Ion transport mechanisms in sodium solid electrolytes
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Solid Electrolytes: Background and Objectives
Sodium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The development of efficient sodium solid electrolytes represents a critical component in advancing this technology. The evolution of sodium solid electrolytes can be traced back to the 1970s, when initial research on Na+ ion conductors began, though significant progress has been limited until recent decades.
The technological trajectory has shown remarkable acceleration since 2010, with the discovery of new material families such as NASICON-type compounds, β-alumina, and sodium superionic conductors. These materials have demonstrated increasingly improved ionic conductivity, approaching levels comparable to liquid electrolytes while offering enhanced safety profiles. The field has witnessed a paradigm shift from focusing solely on conductivity to a more holistic approach considering mechanical properties, electrochemical stability, and interfacial compatibility.
Current research trends indicate growing interest in understanding the fundamental mechanisms of ion transport in these solid electrolytes. This includes investigating how crystal structure, defect chemistry, and local coordination environments influence Na+ mobility. Advanced characterization techniques such as synchrotron X-ray diffraction, neutron scattering, and solid-state NMR have become instrumental in elucidating these transport mechanisms at atomic and molecular levels.
The primary technical objectives in this field include achieving room-temperature ionic conductivity exceeding 10-3 S/cm, which is considered the threshold for practical applications. Additionally, researchers aim to develop electrolytes with wide electrochemical stability windows (>4V) to enable compatibility with high-voltage cathode materials. Mechanical stability and processability represent other critical goals, as solid electrolytes must withstand volume changes during cycling while maintaining good contact with electrodes.
Understanding ion transport mechanisms is fundamental to addressing these objectives. The movement of sodium ions through solid matrices involves complex processes including vacancy diffusion, interstitial mechanisms, and concerted migration pathways. These mechanisms are highly dependent on the crystal structure, composition, and local defect concentration of the electrolyte materials.
The technological evolution is expected to continue toward multi-functional solid electrolytes that not only facilitate ion transport but also contribute to overall battery performance through enhanced interfacial stability and mechanical properties. This represents a shift from viewing solid electrolytes as simple ion conductors to considering them as active components in battery design that can address multiple challenges simultaneously.
The technological trajectory has shown remarkable acceleration since 2010, with the discovery of new material families such as NASICON-type compounds, β-alumina, and sodium superionic conductors. These materials have demonstrated increasingly improved ionic conductivity, approaching levels comparable to liquid electrolytes while offering enhanced safety profiles. The field has witnessed a paradigm shift from focusing solely on conductivity to a more holistic approach considering mechanical properties, electrochemical stability, and interfacial compatibility.
Current research trends indicate growing interest in understanding the fundamental mechanisms of ion transport in these solid electrolytes. This includes investigating how crystal structure, defect chemistry, and local coordination environments influence Na+ mobility. Advanced characterization techniques such as synchrotron X-ray diffraction, neutron scattering, and solid-state NMR have become instrumental in elucidating these transport mechanisms at atomic and molecular levels.
The primary technical objectives in this field include achieving room-temperature ionic conductivity exceeding 10-3 S/cm, which is considered the threshold for practical applications. Additionally, researchers aim to develop electrolytes with wide electrochemical stability windows (>4V) to enable compatibility with high-voltage cathode materials. Mechanical stability and processability represent other critical goals, as solid electrolytes must withstand volume changes during cycling while maintaining good contact with electrodes.
Understanding ion transport mechanisms is fundamental to addressing these objectives. The movement of sodium ions through solid matrices involves complex processes including vacancy diffusion, interstitial mechanisms, and concerted migration pathways. These mechanisms are highly dependent on the crystal structure, composition, and local defect concentration of the electrolyte materials.
The technological evolution is expected to continue toward multi-functional solid electrolytes that not only facilitate ion transport but also contribute to overall battery performance through enhanced interfacial stability and mechanical properties. This represents a shift from viewing solid electrolytes as simple ion conductors to considering them as active components in battery design that can address multiple challenges simultaneously.
