. As sodium ions intercalate and deintercalate, the resulting volume changes create mechanical strain that can lead to delamination between components, cracking of the solid electrolyte, and loss of intimate contact at critical interfaces. These mechanical failures progressively increase cell resistance over multiple cycles.
Space charge layers represent another critical challenge. The accumulation of charged species at interfaces creates localized electric fields that impede ion transport, effectively raising the energy barrier for sodium ion migration across interfaces. This phenomenon is particularly pronounced in ceramic electrolytes with high ionic conductivity but poor interfacial properties.
Dendrite formation at the sodium metal anode/electrolyte interface poses both performance and safety concerns. Unlike lithium, sodium's lower surface energy makes it more prone to non-uniform deposition, leading to dendrite growth that can penetrate the solid electrolyte, causing short circuits and catastrophic failure.
The stability of interfaces under electrochemical cycling presents ongoing challenges. Side reactions between cell components during operation can continuously generate new interfacial phases with poor ionic conductivity. These reaction products accumulate over time, progressively increasing cell impedance and degrading performance.
Temperature sensitivity further complicates interfacial resistance management. Many solid electrolytes exhibit dramatically different conductivity and mechanical properties across operating temperature ranges, leading to variable interfacial behavior that makes consistent performance difficult to achieve across practical usage conditions.
Addressing these interfacial challenges requires multidisciplinary approaches combining materials science, electrochemistry, and engineering solutions. The development of stable interfaces that maintain both chemical compatibility and physical contact throughout battery operation remains one of the most critical research directions for advancing solid sodium battery technology.
The interfacial resistance in solid sodium cells stems from several interconnected factors. Chemical incompatibility between cell components leads to the formation of resistive interphases that impede sodium ion migration. Physical contact issues, including voids and gaps at interfaces due to volume changes during cycling, further exacerbate the problem by reducing the effective contact area for ion transfer.
Mechanical stress during battery operation contributes significantly to interfacial degradation. As sodium ions intercalate and deintercalate, the resulting volume changes create mechanical strain that can lead to delamination between components, cracking of the solid electrolyte, and loss of intimate contact at critical interfaces. These mechanical failures progressively increase cell resistance over multiple cycles.
Space charge layers represent another critical challenge. The accumulation of charged species at interfaces creates localized electric fields that impede ion transport, effectively raising the energy barrier for sodium ion migration across interfaces. This phenomenon is particularly pronounced in ceramic electrolytes with high ionic conductivity but poor interfacial properties.
Dendrite formation at the sodium metal anode/electrolyte interface poses both performance and safety concerns. Unlike lithium, sodium's lower surface energy makes it more prone to non-uniform deposition, leading to dendrite growth that can penetrate the solid electrolyte, causing short circuits and catastrophic failure.
The stability of interfaces under electrochemical cycling presents ongoing challenges. Side reactions between cell components during operation can continuously generate new interfacial phases with poor ionic conductivity. These reaction products accumulate over time, progressively increasing cell impedance and degrading performance.
Temperature sensitivity further complicates interfacial resistance management. Many solid electrolytes exhibit dramatically different conductivity and mechanical properties across operating temperature ranges, leading to variable interfacial behavior that makes consistent performance difficult to achieve across practical usage conditions.
Addressing these interfacial challenges requires multidisciplinary approaches combining materials science, electrochemistry, and engineering solutions. The development of stable interfaces that maintain both chemical compatibility and physical contact throughout battery operation remains one of the most critical research directions for advancing solid sodium battery technology.
