Electrochemical window determination of sodium solid electrolytes
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Solid Electrolytes Electrochemical Window Background and Objectives
The evolution of sodium-ion batteries (SIBs) has gained significant momentum as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. Within this technological landscape, solid-state electrolytes have emerged as critical components that could potentially address safety concerns associated with conventional liquid electrolytes while offering improved electrochemical performance. The electrochemical window, which defines the voltage range within which an electrolyte remains stable without decomposition, is a fundamental parameter that determines the practical energy density and long-term stability of sodium-ion batteries.
Historically, the development of sodium solid electrolytes can be traced back to the 1970s with the discovery of Na-β-alumina. However, significant advancements in this field have only materialized in the past decade, driven by increasing demands for safer and higher-energy-density energy storage solutions. The technological trajectory has evolved from ceramic-based electrolytes to polymer-based systems, and more recently, to composite electrolytes that combine the advantages of both materials.
The primary objective of investigating the electrochemical window determination of sodium solid electrolytes is to establish reliable methodologies for accurately measuring and characterizing the voltage stability limits of these materials. This is crucial because an underestimated electrochemical window may lead to premature battery failure, while an overestimated window might result in unexpected electrolyte decomposition during battery operation, compromising safety and performance.
Current research aims to standardize testing protocols for electrochemical window determination, as inconsistencies in measurement techniques have led to significant variations in reported values across the scientific literature. Additionally, there is a growing focus on understanding the interfacial phenomena between sodium solid electrolytes and electrodes, as these interactions often dictate the practical electrochemical stability window in full-cell configurations.
The technological goals include developing sodium solid electrolytes with wider electrochemical windows (ideally exceeding 4V vs. Na/Na+), improved ionic conductivity at room temperature (>10^-3 S/cm), and enhanced mechanical properties to prevent dendrite formation. These advancements would enable the implementation of high-voltage cathode materials, thereby increasing the energy density of sodium-ion batteries to levels competitive with current lithium-ion technologies.
Furthermore, researchers are exploring the correlation between chemical composition, crystal structure, and electrochemical stability to establish design principles for next-generation sodium solid electrolytes. This fundamental understanding is expected to accelerate the development of tailored materials with optimized properties for specific battery applications, ranging from grid-scale energy storage to electric vehicles.
Historically, the development of sodium solid electrolytes can be traced back to the 1970s with the discovery of Na-β-alumina. However, significant advancements in this field have only materialized in the past decade, driven by increasing demands for safer and higher-energy-density energy storage solutions. The technological trajectory has evolved from ceramic-based electrolytes to polymer-based systems, and more recently, to composite electrolytes that combine the advantages of both materials.
The primary objective of investigating the electrochemical window determination of sodium solid electrolytes is to establish reliable methodologies for accurately measuring and characterizing the voltage stability limits of these materials. This is crucial because an underestimated electrochemical window may lead to premature battery failure, while an overestimated window might result in unexpected electrolyte decomposition during battery operation, compromising safety and performance.
Current research aims to standardize testing protocols for electrochemical window determination, as inconsistencies in measurement techniques have led to significant variations in reported values across the scientific literature. Additionally, there is a growing focus on understanding the interfacial phenomena between sodium solid electrolytes and electrodes, as these interactions often dictate the practical electrochemical stability window in full-cell configurations.
The technological goals include developing sodium solid electrolytes with wider electrochemical windows (ideally exceeding 4V vs. Na/Na+), improved ionic conductivity at room temperature (>10^-3 S/cm), and enhanced mechanical properties to prevent dendrite formation. These advancements would enable the implementation of high-voltage cathode materials, thereby increasing the energy density of sodium-ion batteries to levels competitive with current lithium-ion technologies.
Furthermore, researchers are exploring the correlation between chemical composition, crystal structure, and electrochemical stability to establish design principles for next-generation sodium solid electrolytes. This fundamental understanding is expected to accelerate the development of tailored materials with optimized properties for specific battery applications, ranging from grid-scale energy storage to electric vehicles.
