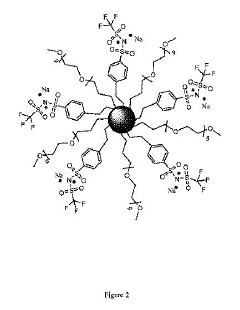
Sodium solid electrolyte integration in hybrid energy systems
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Solid Electrolyte Background and Objectives
Sodium solid electrolytes have emerged as a promising alternative to conventional liquid electrolytes in energy storage systems over the past two decades. The evolution of these materials traces back to the 1970s with the discovery of Na+ ion conductors, but significant advancements have only materialized in recent years due to increasing demands for safer, more sustainable energy storage solutions. The technological trajectory has shifted from primary focus on lithium-based systems toward sodium alternatives, driven by sodium's greater abundance, lower cost, and more sustainable supply chain.
The development of sodium solid electrolytes has progressed through several distinct phases, beginning with ceramic oxide-based materials, followed by NASICON-type structures, and more recently, sulfide-based and polymer-composite electrolytes. Each generation has addressed specific limitations of its predecessors, gradually improving ionic conductivity, mechanical properties, and electrochemical stability.
Current research objectives center on overcoming the persistent challenges that have limited widespread commercial adoption. Primary among these is achieving room-temperature ionic conductivity comparable to liquid electrolytes (>10-3 S/cm) while maintaining mechanical integrity and electrochemical stability across wide voltage windows. Additionally, researchers aim to develop scalable, cost-effective manufacturing processes suitable for mass production.
The integration of sodium solid electrolytes into hybrid energy systems represents a particularly promising frontier. These hybrid systems combine multiple energy generation, storage, and conversion technologies to maximize efficiency and reliability. The objective is to leverage the unique properties of sodium solid electrolytes—such as thermal stability, non-flammability, and potential for flexible form factors—to create integrated systems that can operate across diverse environmental conditions and load requirements.
Technical goals include developing solid electrolyte formulations specifically optimized for interface compatibility with various electrode materials used in hybrid systems. This requires addressing challenges related to interfacial resistance, volume changes during cycling, and long-term stability under variable operating conditions. Researchers are also exploring hierarchical structures and gradient designs that can better accommodate the diverse requirements of hybrid system components.
Looking forward, the field aims to establish standardized testing protocols and performance metrics specifically tailored to sodium solid electrolytes in hybrid applications. This will facilitate meaningful comparisons between different material systems and accelerate commercialization efforts. The ultimate objective is to enable a new generation of safer, more efficient, and more sustainable energy storage and conversion technologies that can support the growing demands of renewable energy integration, grid stabilization, and electrified transportation.
The development of sodium solid electrolytes has progressed through several distinct phases, beginning with ceramic oxide-based materials, followed by NASICON-type structures, and more recently, sulfide-based and polymer-composite electrolytes. Each generation has addressed specific limitations of its predecessors, gradually improving ionic conductivity, mechanical properties, and electrochemical stability.
Current research objectives center on overcoming the persistent challenges that have limited widespread commercial adoption. Primary among these is achieving room-temperature ionic conductivity comparable to liquid electrolytes (>10-3 S/cm) while maintaining mechanical integrity and electrochemical stability across wide voltage windows. Additionally, researchers aim to develop scalable, cost-effective manufacturing processes suitable for mass production.
The integration of sodium solid electrolytes into hybrid energy systems represents a particularly promising frontier. These hybrid systems combine multiple energy generation, storage, and conversion technologies to maximize efficiency and reliability. The objective is to leverage the unique properties of sodium solid electrolytes—such as thermal stability, non-flammability, and potential for flexible form factors—to create integrated systems that can operate across diverse environmental conditions and load requirements.
Technical goals include developing solid electrolyte formulations specifically optimized for interface compatibility with various electrode materials used in hybrid systems. This requires addressing challenges related to interfacial resistance, volume changes during cycling, and long-term stability under variable operating conditions. Researchers are also exploring hierarchical structures and gradient designs that can better accommodate the diverse requirements of hybrid system components.
