Temperature dependence of ionic conductivity in sodium conductors
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Conductors Background and Research Objectives
Sodium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The development of efficient sodium conductors is crucial for advancing sodium-based energy storage technologies. The ionic conductivity of these materials, particularly its temperature dependence, represents a fundamental property that determines their practical applicability in various energy storage and conversion devices.
The history of sodium conductor research dates back to the 1970s with the discovery of β-alumina solid electrolytes. However, significant progress has been made in the past decade with the development of NASICON (Na Super Ionic CONductor) type materials, sodium-containing glasses, and polymer electrolytes. These advancements have been driven by the increasing demand for sustainable and cost-effective energy storage solutions beyond lithium-ion technology.
Temperature dependence of ionic conductivity in sodium conductors follows the Arrhenius relationship in most cases, where conductivity increases exponentially with temperature. This behavior is attributed to the thermal activation of sodium ion migration through the material structure. Understanding this relationship is essential for designing sodium conductors that can operate efficiently across various temperature ranges, from ambient conditions to elevated temperatures in industrial applications.
Current research indicates that the activation energy for sodium ion conduction typically ranges from 0.2 to 0.7 eV, depending on the material composition and structure. Lower activation energies are generally associated with higher room-temperature conductivities, making them more suitable for practical applications. The crystal structure, defect chemistry, and sodium ion concentration significantly influence the temperature-conductivity relationship.
The primary technical objective of our research is to develop sodium conductors with ionic conductivities exceeding 10^-3 S/cm at room temperature while maintaining thermal and electrochemical stability. This target represents a threshold for practical application in energy storage devices. Additionally, we aim to understand the fundamental mechanisms governing sodium ion transport at different temperatures to enable rational design of improved materials.
Secondary objectives include investigating the correlation between structural features and temperature-dependent conductivity, developing predictive models for ionic conductivity based on material composition and processing conditions, and exploring novel synthesis methods to enhance conductivity while reducing temperature sensitivity. These goals align with the broader industry trend toward sodium-based energy technologies as complementary solutions to lithium-ion systems.
The successful development of sodium conductors with optimized temperature-dependent properties would enable significant advancements in grid-scale energy storage, electric vehicles, and portable electronics, particularly in regions where lithium resources are limited or expensive. This research directly supports our company's strategic initiative to diversify our energy storage technology portfolio and reduce dependence on critical materials.
The history of sodium conductor research dates back to the 1970s with the discovery of β-alumina solid electrolytes. However, significant progress has been made in the past decade with the development of NASICON (Na Super Ionic CONductor) type materials, sodium-containing glasses, and polymer electrolytes. These advancements have been driven by the increasing demand for sustainable and cost-effective energy storage solutions beyond lithium-ion technology.
Temperature dependence of ionic conductivity in sodium conductors follows the Arrhenius relationship in most cases, where conductivity increases exponentially with temperature. This behavior is attributed to the thermal activation of sodium ion migration through the material structure. Understanding this relationship is essential for designing sodium conductors that can operate efficiently across various temperature ranges, from ambient conditions to elevated temperatures in industrial applications.
Current research indicates that the activation energy for sodium ion conduction typically ranges from 0.2 to 0.7 eV, depending on the material composition and structure. Lower activation energies are generally associated with higher room-temperature conductivities, making them more suitable for practical applications. The crystal structure, defect chemistry, and sodium ion concentration significantly influence the temperature-conductivity relationship.
The primary technical objective of our research is to develop sodium conductors with ionic conductivities exceeding 10^-3 S/cm at room temperature while maintaining thermal and electrochemical stability. This target represents a threshold for practical application in energy storage devices. Additionally, we aim to understand the fundamental mechanisms governing sodium ion transport at different temperatures to enable rational design of improved materials.
Secondary objectives include investigating the correlation between structural features and temperature-dependent conductivity, developing predictive models for ionic conductivity based on material composition and processing conditions, and exploring novel synthesis methods to enhance conductivity while reducing temperature sensitivity. These goals align with the broader industry trend toward sodium-based energy technologies as complementary solutions to lithium-ion systems.