Market Analysis for Sodium-ion Battery Technologies
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion technologies, driven by increasing concerns about lithium supply chain vulnerabilities and cost fluctuations. Current market projections indicate the global sodium-ion battery market could reach $1.2 billion by 2025, with a compound annual growth rate exceeding 25% through 2030, particularly accelerated by grid storage applications and electric mobility solutions in emerging markets.
Sodium solid electrolytes represent a critical component within this expanding market, with their electrochemical window properties directly influencing battery safety, longevity, and performance characteristics. Market research indicates that manufacturers prioritizing wider electrochemical windows in their sodium solid electrolytes can command premium pricing, with current high-performance formulations selling at 30-40% above standard offerings.
Regional analysis reveals China leading the sodium-ion battery development landscape, accounting for approximately 45% of patents and commercial initiatives in this space. European markets follow with strong research programs focused specifically on solid electrolyte innovations, while North American companies have recently increased investment in sodium-based energy storage technologies by 65% year-over-year.
Consumer electronics represents an emerging application segment, with manufacturers exploring sodium-ion batteries for portable devices where cost sensitivity outweighs energy density requirements. However, grid-scale energy storage remains the dominant market driver, representing nearly 60% of current sodium-ion battery deployments, with particular growth in regions facing lithium supply constraints.
Market segmentation analysis reveals three distinct tiers of sodium solid electrolyte products: budget solutions with narrower electrochemical windows for non-critical applications, mid-range formulations balancing performance and cost, and premium solutions featuring expanded electrochemical stability ranges for high-reliability applications. This tiered market structure has emerged over the past 18 months as manufacturers refine their product positioning.
Investor interest in sodium-ion battery technologies has surged, with venture capital funding increasing by 85% in the past year. Strategic partnerships between electrolyte developers and battery manufacturers have become increasingly common, creating integrated supply chains that accelerate commercialization timelines and reduce market entry barriers for new technologies with superior electrochemical window properties.
Customer adoption patterns indicate growing acceptance of sodium-ion solutions, particularly in price-sensitive markets and applications where the wider electrochemical windows of advanced solid electrolytes address specific safety and longevity requirements that lithium-ion alternatives cannot economically match.
Sodium solid electrolytes represent a critical component within this expanding market, with their electrochemical window properties directly influencing battery safety, longevity, and performance characteristics. Market research indicates that manufacturers prioritizing wider electrochemical windows in their sodium solid electrolytes can command premium pricing, with current high-performance formulations selling at 30-40% above standard offerings.
Regional analysis reveals China leading the sodium-ion battery development landscape, accounting for approximately 45% of patents and commercial initiatives in this space. European markets follow with strong research programs focused specifically on solid electrolyte innovations, while North American companies have recently increased investment in sodium-based energy storage technologies by 65% year-over-year.
Consumer electronics represents an emerging application segment, with manufacturers exploring sodium-ion batteries for portable devices where cost sensitivity outweighs energy density requirements. However, grid-scale energy storage remains the dominant market driver, representing nearly 60% of current sodium-ion battery deployments, with particular growth in regions facing lithium supply constraints.
Market segmentation analysis reveals three distinct tiers of sodium solid electrolyte products: budget solutions with narrower electrochemical windows for non-critical applications, mid-range formulations balancing performance and cost, and premium solutions featuring expanded electrochemical stability ranges for high-reliability applications. This tiered market structure has emerged over the past 18 months as manufacturers refine their product positioning.
Investor interest in sodium-ion battery technologies has surged, with venture capital funding increasing by 85% in the past year. Strategic partnerships between electrolyte developers and battery manufacturers have become increasingly common, creating integrated supply chains that accelerate commercialization timelines and reduce market entry barriers for new technologies with superior electrochemical window properties.