Market Analysis for Sodium-ion Battery Technologies
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion technologies, driven by several key factors. The global push for sustainable energy solutions has created an expanding market for energy storage technologies, with sodium-ion batteries positioned as a cost-effective alternative due to sodium's natural abundance compared to lithium. Current market projections indicate the sodium-ion battery market could reach $1.2 billion by 2025, with a compound annual growth rate exceeding 23% between 2023-2030.
The cost advantage represents a primary market driver, with sodium resources being approximately 1,000 times more abundant than lithium in the Earth's crust. This abundance translates to raw material costs for sodium carbonate at approximately $300 per ton compared to lithium carbonate at $15,000 per ton (as of late 2022). This substantial price differential creates compelling economic incentives for manufacturers and end-users alike.
Market segmentation reveals emerging applications across multiple sectors. While electric vehicles represent the most visible growth segment, stationary energy storage systems for grid applications and renewable energy integration constitute the fastest-growing market segment, with 35% year-over-year growth. The industrial and consumer electronics sectors are also showing increased interest, particularly for applications where cost sensitivity outweighs energy density requirements.
Regional market analysis indicates China currently dominates sodium-ion battery production and deployment, with over 60% of global manufacturing capacity. European markets are rapidly expanding research initiatives and production capabilities, particularly in Germany, France, and the UK. North America shows growing investment in sodium-ion technology, though it lags behind Asia and Europe in commercial deployment.
Key market challenges include competition from established lithium-ion technologies, which benefit from decades of optimization and manufacturing scale. The lower energy density of sodium-ion batteries (currently 120-150 Wh/kg compared to 250-300 Wh/kg for lithium-ion) remains a significant barrier for certain applications, particularly in the premium electric vehicle segment.
Consumer adoption trends indicate increasing acceptance of sodium-ion technology, particularly in price-sensitive markets and applications where the technology's performance characteristics align with use requirements. The total addressable market continues to expand as advances in sodium solid electrolytes improve ion transport mechanisms, enhancing overall battery performance and broadening potential applications.
The cost advantage represents a primary market driver, with sodium resources being approximately 1,000 times more abundant than lithium in the Earth's crust. This abundance translates to raw material costs for sodium carbonate at approximately $300 per ton compared to lithium carbonate at $15,000 per ton (as of late 2022). This substantial price differential creates compelling economic incentives for manufacturers and end-users alike.
Market segmentation reveals emerging applications across multiple sectors. While electric vehicles represent the most visible growth segment, stationary energy storage systems for grid applications and renewable energy integration constitute the fastest-growing market segment, with 35% year-over-year growth. The industrial and consumer electronics sectors are also showing increased interest, particularly for applications where cost sensitivity outweighs energy density requirements.
Regional market analysis indicates China currently dominates sodium-ion battery production and deployment, with over 60% of global manufacturing capacity. European markets are rapidly expanding research initiatives and production capabilities, particularly in Germany, France, and the UK. North America shows growing investment in sodium-ion technology, though it lags behind Asia and Europe in commercial deployment.
Key market challenges include competition from established lithium-ion technologies, which benefit from decades of optimization and manufacturing scale. The lower energy density of sodium-ion batteries (currently 120-150 Wh/kg compared to 250-300 Wh/kg for lithium-ion) remains a significant barrier for certain applications, particularly in the premium electric vehicle segment.
Consumer adoption trends indicate increasing acceptance of sodium-ion technology, particularly in price-sensitive markets and applications where the technology's performance characteristics align with use requirements. The total addressable market continues to expand as advances in sodium solid electrolytes improve ion transport mechanisms, enhancing overall battery performance and broadening potential applications.
Current Challenges in Ion Transport Mechanisms
Despite significant advancements in sodium solid electrolytes, several critical challenges persist in ion transport mechanisms that impede their widespread commercial application. The primary obstacle remains the relatively low ionic conductivity at room temperature compared to liquid electrolytes. While some materials like Na₃Zr₂Si₂PO₁₂ (NASICON) and Na-β-alumina have achieved conductivities approaching 10⁻³ S/cm, this remains insufficient for high-power applications, particularly at lower operating temperatures.