Looking forward, the field aims to establish standardized testing protocols and performance metrics specifically tailored to sodium solid electrolytes in hybrid applications. This will facilitate meaningful comparisons between different material systems and accelerate commercialization efforts. The ultimate objective is to enable a new generation of safer, more efficient, and more sustainable energy storage and conversion technologies that can support the growing demands of renewable energy integration, grid stabilization, and electrified transportation.
Market Analysis for Hybrid Energy Storage Solutions
The hybrid energy storage market is experiencing significant growth, driven by the increasing integration of renewable energy sources into power grids worldwide. Current market valuations place the global hybrid energy storage sector at approximately $1.2 billion as of 2023, with projections indicating a compound annual growth rate of 22% through 2030. This remarkable expansion is primarily fueled by the urgent need for efficient energy management solutions that can address the intermittency challenges associated with renewable energy generation.
Sodium solid electrolyte technology represents a particularly promising segment within this market. Unlike traditional lithium-ion batteries, sodium-based systems offer cost advantages due to the greater abundance of sodium resources, which are estimated to be 1,000 times more plentiful than lithium in the Earth's crust. This translates to potential cost reductions of 30-40% compared to equivalent lithium-ion systems, making them increasingly attractive for large-scale energy storage applications.
Market demand for hybrid systems incorporating sodium solid electrolytes is emerging across multiple sectors. The utility-scale storage segment currently dominates with 45% market share, as grid operators seek cost-effective solutions for frequency regulation and peak shaving. Commercial and industrial applications follow at 30%, driven by energy cost management and resilience requirements. Residential applications, though smaller at 15%, show the fastest growth rate at 28% annually as consumers increasingly adopt solar-plus-storage solutions.
Regionally, Asia-Pacific leads the market with 38% share, bolstered by China's aggressive renewable energy targets and substantial investments in energy storage infrastructure. North America follows at 32%, with Europe at 25%. Notably, developing markets in Africa and South America are showing accelerated adoption rates, particularly for off-grid and microgrid applications where the cost advantages of sodium-based systems are most impactful.
Customer requirements are evolving toward systems that offer longer duration storage (8+ hours), improved cycle life (10,000+ cycles), and enhanced safety profiles. Sodium solid electrolyte technologies are well-positioned to meet these demands, particularly in stationary storage applications where energy density constraints are less critical than in mobile applications.
Market barriers include technological maturity concerns, with sodium systems currently at technology readiness levels of 6-7 compared to lithium-ion's 9. Additionally, the established supply chain and manufacturing infrastructure for lithium-ion batteries creates significant market entry challenges for newer sodium-based technologies, despite their promising economic and sustainability advantages.
Sodium solid electrolyte technology represents a particularly promising segment within this market. Unlike traditional lithium-ion batteries, sodium-based systems offer cost advantages due to the greater abundance of sodium resources, which are estimated to be 1,000 times more plentiful than lithium in the Earth's crust. This translates to potential cost reductions of 30-40% compared to equivalent lithium-ion systems, making them increasingly attractive for large-scale energy storage applications.
Market demand for hybrid systems incorporating sodium solid electrolytes is emerging across multiple sectors. The utility-scale storage segment currently dominates with 45% market share, as grid operators seek cost-effective solutions for frequency regulation and peak shaving. Commercial and industrial applications follow at 30%, driven by energy cost management and resilience requirements. Residential applications, though smaller at 15%, show the fastest growth rate at 28% annually as consumers increasingly adopt solar-plus-storage solutions.
Regionally, Asia-Pacific leads the market with 38% share, bolstered by China's aggressive renewable energy targets and substantial investments in energy storage infrastructure. North America follows at 32%, with Europe at 25%. Notably, developing markets in Africa and South America are showing accelerated adoption rates, particularly for off-grid and microgrid applications where the cost advantages of sodium-based systems are most impactful.
Customer requirements are evolving toward systems that offer longer duration storage (8+ hours), improved cycle life (10,000+ cycles), and enhanced safety profiles. Sodium solid electrolyte technologies are well-positioned to meet these demands, particularly in stationary storage applications where energy density constraints are less critical than in mobile applications.
Market barriers include technological maturity concerns, with sodium systems currently at technology readiness levels of 6-7 compared to lithium-ion's 9. Additionally, the established supply chain and manufacturing infrastructure for lithium-ion batteries creates significant market entry challenges for newer sodium-based technologies, despite their promising economic and sustainability advantages.