Customer adoption patterns indicate growing acceptance of sodium-ion solutions, particularly in price-sensitive markets and applications where the wider electrochemical windows of advanced solid electrolytes address specific safety and longevity requirements that lithium-ion alternatives cannot economically match.
Current Challenges in Electrochemical Window Determination
Despite significant advancements in sodium solid electrolyte research, determining accurate electrochemical windows remains a persistent challenge. Current methodologies suffer from inconsistencies across different laboratories, leading to widely varying reported stability values for identical materials. This discrepancy stems primarily from non-standardized testing protocols, where parameters such as scan rates, temperature conditions, and electrode configurations differ substantially between research groups.
The intrinsic properties of solid electrolytes themselves present additional complications. Interface dynamics between solid electrolytes and electrodes are fundamentally different from liquid systems, with limited contact areas and complex charge transfer mechanisms. These solid-solid interfaces often exhibit high impedance and unpredictable behavior under testing conditions, making traditional electrochemical measurement techniques less reliable.
Contamination issues further compromise accuracy, as trace moisture or atmospheric contaminants can significantly alter electrochemical responses. Even minor exposure during sample preparation or testing can introduce redox reactions unrelated to the electrolyte's intrinsic stability, leading to misinterpretation of stability limits.
Current instrumentation limitations also contribute to measurement challenges. Many potentiostats struggle to accurately measure the extremely low current densities characteristic of solid electrolytes, particularly at stability boundaries. This technical limitation often results in noise-dominated signals that obscure the true onset of decomposition reactions.
Reference electrode implementation remains problematic in solid-state configurations. Unlike liquid systems where reference electrodes can be positioned with relative ease, solid-state cells require complex engineering solutions to incorporate reliable reference points. This often leads to uncompensated resistance effects and potential shifts that distort measurement accuracy.
Data interpretation presents another significant hurdle. Researchers frequently disagree on criteria for determining stability limits, with some using arbitrary current density thresholds while others employ tangent methods or derivative analysis. This methodological inconsistency makes cross-study comparisons nearly impossible and hampers technology development.
Temperature dependence of electrochemical windows adds another layer of complexity. Most measurements are conducted at room temperature despite sodium batteries typically operating at elevated temperatures. This disconnect between testing and application conditions creates uncertainty about real-world stability performance, as decomposition kinetics and thermodynamics change significantly with temperature.
These challenges collectively underscore the urgent need for standardized protocols and improved methodologies specific to solid electrolyte systems, as current approaches borrowed from liquid electrolyte research prove inadequate for these unique materials.
The intrinsic properties of solid electrolytes themselves present additional complications. Interface dynamics between solid electrolytes and electrodes are fundamentally different from liquid systems, with limited contact areas and complex charge transfer mechanisms. These solid-solid interfaces often exhibit high impedance and unpredictable behavior under testing conditions, making traditional electrochemical measurement techniques less reliable.
Contamination issues further compromise accuracy, as trace moisture or atmospheric contaminants can significantly alter electrochemical responses. Even minor exposure during sample preparation or testing can introduce redox reactions unrelated to the electrolyte's intrinsic stability, leading to misinterpretation of stability limits.
Current instrumentation limitations also contribute to measurement challenges. Many potentiostats struggle to accurately measure the extremely low current densities characteristic of solid electrolytes, particularly at stability boundaries. This technical limitation often results in noise-dominated signals that obscure the true onset of decomposition reactions.
Reference electrode implementation remains problematic in solid-state configurations. Unlike liquid systems where reference electrodes can be positioned with relative ease, solid-state cells require complex engineering solutions to incorporate reliable reference points. This often leads to uncompensated resistance effects and potential shifts that distort measurement accuracy.
Data interpretation presents another significant hurdle. Researchers frequently disagree on criteria for determining stability limits, with some using arbitrary current density thresholds while others employ tangent methods or derivative analysis. This methodological inconsistency makes cross-study comparisons nearly impossible and hampers technology development.
