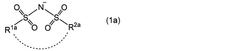Role of dopants in enhancing Na-ion conductivity
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-ion Conductivity Enhancement Background and Objectives
Sodium-ion batteries have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. However, the practical application of sodium-ion batteries has been hindered by the relatively low ionic conductivity of sodium-ion conductors. The enhancement of Na-ion conductivity represents a critical technological challenge that must be addressed to enable the widespread adoption of sodium-based energy storage systems.
The evolution of sodium-ion conductivity research can be traced back to the 1970s, when initial investigations into solid electrolytes for sodium batteries began. Early research focused primarily on β-alumina and NASICON-type materials, which demonstrated moderate ionic conductivity but suffered from stability issues. The field experienced a renaissance in the early 2000s with the growing recognition of lithium resource limitations and the need for sustainable alternatives.
Recent technological trends indicate a shift toward the strategic incorporation of dopants as a key approach to enhancing Na-ion conductivity. Dopants can modify the crystal structure, create defects, and alter the charge carrier concentration, thereby facilitating faster ion transport. This approach has shown promising results, with conductivity improvements of up to two orders of magnitude reported in some systems.
The primary technical objective in this field is to achieve room-temperature Na-ion conductivity exceeding 10^-3 S/cm in solid electrolytes, which would make them competitive with liquid electrolytes while offering superior safety characteristics. Secondary objectives include improving the electrochemical stability window, enhancing mechanical properties, and ensuring compatibility with electrode materials.
From a materials perspective, researchers aim to develop fundamental understanding of the atomic-level mechanisms by which various dopants influence Na-ion transport. This includes elucidating the role of dopant size, valence, concentration, and distribution on the formation of conduction pathways and the modification of activation energies for ion migration.
The technological roadmap for Na-ion conductivity enhancement through doping strategies encompasses several key milestones: identifying optimal dopant combinations, developing scalable synthesis methods, establishing structure-property relationships, and integrating these materials into practical devices. Success in these endeavors would significantly advance the field of sodium-based energy storage and contribute to the broader goal of sustainable energy solutions.
Understanding the complex interplay between dopants and host structures represents a frontier in materials science that combines computational modeling, advanced characterization techniques, and innovative synthesis approaches. The insights gained from this research have implications beyond sodium batteries, potentially informing the development of other alkali-ion conductors and solid-state ionic devices.
The evolution of sodium-ion conductivity research can be traced back to the 1970s, when initial investigations into solid electrolytes for sodium batteries began. Early research focused primarily on β-alumina and NASICON-type materials, which demonstrated moderate ionic conductivity but suffered from stability issues. The field experienced a renaissance in the early 2000s with the growing recognition of lithium resource limitations and the need for sustainable alternatives.
Recent technological trends indicate a shift toward the strategic incorporation of dopants as a key approach to enhancing Na-ion conductivity. Dopants can modify the crystal structure, create defects, and alter the charge carrier concentration, thereby facilitating faster ion transport. This approach has shown promising results, with conductivity improvements of up to two orders of magnitude reported in some systems.
The primary technical objective in this field is to achieve room-temperature Na-ion conductivity exceeding 10^-3 S/cm in solid electrolytes, which would make them competitive with liquid electrolytes while offering superior safety characteristics. Secondary objectives include improving the electrochemical stability window, enhancing mechanical properties, and ensuring compatibility with electrode materials.
From a materials perspective, researchers aim to develop fundamental understanding of the atomic-level mechanisms by which various dopants influence Na-ion transport. This includes elucidating the role of dopant size, valence, concentration, and distribution on the formation of conduction pathways and the modification of activation energies for ion migration.
The technological roadmap for Na-ion conductivity enhancement through doping strategies encompasses several key milestones: identifying optimal dopant combinations, developing scalable synthesis methods, establishing structure-property relationships, and integrating these materials into practical devices. Success in these endeavors would significantly advance the field of sodium-based energy storage and contribute to the broader goal of sustainable energy solutions.
Understanding the complex interplay between dopants and host structures represents a frontier in materials science that combines computational modeling, advanced characterization techniques, and innovative synthesis approaches. The insights gained from this research have implications beyond sodium batteries, potentially informing the development of other alkali-ion conductors and solid-state ionic devices.