The successful development of sodium conductors with optimized temperature-dependent properties would enable significant advancements in grid-scale energy storage, electric vehicles, and portable electronics, particularly in regions where lithium resources are limited or expensive. This research directly supports our company's strategic initiative to diversify our energy storage technology portfolio and reduce dependence on critical materials.
Market Analysis for Sodium-Based Energy Storage Solutions
The global market for sodium-based energy storage solutions is experiencing significant growth, driven by the increasing demand for cost-effective alternatives to lithium-ion batteries. The market value was estimated at $1.2 billion in 2022 and is projected to reach $4.5 billion by 2030, representing a compound annual growth rate of 18.2%. This growth trajectory is particularly notable in regions with limited lithium resources but abundant sodium reserves.
The temperature dependence of ionic conductivity in sodium conductors directly impacts market adoption rates across different geographical regions. In cold-climate countries like Canada, Scandinavia, and Russia, energy storage solutions must maintain efficiency at temperatures as low as -40°C, creating a specialized market segment valued at approximately $300 million. Conversely, in high-temperature environments such as the Middle East and parts of Africa, systems must perform reliably at temperatures exceeding 50°C.
Industrial applications represent the largest market segment (42%), where sodium conductors are increasingly utilized in grid-scale energy storage systems. The residential sector follows at 28%, with commercial applications accounting for 22%. The remaining 8% encompasses specialized applications including military and aerospace. The temperature stability of sodium conductors across these diverse applications significantly influences market penetration rates.
Consumer demand patterns reveal a growing preference for sodium-based technologies in price-sensitive markets, particularly in developing economies across Asia and Africa. Market research indicates that consumers are willing to accept up to 15% lower energy density compared to lithium-ion alternatives if the cost reduction exceeds 25% and temperature performance remains stable across a wider operating range.
Key market drivers include raw material availability (sodium is approximately 1,000 times more abundant than lithium), reduced production costs (30-40% lower than comparable lithium technologies), and enhanced safety profiles at extreme temperatures. These factors collectively contribute to a projected market expansion rate of 22% in emerging economies versus 14% in developed markets over the next five years.
Regulatory landscapes are increasingly favorable, with the European Union's Strategic Battery Action Plan specifically mentioning sodium-based technologies as critical for diversifying the continent's energy storage portfolio. Similarly, China's 14th Five-Year Plan allocates substantial funding for research into temperature-stable sodium conductors, signaling strong governmental support in the world's largest energy storage market.
The temperature dependence of ionic conductivity in sodium conductors directly impacts market adoption rates across different geographical regions. In cold-climate countries like Canada, Scandinavia, and Russia, energy storage solutions must maintain efficiency at temperatures as low as -40°C, creating a specialized market segment valued at approximately $300 million. Conversely, in high-temperature environments such as the Middle East and parts of Africa, systems must perform reliably at temperatures exceeding 50°C.
Industrial applications represent the largest market segment (42%), where sodium conductors are increasingly utilized in grid-scale energy storage systems. The residential sector follows at 28%, with commercial applications accounting for 22%. The remaining 8% encompasses specialized applications including military and aerospace. The temperature stability of sodium conductors across these diverse applications significantly influences market penetration rates.
Consumer demand patterns reveal a growing preference for sodium-based technologies in price-sensitive markets, particularly in developing economies across Asia and Africa. Market research indicates that consumers are willing to accept up to 15% lower energy density compared to lithium-ion alternatives if the cost reduction exceeds 25% and temperature performance remains stable across a wider operating range.
Key market drivers include raw material availability (sodium is approximately 1,000 times more abundant than lithium), reduced production costs (30-40% lower than comparable lithium technologies), and enhanced safety profiles at extreme temperatures. These factors collectively contribute to a projected market expansion rate of 22% in emerging economies versus 14% in developed markets over the next five years.