Temperature dependence of electrochemical windows adds another layer of complexity. Most measurements are conducted at room temperature despite sodium batteries typically operating at elevated temperatures. This disconnect between testing and application conditions creates uncertainty about real-world stability performance, as decomposition kinetics and thermodynamics change significantly with temperature.
These challenges collectively underscore the urgent need for standardized protocols and improved methodologies specific to solid electrolyte systems, as current approaches borrowed from liquid electrolyte research prove inadequate for these unique materials.
Methodologies for Electrochemical Window Measurement
01 Sodium-based solid electrolyte compositions
Various compositions of sodium-based solid electrolytes have been developed for use in electrochemical devices. These compositions typically include sodium ions as the primary charge carrier and may incorporate different structural frameworks such as NASICON-type materials, beta-alumina, or glass-ceramic composites. The specific composition affects ionic conductivity, stability, and electrochemical window properties, which are crucial for battery performance.- Sodium-based solid electrolyte compositions: Various compositions of sodium-based solid electrolytes have been developed for use in batteries and other electrochemical devices. These compositions typically include sodium compounds combined with other elements to form stable electrolyte structures. The compositions are designed to provide high ionic conductivity while maintaining stability within the electrochemical window required for battery applications. Some formulations incorporate additives to enhance performance characteristics and stability.
- Electrochemical window enhancement techniques: Various methods have been developed to enhance the electrochemical window of sodium solid electrolytes. These techniques include doping with specific elements, surface modification, and the incorporation of stabilizing additives. By widening the electrochemical window, these approaches help prevent electrolyte decomposition during battery operation, enabling higher voltage operation and improved energy density in sodium-based battery systems.
- Interface engineering for sodium solid electrolytes: Interface engineering focuses on improving the stability and performance of the interfaces between sodium solid electrolytes and electrodes. This includes developing protective coatings, buffer layers, and gradient structures to minimize interfacial resistance and prevent unwanted reactions. These approaches help maintain the integrity of the electrochemical window by reducing interfacial degradation and enhancing the overall stability of sodium-based electrochemical systems.
- Manufacturing processes for sodium solid electrolytes: Specialized manufacturing processes have been developed for producing sodium solid electrolytes with optimal electrochemical windows. These include various synthesis methods such as solid-state reactions, sol-gel processes, and mechanochemical approaches. Processing parameters are carefully controlled to achieve desired crystal structures, grain boundaries, and microstructural features that influence ionic conductivity and electrochemical stability. Advanced fabrication techniques help create electrolytes with wider electrochemical windows and improved performance.
- Testing and characterization of electrochemical windows: Methods for testing and characterizing the electrochemical windows of sodium solid electrolytes have been developed to evaluate their performance and stability. These include electrochemical techniques such as cyclic voltammetry, impedance spectroscopy, and galvanostatic cycling. Advanced analytical tools are used to investigate degradation mechanisms and identify factors that limit the electrochemical window. These characterization approaches help in the development of electrolytes with wider and more stable electrochemical windows for sodium-based energy storage applications.