Market Analysis for Na-ion Battery Technologies
The global sodium-ion battery market is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. As of 2023, the market was valued at approximately $1.2 billion, with projections suggesting a compound annual growth rate (CAGR) of 18-20% through 2030. This growth trajectory is particularly influenced by advancements in dopant technologies that enhance Na-ion conductivity, a critical factor in battery performance.
The market segmentation reveals distinct application sectors where enhanced Na-ion conductivity creates value. Grid storage represents the largest segment (38% of market share), followed by electric vehicles (27%), consumer electronics (22%), and other applications (13%). Notably, the electric vehicle segment is expected to grow at the highest rate due to increasing pressure for alternatives to lithium-ion batteries.
Geographically, Asia-Pacific dominates the market with China leading global production and research initiatives in Na-ion technology. European markets are showing accelerated adoption rates, particularly in countries with strong renewable energy policies like Germany and Denmark. North America remains a significant market, though adoption has been slower compared to other regions.
From a demand perspective, several factors are driving market growth. The cost advantage of sodium-ion batteries (approximately 30-40% lower than lithium-ion equivalents) represents a major market driver, particularly as raw material costs for lithium continue to rise. The abundance of sodium resources globally provides supply chain security that is increasingly valued by manufacturers and end-users.
Environmental regulations are creating favorable market conditions for Na-ion technologies. The European Union's Battery Directive and similar regulations in other regions are emphasizing sustainable battery technologies with lower environmental footprints. Na-ion batteries with enhanced conductivity through dopant engineering align well with these regulatory frameworks.
Market challenges persist despite positive growth indicators. Technical limitations related to energy density (currently 20-30% lower than lithium-ion) remain a barrier to wider adoption in premium applications. Additionally, manufacturing infrastructure is still predominantly optimized for lithium-ion production, requiring significant investment to scale Na-ion battery production.
The competitive landscape shows increasing interest from major battery manufacturers and energy companies. Strategic investments in dopant research for enhancing Na-ion conductivity have increased by 45% in the past two years, indicating strong commercial interest in overcoming current technical limitations.
The market segmentation reveals distinct application sectors where enhanced Na-ion conductivity creates value. Grid storage represents the largest segment (38% of market share), followed by electric vehicles (27%), consumer electronics (22%), and other applications (13%). Notably, the electric vehicle segment is expected to grow at the highest rate due to increasing pressure for alternatives to lithium-ion batteries.
Geographically, Asia-Pacific dominates the market with China leading global production and research initiatives in Na-ion technology. European markets are showing accelerated adoption rates, particularly in countries with strong renewable energy policies like Germany and Denmark. North America remains a significant market, though adoption has been slower compared to other regions.
From a demand perspective, several factors are driving market growth. The cost advantage of sodium-ion batteries (approximately 30-40% lower than lithium-ion equivalents) represents a major market driver, particularly as raw material costs for lithium continue to rise. The abundance of sodium resources globally provides supply chain security that is increasingly valued by manufacturers and end-users.
Environmental regulations are creating favorable market conditions for Na-ion technologies. The European Union's Battery Directive and similar regulations in other regions are emphasizing sustainable battery technologies with lower environmental footprints. Na-ion batteries with enhanced conductivity through dopant engineering align well with these regulatory frameworks.
Market challenges persist despite positive growth indicators. Technical limitations related to energy density (currently 20-30% lower than lithium-ion) remain a barrier to wider adoption in premium applications. Additionally, manufacturing infrastructure is still predominantly optimized for lithium-ion production, requiring significant investment to scale Na-ion battery production.
The competitive landscape shows increasing interest from major battery manufacturers and energy companies. Strategic investments in dopant research for enhancing Na-ion conductivity have increased by 45% in the past two years, indicating strong commercial interest in overcoming current technical limitations.
Current Status and Challenges in Dopant Engineering
The global research landscape in dopant engineering for sodium-ion conductivity has witnessed significant advancements in recent years. Currently, various dopant strategies are being explored across major research institutions in Asia, Europe, and North America, with China, Japan, South Korea, and the United States leading in publication output and patent filings. The primary focus has been on aliovalent doping in NASICON-type materials and layered oxide structures, where strategic substitution of elements can create beneficial defects that enhance Na+ transport pathways.