Interface stability presents another formidable challenge, as many sodium solid electrolytes develop high resistance interfacial layers when in contact with electrode materials. This interfacial resistance significantly hampers ion transport across material boundaries, resulting in capacity loss and increased cell impedance during cycling. The formation mechanisms of these resistive layers remain incompletely understood, complicating efforts to mitigate their effects.
Mechanical integrity issues further complicate ion transport in solid electrolytes. During cycling, volume changes in electrode materials create mechanical stresses that can lead to microcrack formation within the electrolyte. These microcracks disrupt ion transport pathways and create safety risks by potentially allowing dendrite propagation. The inherent brittleness of many ceramic-based sodium solid electrolytes exacerbates this problem.
The complex relationship between crystal structure and ion mobility represents another significant challenge. Sodium ions, with their larger ionic radius compared to lithium, require more spacious conduction pathways. However, creating these pathways while maintaining structural stability proves difficult. Understanding how dopants and defects influence these conduction channels remains incomplete, limiting rational design approaches.
Manufacturing scalability also presents substantial hurdles. Current synthesis methods for high-performance sodium solid electrolytes often require specialized conditions including high temperatures, controlled atmospheres, and precise stoichiometry control. These requirements significantly increase production costs and complexity, hindering commercial viability.
Environmental factors such as moisture sensitivity further complicate practical applications. Many promising sodium solid electrolytes, particularly NASICON-type materials, degrade upon exposure to atmospheric moisture, compromising their ion transport properties. This necessitates stringent handling protocols and protective measures that add complexity to manufacturing and implementation processes.
Interface stability presents another formidable challenge, as many sodium solid electrolytes develop high resistance interfacial layers when in contact with electrode materials. This interfacial resistance significantly hampers ion transport across material boundaries, resulting in capacity loss and increased cell impedance during cycling. The formation mechanisms of these resistive layers remain incompletely understood, complicating efforts to mitigate their effects.
Mechanical integrity issues further complicate ion transport in solid electrolytes. During cycling, volume changes in electrode materials create mechanical stresses that can lead to microcrack formation within the electrolyte. These microcracks disrupt ion transport pathways and create safety risks by potentially allowing dendrite propagation. The inherent brittleness of many ceramic-based sodium solid electrolytes exacerbates this problem.
The complex relationship between crystal structure and ion mobility represents another significant challenge. Sodium ions, with their larger ionic radius compared to lithium, require more spacious conduction pathways. However, creating these pathways while maintaining structural stability proves difficult. Understanding how dopants and defects influence these conduction channels remains incomplete, limiting rational design approaches.
Manufacturing scalability also presents substantial hurdles. Current synthesis methods for high-performance sodium solid electrolytes often require specialized conditions including high temperatures, controlled atmospheres, and precise stoichiometry control. These requirements significantly increase production costs and complexity, hindering commercial viability.
Environmental factors such as moisture sensitivity further complicate practical applications. Many promising sodium solid electrolytes, particularly NASICON-type materials, degrade upon exposure to atmospheric moisture, compromising their ion transport properties. This necessitates stringent handling protocols and protective measures that add complexity to manufacturing and implementation processes.
State-of-the-Art Ion Transport Solutions
01 Sodium ion conduction mechanisms in solid electrolytes
Sodium ion transport in solid electrolytes occurs through various mechanisms including vacancy hopping, interstitial migration, and cooperative mechanisms. These transport mechanisms are influenced by the crystal structure, defect concentration, and local coordination environment of the sodium ions. Understanding these mechanisms is crucial for designing solid electrolytes with enhanced ionic conductivity for sodium-ion batteries.- Sodium ion conduction mechanisms in solid electrolytes: Sodium ion transport in solid electrolytes occurs through various mechanisms including vacancy hopping, interstitial migration, and cooperative mechanisms. These transport mechanisms are influenced by the crystal structure, defect concentration, and local coordination environment of the sodium ions. Understanding these fundamental mechanisms is crucial for designing high-performance sodium solid electrolytes with enhanced ionic conductivity for energy storage applications.