02 Electrochemical window enhancement techniques
Various methods have been developed to enhance the electrochemical stability window of sodium solid electrolytes. These techniques include surface modification, doping with stabilizing elements, and creating protective interface layers. An expanded electrochemical window allows for compatibility with higher voltage cathode materials and improves the overall energy density and safety of sodium-based batteries.Expand Specific Solutions03 Interface engineering for sodium solid electrolytes
Interface engineering between sodium solid electrolytes and electrodes is critical for maintaining a wide electrochemical window. This involves designing protective coatings, buffer layers, or gradient compositions that prevent undesirable reactions at the electrolyte-electrode interfaces. Proper interface engineering reduces impedance growth during cycling and prevents electrolyte decomposition, thereby extending battery life and improving performance.Expand Specific Solutions04 Manufacturing processes for sodium solid electrolytes
Specialized manufacturing processes have been developed to produce sodium solid electrolytes with optimal electrochemical windows. These processes include sol-gel methods, solid-state reactions, melt-quenching techniques, and advanced sintering protocols. The manufacturing method significantly impacts the microstructure, density, and grain boundary properties of the electrolyte, which in turn affect its electrochemical stability window and overall performance in battery applications.Expand Specific Solutions05 Testing and characterization of electrochemical windows
Various analytical techniques have been developed to accurately measure and characterize the electrochemical window of sodium solid electrolytes. These methods include cyclic voltammetry, electrochemical impedance spectroscopy, and accelerated stability testing. Advanced characterization helps identify degradation mechanisms and stability limits, enabling the design of electrolytes with wider electrochemical windows suitable for next-generation sodium batteries.Expand Specific Solutions
Leading Research Groups and Industrial Players
The sodium solid electrolyte market is in an early growth phase, characterized by increasing research activity but limited commercial deployment. The market size is projected to expand significantly as sodium-ion battery technology gains traction as a cost-effective alternative to lithium-ion batteries. Technical maturity varies across players, with major electronics manufacturers like Samsung Electronics and LG Energy Solution leading development alongside automotive companies such as Toyota, Hyundai, and Nissan who are investing in this technology for future electric vehicles. Research institutions including MIT and University of Porto are advancing fundamental electrochemical window determination techniques, while battery specialists like CATL and PolyPlus Battery are working to overcome stability and conductivity challenges for commercial applications.
Honeycomb Battery Co.
Technical Solution: Honeycomb Battery has developed a specialized electrochemical window determination methodology for sodium solid electrolytes that focuses on interface engineering. Their approach combines traditional electrochemical techniques with in-situ impedance spectroscopy to monitor interface formation during potential sweeping. The company utilizes custom-designed split-cell configurations that allow for post-test retrieval and analysis of electrolyte-electrode interfaces without atmospheric exposure. Honeycomb's methodology incorporates stepped chronoamperometry measurements where potential is held at progressively increasing values while monitoring current decay patterns to distinguish between transient surface reactions and continuous decomposition processes[4]. Their proprietary algorithm analyzes the current response curves to establish decomposition onset potentials with high precision. Particularly innovative is their use of microelectrode arrays to map spatial variations in electrochemical stability across electrolyte pellets, identifying how grain boundaries and compositional heterogeneities affect local stability windows. This approach has been successfully applied to their portfolio of polymer-ceramic composite sodium electrolytes.
Strengths: Exceptional sensitivity to early-stage decomposition processes; detailed spatial mapping of stability variations; excellent correlation with long-term cycling performance. Weaknesses: Complex methodology requires specialized equipment; interpretation demands significant expertise; testing protocols are time-intensive compared to conventional methods.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed an advanced methodology for electrochemical window determination of sodium solid electrolytes using cyclic voltammetry combined with impedance spectroscopy. Their approach utilizes three-electrode cell configurations with sodium metal as reference electrodes and specialized carbon working electrodes to accurately measure the oxidation and reduction limits of solid electrolytes. The company employs a standardized protocol involving slow scan rates (0.1-0.5 mV/s) and temperature-controlled environments (25-80°C) to ensure reproducible results. Their proprietary analysis software can deconvolute interfacial reactions from bulk electrolyte decomposition, allowing for more precise determination of the true electrochemical stability window[1]. LG has successfully applied this methodology to their NASICON-type and sulfide-based sodium solid electrolytes, demonstrating stability windows exceeding 4V in some formulations.
Strengths: Superior precision in distinguishing between surface reactions and bulk decomposition; comprehensive temperature-dependent analysis capabilities; extensive database of reference materials for validation. Weaknesses: Methodology requires specialized equipment and expertise; testing protocols are time-intensive; results can be influenced by trace impurities in electrolyte materials.