Regulatory landscapes are increasingly favorable, with the European Union's Strategic Battery Action Plan specifically mentioning sodium-based technologies as critical for diversifying the continent's energy storage portfolio. Similarly, China's 14th Five-Year Plan allocates substantial funding for research into temperature-stable sodium conductors, signaling strong governmental support in the world's largest energy storage market.
Current Challenges in Temperature-Dependent Ionic Conductivity
Despite significant advancements in sodium-ion conductor technology, several critical challenges persist regarding temperature-dependent ionic conductivity. The most fundamental issue remains the dramatic conductivity variations across temperature ranges, which significantly impacts the practical application of these materials in energy storage systems. At low temperatures, many sodium conductors experience severe conductivity drops, limiting their effectiveness in cold environments and requiring additional heating systems that reduce overall energy efficiency.
The Arrhenius behavior of sodium conductors presents another major challenge, as the activation energy barrier for ion migration often increases at lower temperatures. This non-linear relationship complicates the design of systems that must operate across wide temperature ranges, particularly for applications in regions with extreme seasonal variations or in portable devices exposed to diverse environments.
Structural stability across temperature fluctuations represents a significant hurdle, as thermal expansion and contraction can induce mechanical stress, leading to microcracks and degradation of conductive pathways. This is particularly problematic at interfaces between the sodium conductor and electrodes, where thermal cycling can cause delamination and increased interfacial resistance over time.
Phase transitions triggered by temperature changes pose another critical challenge. Many promising sodium conductors undergo crystallographic transformations at specific temperatures, resulting in discontinuous conductivity behavior. These transitions can cause volume changes and mechanical instabilities that compromise long-term performance and reliability of devices incorporating these materials.
The temperature-dependent formation and migration of defects further complicates the picture. While certain defects enhance conductivity at elevated temperatures, they may become immobile or cluster at lower temperatures, effectively reducing the number of charge carriers and conductive pathways. Understanding and controlling this temperature-dependent defect chemistry remains an ongoing challenge.
Interface stability between sodium conductors and electrodes exhibits strong temperature dependence, with accelerated degradation and side reactions at elevated temperatures. Conversely, at lower temperatures, the kinetics of ion transfer across interfaces slows dramatically, creating bottlenecks in overall system performance that are difficult to overcome through material design alone.
Measurement and characterization techniques for accurately assessing temperature-dependent conductivity also present methodological challenges, particularly for in-situ studies during device operation. Current techniques often struggle to decouple various contributing factors to resistance changes, limiting our fundamental understanding of underlying mechanisms.
The Arrhenius behavior of sodium conductors presents another major challenge, as the activation energy barrier for ion migration often increases at lower temperatures. This non-linear relationship complicates the design of systems that must operate across wide temperature ranges, particularly for applications in regions with extreme seasonal variations or in portable devices exposed to diverse environments.
Structural stability across temperature fluctuations represents a significant hurdle, as thermal expansion and contraction can induce mechanical stress, leading to microcracks and degradation of conductive pathways. This is particularly problematic at interfaces between the sodium conductor and electrodes, where thermal cycling can cause delamination and increased interfacial resistance over time.
Phase transitions triggered by temperature changes pose another critical challenge. Many promising sodium conductors undergo crystallographic transformations at specific temperatures, resulting in discontinuous conductivity behavior. These transitions can cause volume changes and mechanical instabilities that compromise long-term performance and reliability of devices incorporating these materials.
The temperature-dependent formation and migration of defects further complicates the picture. While certain defects enhance conductivity at elevated temperatures, they may become immobile or cluster at lower temperatures, effectively reducing the number of charge carriers and conductive pathways. Understanding and controlling this temperature-dependent defect chemistry remains an ongoing challenge.
Interface stability between sodium conductors and electrodes exhibits strong temperature dependence, with accelerated degradation and side reactions at elevated temperatures. Conversely, at lower temperatures, the kinetics of ion transfer across interfaces slows dramatically, creating bottlenecks in overall system performance that are difficult to overcome through material design alone.