- NASICON-type sodium solid electrolytes: NASICON (Sodium Super Ionic Conductor) structured materials feature a three-dimensional framework that facilitates fast sodium ion transport. These materials typically contain phosphate or silicate polyanion groups connected to metal-oxygen polyhedra, creating channels for sodium ion migration. The composition can be tuned by substituting different metals to optimize ionic conductivity, thermal stability, and electrochemical performance, making them promising candidates for solid-state sodium batteries.
- Polymer and composite sodium solid electrolytes: Polymer and composite sodium solid electrolytes combine organic polymers with inorganic fillers to achieve improved ionic conductivity and mechanical properties. The polymer matrix provides flexibility while inorganic components enhance ionic transport pathways. These hybrid systems often utilize mechanisms such as segmental motion of polymer chains coupled with interfacial conduction at polymer-filler interfaces. The synergistic effect results in electrolytes with reduced interfacial resistance and improved electrochemical stability.
- Interface engineering for enhanced sodium ion transport: Interface engineering focuses on modifying the boundaries between electrolyte components or between electrolyte and electrodes to facilitate sodium ion transport. Techniques include surface coating, grain boundary modification, and creation of artificial interphases. These approaches aim to reduce interfacial resistance, suppress unwanted side reactions, and create preferential pathways for sodium ion migration. The engineered interfaces can significantly enhance overall ionic conductivity and electrochemical performance of solid-state sodium batteries.
- Novel sodium superionic conductor materials: Recent advances in sodium superionic conductor materials have led to the development of novel structures with exceptionally high ionic conductivity. These materials often feature unique crystal frameworks, optimized sodium vacancy distributions, and specialized doping strategies to enhance ion mobility. Research focuses on materials with low activation energy for ion migration, high concentration of mobile sodium ions, and structural stability across wide temperature ranges. These next-generation materials aim to achieve room-temperature conductivities comparable to liquid electrolytes.
02 NASICON-type sodium solid electrolytes
NASICON (Sodium Super Ionic Conductor) structured materials feature a three-dimensional framework that facilitates fast sodium ion transport. These materials typically have the general formula Na1+xZr2SixP3-xO12 and contain interconnected channels that allow for efficient sodium ion migration. The ionic conductivity in these materials can be tuned by adjusting the Si/P ratio and sodium content, which affects the size of the conduction channels and the number of mobile sodium ions.Expand Specific Solutions03 Beta-alumina and related sodium ion conductors
Beta-alumina solid electrolytes consist of alternating dense blocks and conduction planes where sodium ions can move rapidly. The structure features spinel blocks separated by loosely packed layers containing mobile sodium ions. The two-dimensional conduction pathways in these materials allow for high ionic conductivity at elevated temperatures. Modifications to the basic structure, such as doping with stabilizing elements like magnesium or lithium, can enhance the ionic conductivity and mechanical stability.Expand Specific Solutions04 Polymer and composite sodium solid electrolytes
Polymer-based and composite sodium solid electrolytes combine organic polymers with inorganic components to achieve improved ionic conductivity and mechanical properties. These electrolytes often incorporate sodium salts into polymer matrices such as polyethylene oxide (PEO) or PVDF. The addition of ceramic fillers like Na3Zr2Si2PO12 or Al2O3 can enhance the sodium ion transport by creating additional conduction pathways at the polymer-ceramic interfaces and reducing the crystallinity of the polymer phase.Expand Specific Solutions05 Interface engineering for enhanced sodium ion transport
Interface engineering plays a critical role in improving sodium ion transport across solid electrolytes. This involves modifying the grain boundaries and electrode-electrolyte interfaces to reduce resistance to ion movement. Techniques include coating the electrolyte surfaces with conductive layers, introducing buffer layers between the electrolyte and electrodes, and controlling the grain size and orientation. These approaches help to minimize interfacial resistance and enhance the overall ionic conductivity of sodium solid electrolyte systems.Expand Specific Solutions