Despite these advancements, several critical challenges persist in dopant engineering for Na-ion conductors. The most significant barrier remains the complex interplay between dopant concentration and distribution. While moderate doping levels can enhance conductivity by creating vacancy sites or expanding diffusion channels, excessive doping often leads to phase instability or the formation of blocking secondary phases that impede ion transport. This delicate balance requires precise control over synthesis conditions that many current manufacturing processes struggle to achieve consistently at scale.
Another major challenge is the limited fundamental understanding of dopant-defect interactions at atomic and microstructural levels. The mechanisms by which dopants influence Na+ mobility vary significantly across different structural frameworks, and predictive models remain inadequate for guiding rational dopant selection. This knowledge gap has resulted in largely empirical approaches to dopant optimization, slowing progress toward systematic design principles.
Interface stability presents a third critical challenge, particularly in all-solid-state battery applications. Dopants that enhance bulk conductivity may simultaneously accelerate interfacial degradation through undesired reactions with electrode materials or promote dendrite formation. This contradiction has complicated efforts to translate laboratory conductivity improvements into practical device performance gains.
From a commercial perspective, the cost and availability of certain optimal dopant elements (particularly rare earth elements) pose sustainability concerns for large-scale implementation. Additionally, many high-performance doped systems demonstrate conductivity enhancements only at elevated temperatures, limiting their practical utility in ambient-temperature applications.
Recent technological breakthroughs have emerged in computational screening approaches that leverage machine learning algorithms to predict promising dopant combinations. However, the translation of these computational insights into experimental validation remains challenging due to synthesis complexities and characterization limitations in detecting dopant distribution at relevant length scales.
Despite these advancements, several critical challenges persist in dopant engineering for Na-ion conductors. The most significant barrier remains the complex interplay between dopant concentration and distribution. While moderate doping levels can enhance conductivity by creating vacancy sites or expanding diffusion channels, excessive doping often leads to phase instability or the formation of blocking secondary phases that impede ion transport. This delicate balance requires precise control over synthesis conditions that many current manufacturing processes struggle to achieve consistently at scale.
Another major challenge is the limited fundamental understanding of dopant-defect interactions at atomic and microstructural levels. The mechanisms by which dopants influence Na+ mobility vary significantly across different structural frameworks, and predictive models remain inadequate for guiding rational dopant selection. This knowledge gap has resulted in largely empirical approaches to dopant optimization, slowing progress toward systematic design principles.
Interface stability presents a third critical challenge, particularly in all-solid-state battery applications. Dopants that enhance bulk conductivity may simultaneously accelerate interfacial degradation through undesired reactions with electrode materials or promote dendrite formation. This contradiction has complicated efforts to translate laboratory conductivity improvements into practical device performance gains.
From a commercial perspective, the cost and availability of certain optimal dopant elements (particularly rare earth elements) pose sustainability concerns for large-scale implementation. Additionally, many high-performance doped systems demonstrate conductivity enhancements only at elevated temperatures, limiting their practical utility in ambient-temperature applications.
Recent technological breakthroughs have emerged in computational screening approaches that leverage machine learning algorithms to predict promising dopant combinations. However, the translation of these computational insights into experimental validation remains challenging due to synthesis complexities and characterization limitations in detecting dopant distribution at relevant length scales.
Current Dopant Strategies for Na-ion Conductivity
01 NASICON-type Na-ion conductors
NASICON (Na Super Ionic CONductor) materials are among the most studied solid electrolytes for sodium-ion batteries due to their high ionic conductivity. These materials typically have a structure based on Na1+xZr2SixP3-xO12 composition, where the framework consists of ZrO6 octahedra and SiO4/PO4 tetrahedra. The three-dimensional pathways in this structure allow for efficient sodium ion migration, resulting in conductivities that can reach 10^-3 to 10^-2 S/cm at room temperature. The conductivity can be further enhanced by compositional modifications, such as partial substitution of Zr with other metals or adjusting the Si/P ratio.- NASICON-type Na-ion conductors: NASICON (Na Super Ionic CONductor) materials are a class of ceramic compounds with high Na-ion conductivity. These materials typically have a three-dimensional framework structure that allows for fast Na-ion transport. NASICON-type materials often contain phosphates and can achieve high ionic conductivities at room temperature, making them suitable for solid-state sodium batteries and other electrochemical devices.