Critical Patents and Literature on Stability Assessment
A solid electrolyte glass for lithium or sodium ions conduction
PatentWO2015128834A1
Innovation
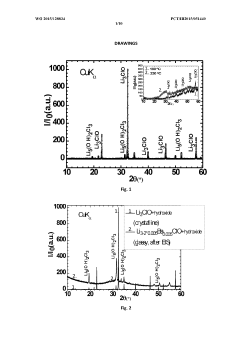
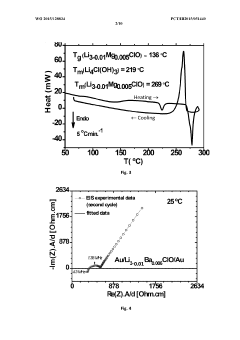
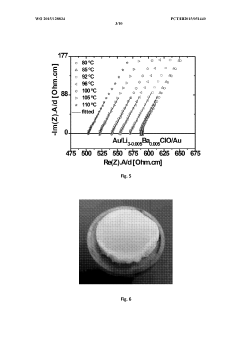
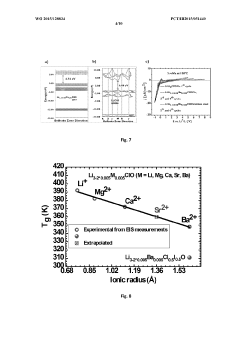
- Development of a novel glassy electrolyte with a disordered amorphous phase, comprising a compound with stoichiometry R3-2xMxHalO, where R is lithium or sodium, M is magnesium, calcium, strontium, or barium, and Hal is fluorine, chlorine, or iodine, exhibiting high ionic conductivity and a wide electrochemical window, making it suitable for lithium or sodium-ion batteries and capacitors.
Facile synthesis of solid sodium ion-conductive electrolytes
PatentActiveUS11685694B2
Innovation
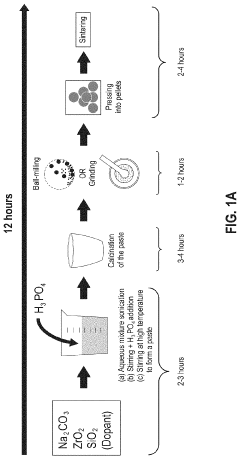
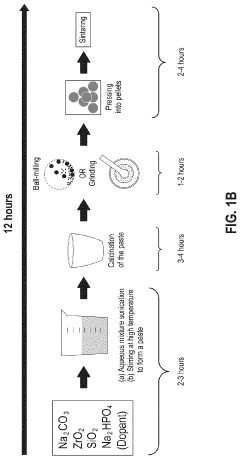
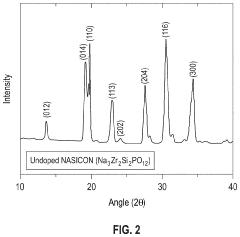
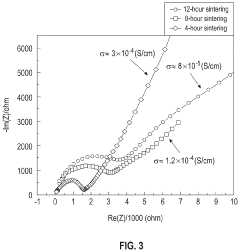
- A solution-based method involving the formation of an alkaline or aqueous mixture with sodium salts, metal oxides, and phosphorous precursors, followed by neutralization, concentration, and sintering at temperatures between 900° C to 1250° C, which significantly reduces processing time and enhances the efficiency of NASICON ceramic fabrication.
Safety and Performance Standards for Solid Electrolytes
The establishment of comprehensive safety and performance standards for solid electrolytes is crucial for the advancement and commercialization of sodium-ion battery technologies. These standards must address the unique electrochemical properties of sodium solid electrolytes, particularly their electrochemical stability windows, which directly impact battery safety and longevity.
Current industry standards primarily focus on lithium-based systems, creating a significant gap for sodium technologies. Organizations such as the International Electrotechnical Commission (IEC) and the American National Standards Institute (ANSI) have begun developing specialized frameworks for sodium solid electrolytes, emphasizing electrochemical window determination as a critical parameter.