Current Interfacial Resistance Reduction Approaches
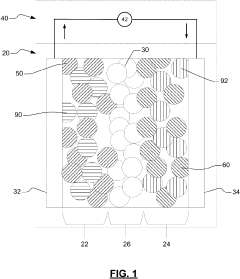
01 Solid electrolyte interfaces for sodium batteries
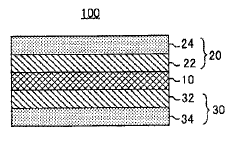
Solid electrolyte interfaces (SEI) play a crucial role in sodium batteries by providing a protective layer between the electrode and electrolyte. These interfaces help reduce interfacial resistance and improve battery performance. Various materials and compositions can be used to form effective SEI layers that enhance sodium ion transport while minimizing unwanted side reactions. Properly engineered SEI layers can significantly improve the cycling stability and efficiency of solid sodium cells.- Solid electrolyte interfaces for sodium batteries: Solid electrolyte interfaces (SEI) play a crucial role in sodium batteries by providing a protective layer between the electrode and electrolyte. These interfaces help reduce interfacial resistance and improve battery performance. Various materials and compositions can be used to form effective SEI layers that enhance sodium ion transport while minimizing unwanted side reactions. The formation and stability of these interfaces directly impact the cycling performance and longevity of solid sodium cells.
- Electrode coatings and surface modifications: Surface modifications and specialized coatings on electrodes can significantly reduce interfacial resistance in solid sodium cells. These modifications create favorable interfaces that facilitate sodium ion transport across electrode-electrolyte boundaries. Techniques include applying thin protective layers, functional coatings, or surface treatments that improve wettability and compatibility between components. Such modifications help prevent unwanted reactions and stabilize the electrode-electrolyte interface during cycling.
- Composite electrolyte systems: Composite electrolyte systems combine different materials to optimize ionic conductivity while reducing interfacial resistance. These systems often incorporate polymer components, ceramic fillers, or other additives that create favorable interfaces with electrodes. The composite approach allows for tailoring of mechanical properties and interfacial characteristics simultaneously. By creating synergistic combinations of materials, these systems can address multiple challenges related to sodium ion transport across interfaces in solid-state batteries.
- Interface engineering and additives: Interface engineering involves the strategic use of additives and interlayers to minimize resistance at electrode-electrolyte boundaries in solid sodium cells. Various compounds can be incorporated to modify interfacial properties, promote sodium ion transport, and suppress side reactions. These additives can form in-situ protective layers, modify surface energies, or create artificial interfaces with optimized properties. Careful selection of these components can significantly improve the overall performance and stability of solid sodium batteries.
- Novel electrode and electrolyte materials: Development of novel materials for electrodes and electrolytes can inherently reduce interfacial resistance in solid sodium cells. These materials are designed with specific crystal structures, compositions, or surface properties that naturally form low-resistance interfaces. Research focuses on materials with high sodium ion conductivity, good mechanical properties, and chemical compatibility with other battery components. Advanced materials can eliminate the need for additional interface modifications while providing stable performance over extended cycling.
02 Electrode coatings to reduce interfacial resistance
Specialized coatings can be applied to electrodes in solid sodium cells to reduce interfacial resistance. These coatings create a stable interface between the electrode and electrolyte, facilitating sodium ion transport while preventing unwanted reactions. Materials such as metal oxides, polymers, and composite materials can be used as effective coatings. The thickness, uniformity, and composition of these coatings are critical factors that determine their effectiveness in reducing interfacial resistance.Expand Specific Solutions03 Electrolyte additives for interface stabilization
Various additives can be incorporated into the electrolyte of solid sodium cells to stabilize the electrode-electrolyte interface and reduce interfacial resistance. These additives can form protective films, scavenge impurities, or modify the surface chemistry of electrodes. Common additives include fluorinated compounds, ionic liquids, and inorganic salts. The selection of appropriate additives depends on the specific electrode materials and operating conditions of the sodium cell.Expand Specific Solutions04 Interface engineering through material selection
The selection of compatible electrode and electrolyte materials is crucial for minimizing interfacial resistance in solid sodium cells. Materials with similar crystal structures or chemical properties at their interfaces tend to have lower resistance. Advanced material combinations can be designed to promote sodium ion transport across interfaces while maintaining structural integrity. Considerations include lattice matching, chemical stability, and mechanical properties at the interface region.Expand Specific Solutions05 Temperature and pressure effects on interfacial resistance
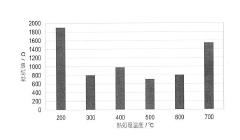
Operating conditions, particularly temperature and pressure, significantly impact the interfacial resistance in solid sodium cells. Higher temperatures generally reduce interfacial resistance by enhancing ion mobility and promoting better contact between components. Applied pressure can improve physical contact at interfaces, reducing resistance. However, these parameters must be carefully controlled to prevent degradation of cell components. Optimized temperature and pressure profiles can be developed for specific cell designs to minimize interfacial resistance.Expand Specific Solutions
Leading Companies and Research Institutions in Solid Sodium Battery Development
The interfacial resistance reduction in solid sodium cells market is currently in an early growth phase, characterized by intensive research and development activities. The market is projected to expand significantly as solid-state battery technology gains traction, with an estimated compound annual growth rate of 25-30% over the next five years. Technologically, the field remains in development with varying maturity levels across different approaches. Leading players include LG Energy Solution and LG Chem focusing on commercial applications, while academic institutions like Shanghai Institute of Ceramics and Beijing Institute of Technology drive fundamental research. Applied Materials and GLOBALFOUNDRIES contribute manufacturing expertise, while companies like Panasonic and Intel invest in proprietary interface engineering solutions to overcome the critical challenges of sodium ion transport across solid-solid interfaces.