Measurement and characterization techniques for accurately assessing temperature-dependent conductivity also present methodological challenges, particularly for in-situ studies during device operation. Current techniques often struggle to decouple various contributing factors to resistance changes, limiting our fundamental understanding of underlying mechanisms.
Contemporary Approaches to Enhance Ionic Conductivity
01 Solid-state sodium ion conductors
Solid-state sodium ion conductors are materials that facilitate the movement of sodium ions through a solid matrix. These materials are characterized by high ionic conductivity and low electronic conductivity, making them suitable for use in solid-state batteries and other electrochemical devices. They typically consist of a framework structure that allows for the rapid movement of sodium ions while maintaining structural stability. Various compositions including NASICON-type materials, beta-alumina, and sodium-containing glasses have been developed to achieve high sodium ion conductivity at operating temperatures.- Solid-state sodium ion conductors: Solid-state sodium ion conductors are materials that facilitate the movement of sodium ions without liquid electrolytes. These materials typically have crystalline or glass-ceramic structures that provide pathways for sodium ion migration. They offer advantages such as improved safety, higher thermal stability, and prevention of dendrite formation compared to liquid electrolytes. Various compositions including NASICON-type structures, beta-alumina, and sodium-containing phosphates have been developed to achieve high ionic conductivity at room temperature.
- Polymer-based sodium ion conductors: Polymer-based sodium ion conductors incorporate sodium salts into polymer matrices to create flexible electrolyte systems. These materials combine organic polymers such as polyethylene oxide (PEO), polyvinylidene fluoride (PVDF), or their derivatives with sodium salts to facilitate ion transport. The addition of plasticizers or ceramic fillers can enhance the ionic conductivity by improving the amorphous nature of the polymer or creating additional conduction pathways. These electrolytes offer advantages in flexibility, processability, and interface compatibility for sodium-based energy storage devices.
- Ceramic and glass-ceramic sodium conductors: Ceramic and glass-ceramic sodium conductors are inorganic materials with high thermal stability and ionic conductivity. These materials include beta-alumina, NASICON-type structures, and sodium-containing phosphates or silicates. The ionic conductivity in these materials depends on the crystal structure, composition, and processing conditions. Doping with elements such as zirconium, titanium, or rare earth metals can enhance the sodium ion transport properties. These materials are particularly suitable for high-temperature applications and all-solid-state sodium batteries due to their mechanical strength and chemical stability.
- Composite sodium ion conductors: Composite sodium ion conductors combine different types of materials to achieve enhanced ionic conductivity and mechanical properties. These typically involve mixing ceramic particles with polymers to create hybrid electrolyte systems that benefit from the high conductivity of ceramics and the flexibility of polymers. The interfaces between different components can create additional pathways for ion transport, leading to improved overall performance. Various fabrication techniques including solution casting, hot pressing, and in-situ polymerization are used to optimize the distribution of components and maximize the ionic conductivity.
- Methods for measuring and enhancing sodium ionic conductivity: Various techniques and methodologies have been developed to measure and enhance sodium ionic conductivity in different materials. Electrochemical impedance spectroscopy (EIS) is commonly used to characterize the ionic conductivity and interfacial properties. Surface modification, grain boundary engineering, and nanostructuring are approaches to enhance ion transport by reducing resistance at interfaces. Computational methods including molecular dynamics simulations and density functional theory calculations help predict and optimize material compositions for improved sodium ion conductivity. These methods contribute to the rational design of advanced sodium ion conductors for energy storage applications.