The electrochemical stability window standard typically requires solid electrolytes to maintain stability between 0V and at least 4V vs. Na/Na+ to accommodate both anode and cathode operating potentials. This wide window ensures compatibility with various electrode materials while preventing decomposition reactions that could lead to capacity fade or safety hazards.
Testing protocols for electrochemical window determination have evolved to include cyclic voltammetry with standardized scan rates (0.1-1 mV/s) and temperature conditions (25-80°C). These protocols must account for the unique challenges of sodium systems, including higher reactivity compared to lithium counterparts and potential formation of dendrites that can compromise safety.
Performance standards also address ionic conductivity requirements, with minimum thresholds typically set at 10^-4 S/cm at room temperature for practical applications. The correlation between conductivity and electrochemical stability is particularly emphasized, as materials with wider stability windows often exhibit lower ionic conductivity, necessitating careful optimization.
Mechanical integrity standards have been established to ensure solid electrolytes maintain performance under various operating conditions. These include resistance to fracture during cycling (minimum fracture toughness of 0.2-0.5 MPa·m^1/2) and dimensional stability across the operating temperature range (-20°C to 60°C).
Environmental and aging standards require accelerated testing protocols to predict long-term stability, with solid electrolytes expected to maintain at least 80% of initial performance metrics after 1000 hours at elevated temperatures. These standards are particularly relevant for sodium systems, which may exhibit different degradation mechanisms compared to lithium technologies.
The harmonization of these standards across international markets remains an ongoing challenge, with efforts underway to establish unified testing methodologies that accurately reflect real-world operating conditions while enabling meaningful comparisons between different electrolyte formulations.
Current industry standards primarily focus on lithium-based systems, creating a significant gap for sodium technologies. Organizations such as the International Electrotechnical Commission (IEC) and the American National Standards Institute (ANSI) have begun developing specialized frameworks for sodium solid electrolytes, emphasizing electrochemical window determination as a critical parameter.
The electrochemical stability window standard typically requires solid electrolytes to maintain stability between 0V and at least 4V vs. Na/Na+ to accommodate both anode and cathode operating potentials. This wide window ensures compatibility with various electrode materials while preventing decomposition reactions that could lead to capacity fade or safety hazards.
Testing protocols for electrochemical window determination have evolved to include cyclic voltammetry with standardized scan rates (0.1-1 mV/s) and temperature conditions (25-80°C). These protocols must account for the unique challenges of sodium systems, including higher reactivity compared to lithium counterparts and potential formation of dendrites that can compromise safety.
Performance standards also address ionic conductivity requirements, with minimum thresholds typically set at 10^-4 S/cm at room temperature for practical applications. The correlation between conductivity and electrochemical stability is particularly emphasized, as materials with wider stability windows often exhibit lower ionic conductivity, necessitating careful optimization.
Mechanical integrity standards have been established to ensure solid electrolytes maintain performance under various operating conditions. These include resistance to fracture during cycling (minimum fracture toughness of 0.2-0.5 MPa·m^1/2) and dimensional stability across the operating temperature range (-20°C to 60°C).
Environmental and aging standards require accelerated testing protocols to predict long-term stability, with solid electrolytes expected to maintain at least 80% of initial performance metrics after 1000 hours at elevated temperatures. These standards are particularly relevant for sodium systems, which may exhibit different degradation mechanisms compared to lithium technologies.
The harmonization of these standards across international markets remains an ongoing challenge, with efforts underway to establish unified testing methodologies that accurately reflect real-world operating conditions while enabling meaningful comparisons between different electrolyte formulations.