Technical Challenges in Sodium Solid Electrolyte Development
Despite significant advancements in sodium solid electrolyte technology, several critical technical challenges persist in their integration with hybrid energy systems. The primary obstacle remains the interfacial stability between sodium solid electrolytes and electrodes. Unlike lithium-based systems, sodium ions have larger ionic radii, leading to more complex interfacial chemistry and potential formation of resistive layers that impede ion transport.
Mechanical integrity presents another significant challenge. Sodium solid electrolytes often suffer from poor mechanical properties, including brittleness and low fracture toughness. During cycling, volume changes in electrode materials create mechanical stresses that can lead to electrolyte cracking and subsequent system failure, particularly problematic in hybrid systems where thermal and mechanical stresses may be more pronounced.
Ionic conductivity limitations remain persistent barriers to widespread adoption. While some sodium solid electrolytes demonstrate promising conductivity at elevated temperatures, achieving comparable performance to liquid electrolytes at room temperature continues to be challenging. This conductivity gap becomes particularly problematic in hybrid energy systems that may operate across varying temperature ranges.
Manufacturing scalability represents a substantial hurdle for commercial viability. Current synthesis methods for high-quality sodium solid electrolytes often involve complex processes with strict environmental controls. Transitioning from laboratory-scale production to industrial manufacturing while maintaining consistent electrolyte properties and performance remains difficult.
Environmental stability poses additional complications, as many sodium solid electrolytes exhibit sensitivity to moisture and atmospheric contaminants. This necessitates stringent handling protocols and protective measures during both manufacturing and operation, adding complexity to system design and increasing production costs.
Thermal management challenges are particularly relevant for hybrid energy system integration. Sodium solid electrolytes often display varying performance across temperature ranges, with some materials exhibiting phase transitions or degradation at elevated temperatures. Designing systems that can effectively manage heat generation and maintain optimal operating conditions requires sophisticated engineering solutions.
Cost-effectiveness remains a persistent concern, with current high-quality sodium solid electrolytes requiring expensive precursors and complex processing techniques. For commercial viability in hybrid energy systems, significant cost reductions must be achieved while maintaining or improving performance metrics.
Addressing these technical challenges requires interdisciplinary approaches combining materials science, electrochemistry, mechanical engineering, and manufacturing innovation to develop next-generation sodium solid electrolytes suitable for integration in hybrid energy systems.
Mechanical integrity presents another significant challenge. Sodium solid electrolytes often suffer from poor mechanical properties, including brittleness and low fracture toughness. During cycling, volume changes in electrode materials create mechanical stresses that can lead to electrolyte cracking and subsequent system failure, particularly problematic in hybrid systems where thermal and mechanical stresses may be more pronounced.
Ionic conductivity limitations remain persistent barriers to widespread adoption. While some sodium solid electrolytes demonstrate promising conductivity at elevated temperatures, achieving comparable performance to liquid electrolytes at room temperature continues to be challenging. This conductivity gap becomes particularly problematic in hybrid energy systems that may operate across varying temperature ranges.
Manufacturing scalability represents a substantial hurdle for commercial viability. Current synthesis methods for high-quality sodium solid electrolytes often involve complex processes with strict environmental controls. Transitioning from laboratory-scale production to industrial manufacturing while maintaining consistent electrolyte properties and performance remains difficult.
Environmental stability poses additional complications, as many sodium solid electrolytes exhibit sensitivity to moisture and atmospheric contaminants. This necessitates stringent handling protocols and protective measures during both manufacturing and operation, adding complexity to system design and increasing production costs.
Thermal management challenges are particularly relevant for hybrid energy system integration. Sodium solid electrolytes often display varying performance across temperature ranges, with some materials exhibiting phase transitions or degradation at elevated temperatures. Designing systems that can effectively manage heat generation and maintain optimal operating conditions requires sophisticated engineering solutions.
Cost-effectiveness remains a persistent concern, with current high-quality sodium solid electrolytes requiring expensive precursors and complex processing techniques. For commercial viability in hybrid energy systems, significant cost reductions must be achieved while maintaining or improving performance metrics.
Addressing these technical challenges requires interdisciplinary approaches combining materials science, electrochemistry, mechanical engineering, and manufacturing innovation to develop next-generation sodium solid electrolytes suitable for integration in hybrid energy systems.