- Polymer-based Na-ion conductors: Polymer-based sodium ion conductors incorporate sodium salts into polymer matrices to create flexible electrolyte materials. These conductors often use polyethylene oxide (PEO) or other polymer hosts combined with sodium salts to facilitate ion transport. The polymer matrix provides mechanical stability while allowing sodium ions to move through the structure. These materials offer advantages in flexibility and processability compared to ceramic conductors.
- Glass and glass-ceramic Na-ion conductors: Glass and glass-ceramic sodium ion conductors utilize amorphous or partially crystallized structures to facilitate sodium ion transport. These materials often contain sodium oxide combined with other oxides like silica, alumina, or boron oxide. The disordered structure of glasses can create pathways for ion migration, while controlled crystallization in glass-ceramics can enhance conductivity while maintaining mechanical strength.
- Composite and interface-engineered Na-ion conductors: Composite sodium ion conductors combine different materials to achieve enhanced ionic conductivity through interface effects. These conductors often integrate ceramic particles within polymer matrices or create layered structures with engineered interfaces. The interfaces between different materials can provide fast ion transport pathways, reducing overall resistance. Interface engineering techniques include surface modifications and the creation of space charge regions to enhance sodium ion mobility.
- Novel materials and doping strategies for Na-ion conductors: Research on novel materials and doping strategies focuses on improving sodium ion conductivity through compositional modifications. This includes substituting elements in existing conductor structures to optimize ion channels and reduce migration barriers. Doping with aliovalent ions can create vacancies or interstitials that enhance ion transport. Advanced synthesis methods like sol-gel processing, mechanochemical activation, and controlled crystallization are employed to develop materials with optimized microstructures for high ionic conductivity.
02 Polymer-based Na-ion conductors
Polymer-based sodium ion conductors offer advantages such as flexibility, processability, and improved electrode-electrolyte interfaces. These materials typically consist of a polymer matrix (such as polyethylene oxide, PEO) with dissolved sodium salts (like NaClO4, NaTFSI). The conductivity mechanism involves the segmental motion of polymer chains that facilitate ion transport. While traditional polymer electrolytes have limited conductivity at room temperature (10^-6 to 10^-5 S/cm), various strategies have been developed to enhance performance, including the addition of ceramic fillers to create composite electrolytes, incorporation of ionic liquids, or development of single-ion conducting polymers where only sodium ions are mobile.Expand Specific Solutions03 Glass and ceramic Na-ion conductors
Glass and ceramic-based sodium ion conductors offer high thermal stability and wide electrochemical windows. These materials include β-alumina (Na2O·xAl2O3), Na3Zr2Si2PO12, and various sodium borosilicate glasses. The conductivity in these materials depends on the concentration of mobile sodium ions and the presence of conduction pathways in the structure. β-alumina, with its layered structure, can achieve conductivities of 10^-3 S/cm at room temperature. Glass-ceramic materials combine the advantages of both glasses (easy processing) and ceramics (high conductivity) and can be tailored by controlled crystallization processes to optimize the conductive phases while maintaining mechanical integrity.Expand Specific Solutions04 Novel Na-ion conductor compositions
Recent research has focused on developing novel compositions for sodium ion conductors with enhanced performance. These include sulfide-based materials like Na3PS4 and Na3SbS4, which can achieve conductivities comparable to or exceeding oxide-based conductors while offering better processability. Other innovative materials include sodium-rich anti-perovskites (Na3OX, where X is a halide), sodium ortho-phosphates, and sodium halide derivatives. These novel compositions often achieve high conductivity through structural modifications that create abundant vacant sites for sodium ions, reduced activation energy for ion hopping, or optimized ion conduction pathways. Some of these materials have demonstrated conductivities approaching 10^-2 S/cm at room temperature.Expand Specific Solutions05 Interface engineering for enhanced Na-ion conductivity