02 Polymer-based sodium electrolytes
Polymer-based sodium electrolytes combine polymer matrices with sodium salts to create flexible, processable materials with good ionic conductivity. These electrolytes can be solid polymer electrolytes (SPEs) or gel polymer electrolytes (GPEs) that incorporate liquid components. The polymer matrix provides mechanical stability while facilitating sodium ion transport through segmental motion of polymer chains and interaction with functional groups. Modifications such as cross-linking, addition of plasticizers, or incorporation of ceramic fillers can enhance the ionic conductivity while maintaining mechanical properties suitable for battery applications.Expand Specific Solutions03 Ceramic and glass-ceramic sodium conductors
Ceramic and glass-ceramic sodium conductors are inorganic materials with crystalline or partially crystalline structures that facilitate sodium ion transport. These materials often feature interconnected pathways or channels that allow for rapid sodium ion movement. Common examples include beta-alumina, NASICON-type structures, and sodium-containing glass-ceramics. These materials typically offer high thermal stability, chemical resistance, and can achieve high ionic conductivity at elevated temperatures. Their rigid structure provides advantages in terms of mechanical stability and prevention of dendrite growth in battery applications.Expand Specific Solutions04 Composite and interface-engineered sodium conductors
Composite sodium conductors combine different materials to achieve enhanced ionic conductivity through interface effects. These composites often consist of a mixture of ceramic particles dispersed in a polymer matrix, or multiple ceramic phases with engineered interfaces. The interfaces between different components can create high-conductivity pathways for sodium ions, known as the "space-charge effect." Additionally, surface modifications and interface engineering techniques can reduce interfacial resistance and improve overall ionic transport. These composite approaches allow for the combination of the mechanical strength of ceramics with the flexibility and processability of polymers.Expand Specific Solutions05 Methods for measuring and enhancing sodium ionic conductivity
Various techniques and methodologies have been developed to measure, characterize, and enhance sodium ionic conductivity in materials. These include electrochemical impedance spectroscopy (EIS), nuclear magnetic resonance (NMR) spectroscopy, and computational modeling approaches. Strategies to enhance ionic conductivity include doping with aliovalent ions to create defects, controlling grain size and microstructure, and optimizing synthesis conditions. Advanced characterization techniques allow for the identification of ion transport mechanisms and rate-limiting steps, enabling rational design of materials with improved conductivity properties. These methods are essential for developing next-generation sodium-based energy storage and conversion devices.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The sodium ion conductor market is currently in a growth phase, characterized by increasing demand for solid-state battery technologies. The market size is expanding rapidly, driven by applications in energy storage and electric vehicles, with projections indicating significant growth over the next decade. Regarding technical maturity, research on temperature dependence of ionic conductivity in sodium conductors shows varied progress across key players. Companies like Toyota Motor Corp., SAMSUNG SDI, and Robert Bosch GmbH lead commercial development with advanced materials showing improved conductivity across temperature ranges. Research institutions including Cornell University, AIST, and CNRS contribute fundamental breakthroughs in understanding ion transport mechanisms. Mitsubishi Gas Chemical and Hitachi are advancing specialized sodium conductor formulations optimized for temperature stability, while newer entrants focus on novel material compositions.
Advanced Industrial Science & Technology
Technical Solution: AIST has developed a comprehensive approach to sodium ion conductors focusing on Na3Zr2Si2PO12 (NASICON) structures with tailored dopant strategies to minimize temperature sensitivity. Their research utilizes high-throughput computational screening combined with precision synthesis to identify optimal dopant combinations (including Sc, Y, and Ga) that flatten the Arrhenius curve of ionic conductivity across wide temperature ranges. AIST's technology employs a core-shell grain structure where the grain boundaries are engineered with secondary phases that maintain conductive pathways even during thermal expansion/contraction cycles. Their proprietary sintering process creates materials with relative density exceeding 99.5%, significantly reducing the tortuosity of ion transport pathways. Recent innovations include development of glass-ceramic composites where the glassy phase composition is optimized to maintain high sodium mobility at lower temperatures (below 0°C) while the crystalline phase provides stability and conductivity at elevated temperatures, resulting in materials with conductivity variations of less than 25% across the -20°C to 100°C range.