Panasonic Intellectual Property Management Co. Ltd.
Technical Solution: Panasonic has developed a comprehensive interfacial resistance reduction strategy for solid sodium batteries centered around their proprietary "Adaptive Interface Technology" (AIT). This approach utilizes specially formulated interlayers containing sodium superionic conductor materials that are applied between electrodes and solid electrolytes. The interlayers are designed to maintain excellent contact during volume changes while facilitating rapid sodium ion transport. Panasonic's technology incorporates pressure-sensitive adhesive components that ensure continuous physical contact at interfaces even during thermal expansion and contraction cycles. Their research has demonstrated that these adaptive interfaces can reduce contact resistance by up to 60% compared to conventional designs. Additionally, Panasonic has pioneered surface treatment methods using plasma-assisted deposition techniques that modify the surface chemistry of electrodes to enhance wettability with solid electrolytes. The company has also developed specialized heat treatment protocols that promote interfacial bonding without degrading the electrochemical properties of active materials.
Strengths: Highly effective at maintaining interface contact during cycling; compatible with existing manufacturing infrastructure; addresses both chemical and mechanical aspects of interfacial resistance. Weaknesses: Requires additional processing steps that may increase production costs; some components have limited temperature stability; optimization needed for different electrode chemistries.
LG Chem Ltd.
Technical Solution: LG Chem has developed innovative interfacial engineering strategies for solid sodium batteries focusing on composite electrolyte systems. Their approach combines polymer and ceramic electrolytes to create flexible interfaces that maintain good ionic conductivity while accommodating volume changes during cycling. The company has pioneered the use of sodium-conducting solid electrolyte interphases (SEIs) that are artificially constructed using specialized coating techniques. These engineered interfaces incorporate sodium-ion conducting additives and surface modifiers that significantly reduce interfacial resistance. LG Chem has also developed proprietary pressure-application systems during cell assembly that ensure optimal contact between electrodes and solid electrolytes, addressing one of the fundamental challenges in solid-state battery technology. Their research indicates that these combined approaches can reduce interfacial resistance by up to 70% compared to conventional solid sodium cell designs.
Strengths: Comprehensive approach combining materials science and engineering solutions; scalable manufacturing processes suitable for mass production; significant reduction in interfacial resistance without compromising safety. Weaknesses: Higher production costs compared to liquid electrolyte systems; potential long-term stability issues at high cycling rates; requires precise control of manufacturing conditions.
Key Patents and Research on Sodium Ion Transport at Interfaces
Methods to reduce interfacial resistance in solid-state battery
PatentInactiveUS20230246241A1
Innovation
- A method involving heating a cell with a solid-state electrolyte adjacent to a lithium-metal electrode to a temperature between 50°C and 175°C, while cycling it, to improve interfacial contact and reduce resistance, potentially including impurities like Li2O and Li2CO3, and cycling at elevated temperatures to enhance contact area and electrochemical plating.
Manufacturing method of positive electrode of sodium all-solid-state battery
PatentActiveJP2021068672A
Innovation
- A manufacturing method for a sodium all-solid-state battery positive electrode involves mixing a P2-type layered crystal structure positive electrode active material with a NASICON-type phosphate compound and subjecting the mixture to heat treatment between 300°C and 600°C to form a film that suppresses the formation of by-products and reduces interfacial resistance.