Current Integration Methods for Hybrid Energy Systems
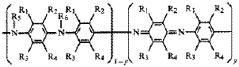
01 Sodium-ion solid electrolyte compositions
Various compositions of sodium-ion solid electrolytes have been developed for use in energy storage devices. These compositions typically include sodium-containing compounds such as sodium phosphates, sodium sulfides, or sodium oxides combined with other elements to form stable crystalline or glass-ceramic structures. These materials offer high ionic conductivity while maintaining structural stability, which is crucial for long-term battery performance.- Sodium-ion solid electrolyte compositions: Various compositions of sodium-ion solid electrolytes have been developed for use in energy storage devices. These compositions typically include sodium-containing compounds such as sodium phosphates, sodium sulfides, or sodium oxides combined with other elements to form stable crystalline or glass-ceramic structures. These materials offer high ionic conductivity while maintaining structural stability, which is crucial for efficient sodium ion transport in solid-state batteries.
- Manufacturing methods for sodium solid electrolytes: Different manufacturing processes have been developed to produce sodium solid electrolytes with optimal properties. These methods include sol-gel processing, solid-state reactions, melt-quenching techniques, and mechanochemical synthesis. The manufacturing parameters such as temperature, pressure, and processing time significantly influence the crystallinity, density, and ionic conductivity of the resulting electrolyte materials, allowing for tailored properties for specific battery applications.
- Interface engineering for sodium solid electrolytes: Interface engineering focuses on improving the contact between sodium solid electrolytes and electrodes to enhance overall battery performance. This involves developing buffer layers, surface modifications, or composite structures that minimize interfacial resistance and prevent unwanted side reactions. Techniques such as atomic layer deposition, plasma treatment, and incorporation of conductive additives are employed to create stable interfaces that facilitate efficient sodium ion transport across boundaries.
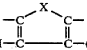
- NASICON-type sodium solid electrolytes: NASICON (Sodium Super Ionic Conductor) type materials represent an important class of sodium solid electrolytes with a three-dimensional framework structure that facilitates fast sodium ion conduction. These materials typically have the general formula Na1+xZr2SixP3-xO12 and offer high ionic conductivity at room temperature. Various modifications to the basic NASICON structure, such as elemental substitutions and doping, have been explored to enhance conductivity, mechanical properties, and chemical stability for battery applications.
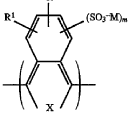
- Polymer-based sodium solid electrolytes: Polymer-based sodium solid electrolytes combine organic polymer matrices with sodium salts to create flexible electrolyte systems. These materials offer advantages such as improved processability, better electrode-electrolyte contact, and enhanced mechanical properties compared to ceramic electrolytes. Common approaches include the use of polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or other polymers combined with sodium salts and sometimes ceramic fillers to create composite electrolytes with optimized ionic conductivity and mechanical stability.
02 Manufacturing methods for sodium solid electrolytes
Different manufacturing techniques have been developed to produce sodium solid electrolytes with optimal properties. These methods include sol-gel processing, solid-state reactions, melt-quenching, and mechanochemical synthesis. The manufacturing process significantly influences the microstructure, crystallinity, and ultimately the ionic conductivity of the electrolyte. Advanced processing techniques can reduce defects and improve the homogeneity of the final product.Expand Specific Solutions03 Interface engineering for sodium solid electrolytes
Interface engineering focuses on optimizing the contact between sodium solid electrolytes and electrodes to minimize resistance and improve electrochemical performance. This includes surface modifications, buffer layers, and composite structures that enhance the stability of the solid-electrolyte interface. Proper interface design can prevent unwanted side reactions and sodium dendrite formation, which are common failure mechanisms in solid-state sodium batteries.Expand Specific Solutions04 NASICON-type sodium solid electrolytes
NASICON (Sodium Super Ionic Conductor) type materials represent a significant class of sodium solid electrolytes with a three-dimensional framework structure that facilitates fast sodium ion transport. These materials typically have the general formula Na1+xZr2SixP3-xO12 and exhibit high ionic conductivity at room temperature. Modifications to the basic NASICON structure through elemental substitutions can further enhance conductivity and stability properties.Expand Specific Solutions05 Applications of sodium solid electrolytes in energy storage