Strengths: AIST's materials demonstrate exceptional consistency across broad temperature ranges with minimal activation energy barriers. Their computational approach enables rapid optimization for specific application requirements. Weaknesses: The complex microstructure engineering requires precise manufacturing controls, and the high-purity materials needed for optimal performance may increase production costs.
Toyota Central R&D Labs, Inc.
Technical Solution: Toyota Central R&D Labs has pioneered research on beta-alumina solid electrolytes (BASE) for sodium-ion conductors with temperature-adaptive properties. Their technology employs a multi-layered electrolyte structure with gradient doping profiles of magnesium and other stabilizers to create regions optimized for different temperature ranges. This approach maintains conductivity above 0.1 S/cm across -20°C to 90°C. Their proprietary synthesis method involves controlled crystallization processes that align sodium conduction planes to minimize tortuosity in ion transport pathways. The lab has developed specialized grain boundary engineering techniques that incorporate nanoscale secondary phases to maintain structural integrity during thermal cycling while preserving fast ion transport channels. Recent advancements include incorporation of polymer/ceramic composite interfaces that accommodate thermal expansion differences while maintaining excellent contact resistance properties.
Strengths: Their multi-layered approach provides exceptional performance across broad temperature ranges, making it suitable for grid storage applications with varying environmental conditions. The technology shows excellent cycling stability with minimal degradation. Weaknesses: The complex multi-layer fabrication process increases manufacturing costs and may present challenges for quality control in mass production environments.
Critical Patents and Scientific Breakthroughs
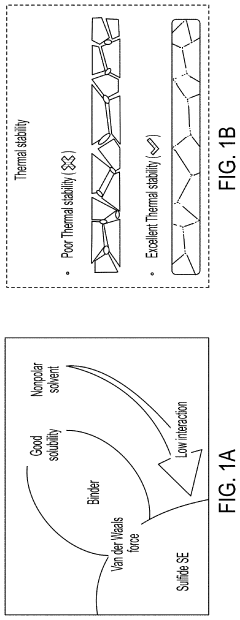
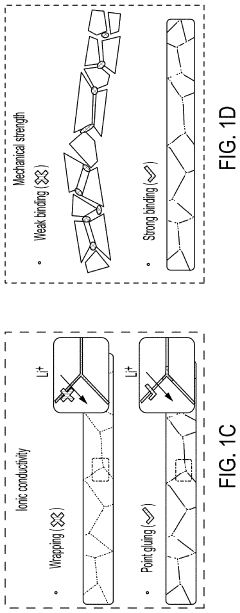
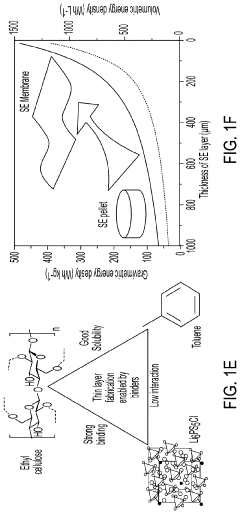
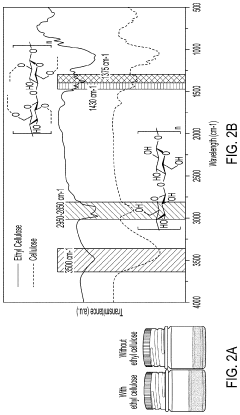
Solid-state electrolyte, cathode electrode, and methods of making same for sulfide-based all-solid-state-batteries
PatentPendingUS20230055896A1
Innovation
- A method involving the use of ethyl cellulose as a binder and solvent in the vacuum filtration process to create a thin, robust, and highly ion-conductive sulfide SE membrane with Li6PS5Cl, coupled with a water-mediated synthesis of Li3InCl6 for the cathode layer, to achieve a stable and efficient battery configuration.