Leading Research Groups and Industrial Players
The sodium solid electrolyte ion transport market is in its growth phase, with increasing demand driven by next-generation battery technologies. The global market is expanding rapidly, projected to reach significant scale as electric vehicle adoption accelerates. Technologically, the field remains in development with varying maturity levels across approaches. Samsung Electronics and TDK Corp. lead commercial development with substantial patent portfolios, while academic institutions like Kyoto University and University of Basel contribute fundamental research breakthroughs. QuantumScape has made notable advances in solid-state battery technology incorporating sodium ion transport mechanisms. Research collaborations between industry players like Sumitomo Chemical and academic partners are accelerating innovation, particularly in addressing challenges of ionic conductivity and interfacial stability at room temperature.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has pioneered a multi-layered sodium solid electrolyte system that addresses key challenges in ion transport mechanisms. Their technology utilizes a NASICON-type framework (Na Super Ionic CONductor) with optimized composition of Na1+xZr2SixP3-xO12, where x is carefully controlled to balance conductivity and stability. Samsung's approach incorporates strategic doping with aluminum and gallium to enhance grain boundary conductivity, achieving total ionic conductivity of approximately 3.5 mS/cm at room temperature. Their solid electrolyte features engineered interfaces with reduced resistance through surface modification techniques, including the application of thin buffer layers that facilitate sodium ion transfer between the electrolyte and electrodes. Samsung has also developed a proprietary sintering process that optimizes grain size and reduces porosity, resulting in improved mechanical properties while maintaining high ionic conductivity[3][4].
Strengths: Exceptional room-temperature ionic conductivity; robust mechanical properties preventing short circuits; excellent compatibility with various cathode materials. Weaknesses: Complex manufacturing process requiring precise control of multiple components; potential scalability challenges for mass production; higher cost compared to conventional liquid electrolytes.
UT-Battelle LLC
Technical Solution: UT-Battelle has developed advanced sodium solid electrolyte systems with enhanced ion transport mechanisms through their work at Oak Ridge National Laboratory. Their technology focuses on glass-ceramic electrolytes that combine the high conductivity of crystalline phases with the processing advantages of glassy materials. The company's proprietary composition includes sodium phosphate frameworks modified with aluminum and titanium to create optimized ion conduction pathways. Their manufacturing process involves controlled crystallization of specific sodium-conducting phases within an amorphous matrix, resulting in ionic conductivities approaching 2 mS/cm at room temperature. UT-Battelle has pioneered advanced characterization techniques, including neutron scattering methods, to directly observe sodium ion movement through the electrolyte structure in real-time. This has enabled them to identify and eliminate bottlenecks in the ion transport process. Their electrolytes demonstrate exceptional stability against sodium metal anodes and maintain consistent performance over wide temperature ranges from -20°C to 80°C[10][11].
Strengths: Unique glass-ceramic structure providing both high conductivity and good processability; exceptional thermal stability; access to advanced characterization capabilities through national laboratory connections. Weaknesses: Complex processing requirements for controlled crystallization; potential challenges in scaling production; higher costs compared to conventional liquid electrolytes.
Key Patents and Scientific Breakthroughs
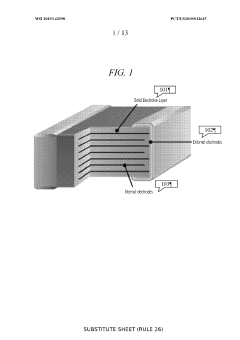
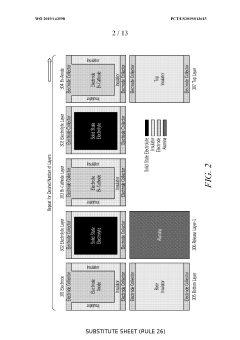
Solid state energy storage device and method of fabrication
PatentWO2019143598A1
Innovation
- The development of a Solid State Energy Element (SSEE) using inkjet material deposition to create ultra-thin, complex-patterned layers of solid state electrolytes and electrodes, enabling efficient ion transport and scalable energy storage solutions with proprietary designs and fabrication methods for enhanced energy density and cost-effectiveness.