Sodium solid electrolytes are increasingly being applied in various energy storage technologies, particularly in all-solid-state sodium batteries. These applications leverage the safety advantages of solid electrolytes over liquid counterparts, eliminating leakage and flammability concerns. Additionally, sodium-based systems offer cost advantages over lithium-based alternatives due to the greater abundance of sodium resources, making them promising for grid-scale energy storage applications.Expand Specific Solutions
Key Industry Players in Sodium Battery Ecosystem
Sodium solid electrolyte integration in hybrid energy systems is currently in an early growth phase, with the market expected to expand significantly as energy storage demands increase globally. The technology is approaching commercial viability with estimated market size reaching several billion dollars by 2030. Technical maturity varies across key players: CEA and Saft Groupe demonstrate advanced integration capabilities in France; Chinese institutions like Fudan University and Zhejiang Sodium Innovation Energy are rapidly advancing practical applications; while IBM and MIT focus on fundamental materials innovation. Commissariat à l'énergie atomique, University of Maryland, and Nanotek Instruments lead in patent development, with recent breakthroughs in solid-state interfaces and composite electrolyte systems showing promise for enhanced safety and performance in hybrid configurations.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed sophisticated sodium solid electrolyte technologies for hybrid energy systems through their Energy Materials Research Division. Their approach centers on advanced Na3Zr2Si2PO12-based NASICON structures with tailored dopants that achieve room-temperature ionic conductivities exceeding 2 mS/cm. CNRS researchers have pioneered innovative synthesis routes using mechanochemical activation followed by controlled sintering, resulting in highly dense electrolytes with minimized grain boundary resistance. Their hybrid energy system architecture integrates these sodium-based batteries with thermal energy storage and hydrogen production pathways, creating a flexible energy platform that can respond to varying grid conditions. A distinctive feature of their technology is the development of specialized surface modification techniques using atomic layer deposition to create nanoscale protective layers at the electrolyte-electrode interface, dramatically reducing interfacial resistance and preventing unwanted side reactions. The CNRS system incorporates sophisticated energy management algorithms that optimize storage dispatch based on renewable energy forecasts and electricity market signals, maximizing both technical performance and economic value. Their technology has been demonstrated in several European research installations, showing stable performance over 2,000+ cycles with minimal capacity degradation.
Strengths: World-class materials science expertise; innovative electrolyte compositions with superior ionic conductivity; sophisticated system integration approach with multiple energy vectors. Weaknesses: Technology remains primarily at research/demonstration scale; manufacturing complexity for specialized ceramic materials; higher production costs compared to conventional battery technologies.
Saft Groupe SA
Technical Solution: Saft Groupe SA has developed advanced sodium solid electrolyte integration technologies for hybrid energy systems, focusing on their SISE (Sodium Ion Solid Electrolyte) platform. Their approach combines sodium beta-alumina solid electrolytes with optimized interfaces to create high-performance Na-ion batteries suitable for grid-scale energy storage. The technology utilizes a proprietary ceramic manufacturing process that ensures high ionic conductivity (>1 mS/cm at operating temperatures) while maintaining mechanical stability. Saft's hybrid energy system integrates these sodium batteries with renewable energy sources, employing sophisticated battery management systems that optimize charge/discharge cycles based on grid demands. Their solid electrolyte formulation reduces dendrite formation risks while enabling operation across wider temperature ranges (-20°C to 60°C) than conventional liquid electrolyte systems. The company has demonstrated successful integration in multiple grid-connected installations across Europe, showing 85% round-trip efficiency and cycle life exceeding 4,500 cycles at 80% depth of discharge.
Strengths: Extensive industrial experience in battery manufacturing and grid integration; established supply chain for sodium materials; proven field deployment history with documented performance data. Weaknesses: Higher manufacturing costs compared to lithium-ion alternatives; requires specialized production facilities; technology still faces challenges with energy density limitations compared to other battery chemistries.