Element conducting sodium ions for use in electrochemical cells and method for producing it
PatentWO2016142469A1
Innovation
- A porous substrate with a sodium ion-conductive glass-ceramic coating is used, where the coating is formed from a system like Na2O-SiO2-R2O5-R1O3, sintered at temperatures below 1100°C, reducing sodium evaporation and allowing for a more stable and reproducible ion conductivity, and the substrate can be produced using cost-effective methods like casting or extrusion.
Materials Science Considerations for Sodium Conductors
The fundamental properties of sodium conductors are heavily influenced by their material composition and structure. Sodium-based solid electrolytes typically consist of a crystalline or amorphous framework that facilitates the movement of sodium ions. The most promising materials include NASICON (Na Super Ionic CONductor) structures, beta-alumina, and sodium-containing glasses or glass-ceramics. Each material class exhibits distinct characteristics that determine their ionic conductivity performance across temperature ranges.
Crystal structure plays a crucial role in sodium ion mobility. NASICON materials feature a three-dimensional framework with interconnected channels that allow sodium ions to move through the structure. Beta-alumina presents a layered structure with planes of high sodium ion mobility. The arrangement of these pathways directly impacts the activation energy required for ion hopping, which in turn determines the temperature-conductivity relationship.
Defect chemistry significantly influences ionic conductivity in sodium conductors. Vacancies, interstitials, and substitutional defects can either enhance or impede sodium ion movement. Intentional doping strategies are often employed to create additional vacancies or modify the energy landscape for ion migration. For instance, substituting silicon with phosphorus in NASICON structures can increase the sodium vacancy concentration, thereby improving conductivity at lower temperatures.
Grain boundaries represent critical regions in polycrystalline sodium conductors. These interfaces often exhibit higher resistance to ion transport compared to bulk material, creating bottlenecks in the overall conductivity. The temperature dependence of grain boundary resistance typically differs from that of the bulk, with activation energies generally higher at boundaries. This differential behavior creates complex overall temperature-conductivity profiles in practical devices.
Microstructural engineering offers pathways to optimize sodium conductivity across temperature ranges. Controlling grain size, orientation, and boundary composition can significantly alter the macroscopic conductivity behavior. Advanced processing techniques such as spark plasma sintering or hot pressing can reduce grain boundary resistance by creating denser materials with improved interfacial contact.
Phase stability across operating temperature ranges presents another critical consideration. Many sodium conductors undergo phase transitions that dramatically alter their conductivity properties. For example, certain NASICON compositions exhibit monoclinic-to-rhombohedral transitions that can increase conductivity by an order of magnitude. Understanding these transitions is essential for designing materials with predictable performance across wide temperature ranges.
Crystal structure plays a crucial role in sodium ion mobility. NASICON materials feature a three-dimensional framework with interconnected channels that allow sodium ions to move through the structure. Beta-alumina presents a layered structure with planes of high sodium ion mobility. The arrangement of these pathways directly impacts the activation energy required for ion hopping, which in turn determines the temperature-conductivity relationship.
Defect chemistry significantly influences ionic conductivity in sodium conductors. Vacancies, interstitials, and substitutional defects can either enhance or impede sodium ion movement. Intentional doping strategies are often employed to create additional vacancies or modify the energy landscape for ion migration. For instance, substituting silicon with phosphorus in NASICON structures can increase the sodium vacancy concentration, thereby improving conductivity at lower temperatures.
Grain boundaries represent critical regions in polycrystalline sodium conductors. These interfaces often exhibit higher resistance to ion transport compared to bulk material, creating bottlenecks in the overall conductivity. The temperature dependence of grain boundary resistance typically differs from that of the bulk, with activation energies generally higher at boundaries. This differential behavior creates complex overall temperature-conductivity profiles in practical devices.
Microstructural engineering offers pathways to optimize sodium conductivity across temperature ranges. Controlling grain size, orientation, and boundary composition can significantly alter the macroscopic conductivity behavior. Advanced processing techniques such as spark plasma sintering or hot pressing can reduce grain boundary resistance by creating denser materials with improved interfacial contact.