Materials Compatibility and Stability Considerations
The compatibility and stability of materials in solid sodium cells represent critical factors determining long-term performance and safety. When selecting materials for solid electrolytes, electrodes, and interfaces, chemical stability must be prioritized to prevent unwanted side reactions that could lead to performance degradation. Sodium metal anodes, while offering high theoretical capacity, are particularly reactive with most solid electrolytes, forming interphases that increase interfacial resistance over time.
Thermodynamic stability between solid electrolytes and electrode materials requires careful consideration of their electrochemical potential windows. Materials like Na3PS4 and Na3SbS4 sulfide electrolytes demonstrate excellent ionic conductivity but suffer from narrow electrochemical windows, limiting their compatibility with high-voltage cathodes. Oxide-based electrolytes such as NASICON-type Na3Zr2Si2PO12 offer wider stability windows but typically exhibit lower ionic conductivity at room temperature.
Mechanical stability at interfaces presents another significant challenge. Volume changes during sodium insertion/extraction can create mechanical stress, leading to contact loss and increased interfacial resistance. This is particularly problematic at the sodium metal anode interface, where continuous plating and stripping processes can cause mechanical degradation of the solid electrolyte surface. Strategies incorporating buffer layers with appropriate mechanical properties have shown promise in accommodating these volume changes.
Environmental stability factors, including moisture and oxygen sensitivity, significantly impact material selection and cell design. Many sodium-ion conducting materials, particularly sulfide-based electrolytes, are highly hygroscopic and react readily with atmospheric moisture, forming hydrogen sulfide gas and degrading rapidly. This necessitates stringent manufacturing conditions and robust encapsulation technologies to ensure long-term stability.
Temperature-dependent compatibility issues further complicate material selection. Thermal expansion coefficient mismatches between cell components can induce mechanical stress during temperature fluctuations, potentially creating microcracks that increase interfacial resistance. Additionally, phase transitions in certain solid electrolytes at elevated temperatures can alter their conductive properties and interfacial contact characteristics.
Addressing these compatibility and stability challenges requires integrated approaches combining protective coatings, buffer layers, and gradient interfaces. Recent advances in artificial solid electrolyte interphase (SEI) engineering have demonstrated significant improvements in interface stability, with thin layers of NaF, Na3N, or sodium phosphates showing promise in protecting reactive interfaces while maintaining sodium-ion transport pathways.
Thermodynamic stability between solid electrolytes and electrode materials requires careful consideration of their electrochemical potential windows. Materials like Na3PS4 and Na3SbS4 sulfide electrolytes demonstrate excellent ionic conductivity but suffer from narrow electrochemical windows, limiting their compatibility with high-voltage cathodes. Oxide-based electrolytes such as NASICON-type Na3Zr2Si2PO12 offer wider stability windows but typically exhibit lower ionic conductivity at room temperature.
Mechanical stability at interfaces presents another significant challenge. Volume changes during sodium insertion/extraction can create mechanical stress, leading to contact loss and increased interfacial resistance. This is particularly problematic at the sodium metal anode interface, where continuous plating and stripping processes can cause mechanical degradation of the solid electrolyte surface. Strategies incorporating buffer layers with appropriate mechanical properties have shown promise in accommodating these volume changes.
Environmental stability factors, including moisture and oxygen sensitivity, significantly impact material selection and cell design. Many sodium-ion conducting materials, particularly sulfide-based electrolytes, are highly hygroscopic and react readily with atmospheric moisture, forming hydrogen sulfide gas and degrading rapidly. This necessitates stringent manufacturing conditions and robust encapsulation technologies to ensure long-term stability.
Temperature-dependent compatibility issues further complicate material selection. Thermal expansion coefficient mismatches between cell components can induce mechanical stress during temperature fluctuations, potentially creating microcracks that increase interfacial resistance. Additionally, phase transitions in certain solid electrolytes at elevated temperatures can alter their conductive properties and interfacial contact characteristics.