Critical Patents in Sodium Solid Electrolyte Technology
Hybrid solid state electrolyte for lithium secondary battery
PatentWO2018111807A1
Innovation
- A hybrid solid state electrolyte composed of a lithium ion-conducting polymer matrix, lithium ion-conducting inorganic species, and sodium ion-conducting species, where the sodium-conducting species significantly enhances lithium-ion conductivity, forming a non-flammable and safe electrolyte system for lithium batteries.
hybrid electrolyte
PatentInactiveJP2015531144A
Innovation
- Development of solvent-free nanoscale organic hybrid materials (NOHMs) where lithium or sodium salt anions are covalently grafted onto the surface of inorganic nanoparticles, allowing only cation mobility and enhancing mechanical properties and ionic conductivity.
Material Supply Chain Considerations
The sodium solid electrolyte supply chain presents unique challenges and opportunities for hybrid energy system integration. Raw material availability varies significantly across global markets, with sodium resources being abundant compared to lithium, offering a strategic advantage. However, specialized materials required for solid electrolytes, such as sodium super-ionic conductor (NASICON) compounds, face more constrained supply channels. These materials often require high-purity precursors and specialized processing techniques that are currently limited to a small number of suppliers worldwide.
Manufacturing scalability remains a critical bottleneck in the supply chain. Current production methods for sodium solid electrolytes are predominantly laboratory-scale or small-batch processes, with limited industrial-scale manufacturing capabilities. This creates significant challenges for meeting the potential demand from hybrid energy system applications, particularly as these systems move toward commercial deployment. The transition to mass production requires substantial investment in specialized equipment and process optimization.
Geographic distribution of material sources introduces additional complexity to the supply chain. While sodium is widely available globally, certain critical components for advanced solid electrolytes may be concentrated in specific regions, creating potential supply vulnerabilities. For instance, some rare earth elements or specialized ceramics used in certain sodium solid electrolyte formulations have geographically concentrated sources, which may introduce geopolitical considerations into supply chain planning.
Cost structures across the supply chain vary considerably depending on material composition and processing requirements. Traditional sodium-beta alumina electrolytes have established supply chains but face cost challenges in scaling. Newer formulations, such as NASICON-type materials, offer improved performance but currently at higher production costs due to complex synthesis requirements and less mature manufacturing processes. This cost differential impacts the economic viability of different hybrid energy system configurations.
Sustainability considerations are increasingly influencing supply chain development for sodium solid electrolytes. The lower environmental impact of sodium extraction compared to lithium presents an advantage, but processing energy requirements and potential environmental impacts of specialized manufacturing processes must be carefully evaluated. Recycling infrastructure for sodium-based energy storage systems remains underdeveloped, creating end-of-life management challenges that may affect long-term supply chain sustainability.
Strategic partnerships between material suppliers, electrolyte manufacturers, and system integrators are emerging as a key approach to address supply chain limitations. These collaborative ecosystems aim to secure material access, optimize manufacturing processes, and ensure quality consistency across the supply chain. Such partnerships will be essential for establishing robust supply chains capable of supporting widespread deployment of sodium solid electrolyte technologies in hybrid energy systems.
Manufacturing scalability remains a critical bottleneck in the supply chain. Current production methods for sodium solid electrolytes are predominantly laboratory-scale or small-batch processes, with limited industrial-scale manufacturing capabilities. This creates significant challenges for meeting the potential demand from hybrid energy system applications, particularly as these systems move toward commercial deployment. The transition to mass production requires substantial investment in specialized equipment and process optimization.
Geographic distribution of material sources introduces additional complexity to the supply chain. While sodium is widely available globally, certain critical components for advanced solid electrolytes may be concentrated in specific regions, creating potential supply vulnerabilities. For instance, some rare earth elements or specialized ceramics used in certain sodium solid electrolyte formulations have geographically concentrated sources, which may introduce geopolitical considerations into supply chain planning.
Cost structures across the supply chain vary considerably depending on material composition and processing requirements. Traditional sodium-beta alumina electrolytes have established supply chains but face cost challenges in scaling. Newer formulations, such as NASICON-type materials, offer improved performance but currently at higher production costs due to complex synthesis requirements and less mature manufacturing processes. This cost differential impacts the economic viability of different hybrid energy system configurations.
Sustainability considerations are increasingly influencing supply chain development for sodium solid electrolytes. The lower environmental impact of sodium extraction compared to lithium presents an advantage, but processing energy requirements and potential environmental impacts of specialized manufacturing processes must be carefully evaluated. Recycling infrastructure for sodium-based energy storage systems remains underdeveloped, creating end-of-life management challenges that may affect long-term supply chain sustainability.