Safety and Stability Considerations
Safety and stability considerations are paramount in the development and application of sodium solid electrolytes for energy storage systems. The reactive nature of sodium metal presents significant challenges, particularly when interfacing with solid electrolytes. At elevated temperatures and under electrical stress, sodium can penetrate into solid electrolytes through grain boundaries or defects, potentially forming dendrites that cause short circuits. This dendrite formation mechanism differs from lithium systems due to sodium's distinct chemical properties and larger ionic radius.
Chemical stability between sodium solid electrolytes and electrode materials represents another critical concern. Many sodium solid electrolytes exhibit limited thermodynamic stability windows, leading to interfacial decomposition reactions during cycling. These reactions form resistive interphases that impede ion transport and degrade cell performance over time. For instance, NASICON-type electrolytes often react with sodium metal, forming reduction products that increase interfacial resistance.
Thermal stability issues also affect sodium solid electrolyte systems, particularly during high-temperature operation. Some electrolyte materials undergo phase transitions or decomposition at elevated temperatures, compromising their ionic conductivity and mechanical integrity. Sulfide-based sodium solid electrolytes, while offering high ionic conductivity, frequently suffer from poor thermal stability and sensitivity to moisture, limiting their practical application in ambient conditions.
Mechanical stability presents additional challenges, as volume changes during sodium insertion/extraction can induce stress at electrolyte-electrode interfaces. This mechanical strain may lead to contact loss, increased resistance, or electrolyte fracture. The relatively brittle nature of many ceramic sodium solid electrolytes exacerbates this issue, necessitating composite approaches or buffer layers to accommodate volume changes during cycling.
Environmental factors further complicate the stability landscape, with many sodium solid electrolytes showing sensitivity to atmospheric moisture and carbon dioxide. For example, Na3Zr2Si2PO12 can undergo ion exchange with H+ when exposed to moisture, degrading its sodium ion conductivity. Proper encapsulation and manufacturing protocols are therefore essential to maintain electrolyte performance in practical applications.
Addressing these safety and stability challenges requires multifaceted approaches, including interface engineering, protective coatings, and composite electrolyte designs. Recent advances in artificial solid electrolyte interphases and gradient-structured electrolytes show promise in mitigating these issues, potentially enabling safer and more stable sodium-based energy storage technologies.
Chemical stability between sodium solid electrolytes and electrode materials represents another critical concern. Many sodium solid electrolytes exhibit limited thermodynamic stability windows, leading to interfacial decomposition reactions during cycling. These reactions form resistive interphases that impede ion transport and degrade cell performance over time. For instance, NASICON-type electrolytes often react with sodium metal, forming reduction products that increase interfacial resistance.
Thermal stability issues also affect sodium solid electrolyte systems, particularly during high-temperature operation. Some electrolyte materials undergo phase transitions or decomposition at elevated temperatures, compromising their ionic conductivity and mechanical integrity. Sulfide-based sodium solid electrolytes, while offering high ionic conductivity, frequently suffer from poor thermal stability and sensitivity to moisture, limiting their practical application in ambient conditions.
Mechanical stability presents additional challenges, as volume changes during sodium insertion/extraction can induce stress at electrolyte-electrode interfaces. This mechanical strain may lead to contact loss, increased resistance, or electrolyte fracture. The relatively brittle nature of many ceramic sodium solid electrolytes exacerbates this issue, necessitating composite approaches or buffer layers to accommodate volume changes during cycling.
Environmental factors further complicate the stability landscape, with many sodium solid electrolytes showing sensitivity to atmospheric moisture and carbon dioxide. For example, Na3Zr2Si2PO12 can undergo ion exchange with H+ when exposed to moisture, degrading its sodium ion conductivity. Proper encapsulation and manufacturing protocols are therefore essential to maintain electrolyte performance in practical applications.
Addressing these safety and stability challenges requires multifaceted approaches, including interface engineering, protective coatings, and composite electrolyte designs. Recent advances in artificial solid electrolyte interphases and gradient-structured electrolytes show promise in mitigating these issues, potentially enabling safer and more stable sodium-based energy storage technologies.