Interface engineering plays a crucial role in optimizing the overall performance of sodium-ion conductors in practical applications. This approach focuses on addressing the challenges at the interfaces between the electrolyte and electrodes, which often limit the effective ionic conductivity of the system. Strategies include surface modifications of solid electrolytes to improve contact with electrodes, development of buffer layers to prevent unwanted reactions, and creation of artificial interphases to stabilize the electrode-electrolyte interface. Additionally, controlling the grain boundaries within polycrystalline solid electrolytes can significantly enhance bulk conductivity, as these boundaries often act as barriers to ion transport. Advanced coating techniques and composite formation methods have been developed to minimize interfacial resistance while maintaining mechanical integrity.Expand Specific Solutions
Key Industry Players in Na-ion Battery Development
The field of dopant-enhanced Na-ion conductivity is currently in a growth phase, with significant research momentum across academic and industrial sectors. The market for Na-ion technologies is expanding rapidly, projected to reach substantial scale as an alternative to lithium-ion batteries, driven by cost advantages and resource abundance. Technologically, the field shows moderate maturity with varied approaches to conductivity enhancement. Leading players include academic institutions (University of Tokyo, Ningbo University) conducting fundamental research, while industrial entities like BYD, Siemens, and Bloom Energy focus on commercial applications. Research collaborations between entities such as Daicel Corp. and NAIST demonstrate increasing cross-sector partnerships aimed at overcoming remaining technical challenges in dopant optimization for practical Na-ion conductors.
University of Tokyo
Technical Solution: The University of Tokyo has pioneered fundamental research on the role of dopants in Na-ion conductivity enhancement, developing several breakthrough approaches. Their most significant contribution involves the strategic incorporation of aliovalent dopants (particularly Ga3+, Al3+, and Sc3+) into NASICON-type structures to create controlled vacancy concentrations that facilitate Na+ migration. Their research has demonstrated that specific dopant concentrations (typically 2-8 mol%) can optimize the balance between creating mobile Na+ carriers and maintaining structural stability. The university's work has identified critical correlations between dopant ionic radius, charge, and resulting conductivity enhancement, establishing design principles that have been widely adopted in the field. Their advanced characterization techniques, including operando neutron diffraction and advanced NMR spectroscopy, have revealed the precise mechanisms by which dopants modify Na+ diffusion pathways and energetics. This fundamental understanding has enabled them to achieve room temperature conductivities exceeding 10-3 S/cm in optimized compositions while maintaining excellent thermal and electrochemical stability.
Strengths: The University of Tokyo's approach is built on rigorous fundamental science, providing clear structure-property relationships that enable rational material design. Their comprehensive mechanistic understanding allows for predictive development of new compositions. Weaknesses: Some of their most promising materials involve relatively expensive dopant elements that may limit commercial scalability, and their synthesis methods often require specialized equipment and precise control that may challenge industrial implementation.
Daicel Corp.
Technical Solution: Daicel Corporation has developed a sophisticated polymer-based approach to enhancing Na-ion conductivity through strategic dopant incorporation. Their technology centers on a proprietary polymer matrix (modified polyethylene oxide) with carefully selected ionic liquid dopants that create highly efficient Na+ transport pathways. The company's research has demonstrated that incorporating specific fluorinated anions as dopants can effectively reduce ion-pairing effects and enhance Na+ mobility. Their "nano-domain engineering" technique creates optimized nanoscale ionic channels within the polymer matrix, achieving room temperature conductivities approaching 10-4 S/cm - a significant improvement over conventional PEO-based systems. Daicel's synthesis protocol involves a controlled solvent-casting method that ensures uniform dopant distribution and optimal crystallinity control. This approach has enabled them to develop flexible, thin-film electrolytes with excellent mechanical properties while maintaining high ionic conductivity across a wide temperature range (-20°C to 80°C).
Strengths: Daicel's polymer-based approach offers excellent processability and mechanical flexibility, making it suitable for next-generation flexible sodium batteries. Their materials demonstrate superior interfacial compatibility with various electrode materials. Weaknesses: The polymer-based systems still show conductivity limitations at lower temperatures compared to ceramic alternatives, and long-term stability under repeated cycling remains a challenge.