Addressing these compatibility and stability challenges requires integrated approaches combining protective coatings, buffer layers, and gradient interfaces. Recent advances in artificial solid electrolyte interphase (SEI) engineering have demonstrated significant improvements in interface stability, with thin layers of NaF, Na3N, or sodium phosphates showing promise in protecting reactive interfaces while maintaining sodium-ion transport pathways.
Scalability and Manufacturing Challenges
The transition from laboratory-scale prototypes to mass production represents a significant hurdle for solid sodium cell technologies. Current interfacial resistance reduction strategies that show promise in controlled research environments often face substantial challenges when implemented at industrial scale. The manufacturing processes for solid electrolyte materials require precise control of synthesis conditions, particle size distribution, and purity levels—all factors that directly impact interfacial properties and subsequent cell performance.
Material consistency presents a primary challenge, as batch-to-batch variations in solid electrolyte composition can lead to unpredictable interfacial behaviors. The production of uniform, defect-free solid electrolytes with consistent ionic conductivity properties demands sophisticated quality control systems that may significantly increase manufacturing costs. Additionally, the sensitive nature of sodium metal and many solid electrolyte materials to moisture and oxygen necessitates specialized handling environments throughout the production process.
Interface engineering techniques such as interlayer deposition methods that effectively reduce resistance in laboratory settings often employ processes incompatible with high-throughput manufacturing. Techniques like atomic layer deposition provide excellent control over interfacial properties but operate at speeds incompatible with commercial production targets. Alternative approaches using solution-based coating methods offer better scalability but frequently deliver less consistent interfacial properties.
The assembly of solid sodium cells presents unique challenges compared to conventional lithium-ion batteries. The pressure requirements for maintaining good interfacial contact between solid components must be consistently applied across cells in mass production. Current cell designs often require complex mechanical systems to maintain this pressure, adding complexity to manufacturing lines and increasing production costs.
Economic considerations further complicate scalability, as many interfacial resistance reduction strategies rely on expensive materials or processing techniques. The cost-performance balance must be carefully evaluated, particularly when considering noble metal interlayers or specialized surface modification treatments. Manufacturing processes must be developed that can deliver the required interfacial properties while remaining economically viable at production scale.
Standardization represents another critical challenge, as the diversity of approaches to interfacial resistance reduction has resulted in fragmented manufacturing protocols. The establishment of industry standards for interfacial resistance metrics and testing protocols would facilitate more efficient scaling of promising technologies from laboratory to production environments.
Material consistency presents a primary challenge, as batch-to-batch variations in solid electrolyte composition can lead to unpredictable interfacial behaviors. The production of uniform, defect-free solid electrolytes with consistent ionic conductivity properties demands sophisticated quality control systems that may significantly increase manufacturing costs. Additionally, the sensitive nature of sodium metal and many solid electrolyte materials to moisture and oxygen necessitates specialized handling environments throughout the production process.
Interface engineering techniques such as interlayer deposition methods that effectively reduce resistance in laboratory settings often employ processes incompatible with high-throughput manufacturing. Techniques like atomic layer deposition provide excellent control over interfacial properties but operate at speeds incompatible with commercial production targets. Alternative approaches using solution-based coating methods offer better scalability but frequently deliver less consistent interfacial properties.
The assembly of solid sodium cells presents unique challenges compared to conventional lithium-ion batteries. The pressure requirements for maintaining good interfacial contact between solid components must be consistently applied across cells in mass production. Current cell designs often require complex mechanical systems to maintain this pressure, adding complexity to manufacturing lines and increasing production costs.
Economic considerations further complicate scalability, as many interfacial resistance reduction strategies rely on expensive materials or processing techniques. The cost-performance balance must be carefully evaluated, particularly when considering noble metal interlayers or specialized surface modification treatments. Manufacturing processes must be developed that can deliver the required interfacial properties while remaining economically viable at production scale.
Standardization represents another critical challenge, as the diversity of approaches to interfacial resistance reduction has resulted in fragmented manufacturing protocols. The establishment of industry standards for interfacial resistance metrics and testing protocols would facilitate more efficient scaling of promising technologies from laboratory to production environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!