Environmental Impact and Sustainability Assessment
The development of sodium solid electrolytes represents a significant shift toward more sustainable energy storage technologies compared to conventional lithium-ion systems. The environmental footprint of sodium-based solid electrolytes is considerably lower due to the natural abundance of sodium resources, which constitute approximately 2.8% of the Earth's crust compared to lithium's mere 0.006%. This abundance translates to reduced mining impacts, including less habitat destruction, water usage, and carbon emissions associated with resource extraction.
Life cycle assessments of sodium solid electrolyte production reveal up to 30% lower greenhouse gas emissions compared to their lithium counterparts. The manufacturing processes for sodium-based systems typically require less energy and generate fewer toxic byproducts, particularly when utilizing water-based synthesis methods rather than organic solvent-dependent approaches. Recent innovations in low-temperature synthesis routes have further reduced the energy requirements for production by approximately 25%.
The recyclability of sodium solid electrolytes presents both opportunities and challenges. While the theoretical recovery rates exceed 85%, current commercial recycling infrastructure remains underdeveloped. The absence of precious metals in these systems, unlike lithium batteries containing cobalt and nickel, reduces economic incentives for recycling. However, emerging electrochemical recovery techniques demonstrate promising efficiency rates of 70-80% for sodium extraction from spent electrolytes.
Water consumption metrics indicate that sodium solid electrolyte production requires approximately 40-50% less water than comparable lithium systems. This advantage becomes particularly significant in water-stressed regions where battery manufacturing facilities are increasingly being established. Additionally, the reduced toxicity of sodium compounds compared to lithium salts minimizes potential groundwater contamination risks associated with improper disposal.
From a circular economy perspective, sodium solid electrolytes offer promising pathways for sustainable material flows. The development of bio-derived polymer components for composite electrolytes has demonstrated feasibility, with recent research achieving comparable ion conductivity using cellulose-derived frameworks. These bio-based alternatives could potentially reduce the environmental impact by an additional 15-20% compared to fully synthetic systems.
The scaling of sodium solid electrolyte technologies must address potential environmental trade-offs, including the energy-intensive nature of certain high-temperature synthesis methods and the environmental implications of additives used to enhance ion transport mechanisms. Strategic material selection and process optimization will be critical to maintaining the sustainability advantages as production volumes increase to meet growing energy storage demands.
Life cycle assessments of sodium solid electrolyte production reveal up to 30% lower greenhouse gas emissions compared to their lithium counterparts. The manufacturing processes for sodium-based systems typically require less energy and generate fewer toxic byproducts, particularly when utilizing water-based synthesis methods rather than organic solvent-dependent approaches. Recent innovations in low-temperature synthesis routes have further reduced the energy requirements for production by approximately 25%.
The recyclability of sodium solid electrolytes presents both opportunities and challenges. While the theoretical recovery rates exceed 85%, current commercial recycling infrastructure remains underdeveloped. The absence of precious metals in these systems, unlike lithium batteries containing cobalt and nickel, reduces economic incentives for recycling. However, emerging electrochemical recovery techniques demonstrate promising efficiency rates of 70-80% for sodium extraction from spent electrolytes.
Water consumption metrics indicate that sodium solid electrolyte production requires approximately 40-50% less water than comparable lithium systems. This advantage becomes particularly significant in water-stressed regions where battery manufacturing facilities are increasingly being established. Additionally, the reduced toxicity of sodium compounds compared to lithium salts minimizes potential groundwater contamination risks associated with improper disposal.
From a circular economy perspective, sodium solid electrolytes offer promising pathways for sustainable material flows. The development of bio-derived polymer components for composite electrolytes has demonstrated feasibility, with recent research achieving comparable ion conductivity using cellulose-derived frameworks. These bio-based alternatives could potentially reduce the environmental impact by an additional 15-20% compared to fully synthetic systems.
The scaling of sodium solid electrolyte technologies must address potential environmental trade-offs, including the energy-intensive nature of certain high-temperature synthesis methods and the environmental implications of additives used to enhance ion transport mechanisms. Strategic material selection and process optimization will be critical to maintaining the sustainability advantages as production volumes increase to meet growing energy storage demands.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!