Phase stability across operating temperature ranges presents another critical consideration. Many sodium conductors undergo phase transitions that dramatically alter their conductivity properties. For example, certain NASICON compositions exhibit monoclinic-to-rhombohedral transitions that can increase conductivity by an order of magnitude. Understanding these transitions is essential for designing materials with predictable performance across wide temperature ranges.
Sustainability Impact of Sodium-Based Technologies
The adoption of sodium-based technologies represents a significant shift towards more sustainable energy storage and transmission systems. Unlike lithium, sodium is abundant in the Earth's crust and oceans, making it a more environmentally sustainable resource. The extraction of sodium compounds typically requires less energy and produces fewer environmental pollutants compared to lithium mining operations, which often involve extensive water usage and potential habitat disruption.
Sodium conductors, particularly when optimized for temperature-dependent ionic conductivity, can contribute substantially to reducing the carbon footprint of energy storage systems. The lower production energy requirements translate directly into reduced greenhouse gas emissions during manufacturing processes. Additionally, the domestic availability of sodium resources in many countries reduces transportation-related emissions associated with global supply chains.
The recyclability of sodium-based technologies further enhances their sustainability profile. End-of-life sodium batteries and conductors can be processed with less hazardous waste generation compared to their lithium counterparts. This circular economy approach minimizes landfill impact and reduces the need for continuous raw material extraction, creating a more sustainable lifecycle for energy storage technologies.
From a social sustainability perspective, sodium-based technologies promote greater energy equity. The lower cost and greater accessibility of sodium resources can democratize access to energy storage solutions in developing regions, supporting renewable energy integration and electrification efforts in areas previously underserved due to economic constraints.
Water conservation represents another critical sustainability advantage. While lithium extraction can consume up to 500,000 gallons of water per ton of lithium produced, sodium extraction processes typically require significantly less water, an increasingly important consideration in water-stressed regions facing climate change impacts.
The temperature dependence of ionic conductivity in sodium conductors also offers sustainability benefits through improved energy efficiency. By optimizing conductors to perform efficiently across varying temperature conditions, these technologies can maintain performance with less energy input for temperature regulation, reducing operational energy consumption and associated environmental impacts.
As global energy systems transition toward renewables, the sustainability advantages of sodium-based technologies position them as key enablers of this transformation, offering a more environmentally responsible alternative to current dominant technologies while supporting broader climate action goals.
Sodium conductors, particularly when optimized for temperature-dependent ionic conductivity, can contribute substantially to reducing the carbon footprint of energy storage systems. The lower production energy requirements translate directly into reduced greenhouse gas emissions during manufacturing processes. Additionally, the domestic availability of sodium resources in many countries reduces transportation-related emissions associated with global supply chains.
The recyclability of sodium-based technologies further enhances their sustainability profile. End-of-life sodium batteries and conductors can be processed with less hazardous waste generation compared to their lithium counterparts. This circular economy approach minimizes landfill impact and reduces the need for continuous raw material extraction, creating a more sustainable lifecycle for energy storage technologies.
From a social sustainability perspective, sodium-based technologies promote greater energy equity. The lower cost and greater accessibility of sodium resources can democratize access to energy storage solutions in developing regions, supporting renewable energy integration and electrification efforts in areas previously underserved due to economic constraints.
Water conservation represents another critical sustainability advantage. While lithium extraction can consume up to 500,000 gallons of water per ton of lithium produced, sodium extraction processes typically require significantly less water, an increasingly important consideration in water-stressed regions facing climate change impacts.
The temperature dependence of ionic conductivity in sodium conductors also offers sustainability benefits through improved energy efficiency. By optimizing conductors to perform efficiently across varying temperature conditions, these technologies can maintain performance with less energy input for temperature regulation, reducing operational energy consumption and associated environmental impacts.
As global energy systems transition toward renewables, the sustainability advantages of sodium-based technologies position them as key enablers of this transformation, offering a more environmentally responsible alternative to current dominant technologies while supporting broader climate action goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!