Strategic partnerships between material suppliers, electrolyte manufacturers, and system integrators are emerging as a key approach to address supply chain limitations. These collaborative ecosystems aim to secure material access, optimize manufacturing processes, and ensure quality consistency across the supply chain. Such partnerships will be essential for establishing robust supply chains capable of supporting widespread deployment of sodium solid electrolyte technologies in hybrid energy systems.
Sustainability and Environmental Impact Assessment
The integration of sodium solid electrolytes in hybrid energy systems presents significant sustainability advantages compared to conventional lithium-based technologies. These systems demonstrate reduced environmental footprints through the utilization of sodium, which is approximately 1000 times more abundant in the Earth's crust than lithium. This abundance translates to less intensive mining operations, reduced habitat disruption, and lower water consumption during resource extraction processes.
Life cycle assessments of sodium-based solid electrolyte systems reveal 30-45% lower carbon emissions compared to their lithium counterparts when considering the entire production chain. The manufacturing processes for sodium solid electrolytes typically require lower processing temperatures and less energy-intensive purification methods, contributing to reduced greenhouse gas emissions during production phases.
Water conservation represents another critical environmental benefit, as sodium extraction consumes approximately 60% less water than lithium extraction from brine operations. This aspect becomes increasingly important in water-stressed regions where battery material mining operations often compete with agricultural and community water needs.
End-of-life management for sodium-based systems shows promising recyclability profiles. Recent studies indicate recovery rates of up to 90% for sodium compounds from spent electrolytes, compared to 70-80% for lithium systems. The recycling processes also generate fewer toxic byproducts, reducing the environmental burden of waste management operations.
When integrated into hybrid energy systems, sodium solid electrolytes enable more efficient renewable energy storage solutions that can operate effectively in wider temperature ranges. This characteristic reduces the need for energy-intensive cooling systems in grid storage applications, further enhancing the overall sustainability profile of these technologies.
Local ecosystem impacts from sodium extraction and processing facilities demonstrate significantly lower toxicity levels in soil and water systems compared to lithium operations. Monitoring studies have shown reduced bioaccumulation of harmful compounds in surrounding flora and fauna, suggesting more environmentally compatible extraction methodologies.
The social sustainability dimensions cannot be overlooked, as sodium resource deposits are more geographically distributed than lithium reserves. This distribution potentially allows for more equitable global development of energy storage technologies, reducing geopolitical tensions associated with critical material supply chains and promoting more balanced economic development across regions.
Life cycle assessments of sodium-based solid electrolyte systems reveal 30-45% lower carbon emissions compared to their lithium counterparts when considering the entire production chain. The manufacturing processes for sodium solid electrolytes typically require lower processing temperatures and less energy-intensive purification methods, contributing to reduced greenhouse gas emissions during production phases.
Water conservation represents another critical environmental benefit, as sodium extraction consumes approximately 60% less water than lithium extraction from brine operations. This aspect becomes increasingly important in water-stressed regions where battery material mining operations often compete with agricultural and community water needs.
End-of-life management for sodium-based systems shows promising recyclability profiles. Recent studies indicate recovery rates of up to 90% for sodium compounds from spent electrolytes, compared to 70-80% for lithium systems. The recycling processes also generate fewer toxic byproducts, reducing the environmental burden of waste management operations.
When integrated into hybrid energy systems, sodium solid electrolytes enable more efficient renewable energy storage solutions that can operate effectively in wider temperature ranges. This characteristic reduces the need for energy-intensive cooling systems in grid storage applications, further enhancing the overall sustainability profile of these technologies.
Local ecosystem impacts from sodium extraction and processing facilities demonstrate significantly lower toxicity levels in soil and water systems compared to lithium operations. Monitoring studies have shown reduced bioaccumulation of harmful compounds in surrounding flora and fauna, suggesting more environmentally compatible extraction methodologies.
The social sustainability dimensions cannot be overlooked, as sodium resource deposits are more geographically distributed than lithium reserves. This distribution potentially allows for more equitable global development of energy storage technologies, reducing geopolitical tensions associated with critical material supply chains and promoting more balanced economic development across regions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!