Environmental Impact and Sustainability Considerations
The development and implementation of sodium solid electrolytes for energy storage systems necessitate careful consideration of their environmental impact and sustainability profile. The extraction of raw materials for these electrolytes, particularly sodium compounds, generally presents a lower environmental burden compared to lithium-based alternatives. Sodium is approximately 1000 times more abundant in the Earth's crust than lithium, reducing the ecological footprint associated with mining activities and potentially mitigating geopolitical tensions related to resource scarcity.
The manufacturing processes for sodium solid electrolytes require significant energy input, particularly during high-temperature sintering phases that are essential for achieving optimal electrochemical windows. This energy consumption contributes to the carbon footprint of these materials, though innovations in low-temperature synthesis routes are progressively reducing this impact. Additionally, the use of certain dopants and additives to enhance electrochemical stability windows may introduce toxic elements that require careful management throughout the product lifecycle.
Life cycle assessment (LCA) studies indicate that sodium-based energy storage systems generally demonstrate favorable environmental profiles when compared to conventional lithium-ion technologies. The wider electrochemical windows achieved in advanced sodium solid electrolytes contribute to longer cycle life and improved energy efficiency, thereby reducing the environmental impact per unit of energy stored over the system's operational lifetime. This extended durability directly translates to reduced waste generation and resource consumption.
End-of-life considerations for sodium solid electrolytes present both challenges and opportunities. While recycling infrastructure for these materials remains underdeveloped compared to established battery technologies, their composition typically facilitates more straightforward recovery processes. The absence of cobalt and reduced reliance on other critical materials simplifies recycling protocols and diminishes the environmental hazards associated with improper disposal.
The water solubility characteristics of many sodium compounds must be carefully managed to prevent potential contamination of aquatic ecosystems. However, this same property can be advantageous in controlled recycling processes. Research into closed-loop manufacturing systems for sodium solid electrolytes is advancing, with particular focus on recovery methods that preserve the electrochemical window properties in second-life applications.
Regulatory frameworks governing the environmental aspects of sodium solid electrolytes are evolving globally, with increasing emphasis on extended producer responsibility and circular economy principles. Manufacturers are increasingly required to demonstrate comprehensive environmental impact assessments, including specific data on how electrochemical window stability affects the overall sustainability profile of their energy storage solutions.
The manufacturing processes for sodium solid electrolytes require significant energy input, particularly during high-temperature sintering phases that are essential for achieving optimal electrochemical windows. This energy consumption contributes to the carbon footprint of these materials, though innovations in low-temperature synthesis routes are progressively reducing this impact. Additionally, the use of certain dopants and additives to enhance electrochemical stability windows may introduce toxic elements that require careful management throughout the product lifecycle.
Life cycle assessment (LCA) studies indicate that sodium-based energy storage systems generally demonstrate favorable environmental profiles when compared to conventional lithium-ion technologies. The wider electrochemical windows achieved in advanced sodium solid electrolytes contribute to longer cycle life and improved energy efficiency, thereby reducing the environmental impact per unit of energy stored over the system's operational lifetime. This extended durability directly translates to reduced waste generation and resource consumption.
End-of-life considerations for sodium solid electrolytes present both challenges and opportunities. While recycling infrastructure for these materials remains underdeveloped compared to established battery technologies, their composition typically facilitates more straightforward recovery processes. The absence of cobalt and reduced reliance on other critical materials simplifies recycling protocols and diminishes the environmental hazards associated with improper disposal.
The water solubility characteristics of many sodium compounds must be carefully managed to prevent potential contamination of aquatic ecosystems. However, this same property can be advantageous in controlled recycling processes. Research into closed-loop manufacturing systems for sodium solid electrolytes is advancing, with particular focus on recovery methods that preserve the electrochemical window properties in second-life applications.
Regulatory frameworks governing the environmental aspects of sodium solid electrolytes are evolving globally, with increasing emphasis on extended producer responsibility and circular economy principles. Manufacturers are increasingly required to demonstrate comprehensive environmental impact assessments, including specific data on how electrochemical window stability affects the overall sustainability profile of their energy storage solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!