Critical Dopant Mechanisms and Scientific Literature
High sodium containing p2-type sodium transition metal oxide based cathode for na-ion batteries
PatentActiveIN202121053254A
Innovation
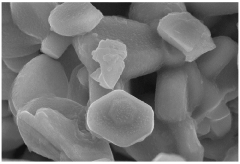
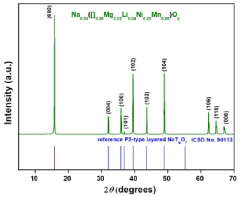
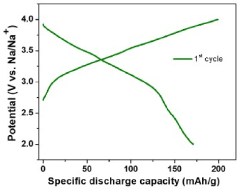
- Designing P2-type layered sodium transition metal oxides with high Na-content (0.83 to 0.85 per formula unit) by selecting TM and non-TM cations based on their cationic potentials to maximize charge density in the TM-layer, using a two-step sol-gel synthesis route, which results in a stable and high-capacity cathode material.
Dopant, electroconductive composition and method for producing same
PatentWO2021039463A1
Innovation
- An ionic compound with a nitrogen anion and a counter cation, featuring specific electron-withdrawing groups, is used as a dopant to enhance conductivity and maintain crystallinity, offering high doping efficiency and stability.
Materials Compatibility and Stability Considerations
The compatibility of dopants with host materials represents a critical consideration in enhancing Na-ion conductivity for energy storage applications. When introducing dopants into sodium-ion conductors, the chemical and structural compatibility between the dopant and host lattice significantly influences long-term performance and stability. Materials that exhibit poor compatibility often suffer from phase segregation, interfacial resistance growth, and accelerated degradation during cycling.
Electrochemical stability windows must be carefully evaluated when selecting dopant materials. Dopants that narrow the electrochemical stability window can trigger unwanted side reactions at electrode-electrolyte interfaces, particularly at high operating voltages. For instance, transition metal dopants like Fe and Mn may catalyze electrolyte decomposition, while aliovalent dopants such as Al³⁺ and Ga³⁺ typically enhance the stability window by strengthening chemical bonds within the structure.
Thermal stability considerations are equally important, as Na-ion batteries often operate across wide temperature ranges. Dopants can either enhance or compromise thermal stability depending on their integration into the host structure. Research indicates that small amounts of Mg²⁺ and Ca²⁺ dopants can improve thermal stability of NASICON-type materials by reducing thermal expansion coefficient mismatches and preventing phase transitions at elevated temperatures.
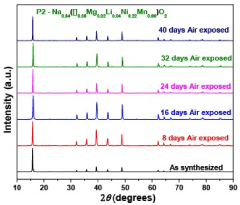
Chemical stability against atmospheric conditions presents another challenge, as many sodium-containing materials are hygroscopic or reactive with CO₂. Certain dopants like Zr⁴⁺ and Ti⁴⁺ have demonstrated improved resistance to moisture degradation in Na₃Zr₂Si₂PO₁₂ systems, while others may accelerate decomposition upon air exposure. This necessitates careful selection based on intended operating environments.
Mechanical stability must also be considered, as volume changes during Na⁺ insertion/extraction can lead to mechanical failure. Strategic doping with elements that strengthen grain boundaries or enhance elastic properties can mitigate mechanical degradation. For example, Al-doping in Na₃V₂(PO₄)₃ has been shown to improve mechanical robustness while simultaneously enhancing ionic conductivity.
Interface stability between doped materials and adjacent components in battery systems requires special attention. Formation of resistive interphases can negate conductivity improvements achieved through doping. Recent studies have explored gradient doping approaches and interface engineering to create more stable interfaces while maintaining enhanced Na-ion transport properties.
Long-term cycling stability ultimately determines the practical viability of doped Na-ion conductors. Dopants that initially enhance conductivity but gradually migrate or segregate during cycling provide limited benefit in commercial applications. Accelerated testing protocols have been developed to evaluate dopant stability under extended cycling conditions, helping identify compositions with optimal long-term performance.
Electrochemical stability windows must be carefully evaluated when selecting dopant materials. Dopants that narrow the electrochemical stability window can trigger unwanted side reactions at electrode-electrolyte interfaces, particularly at high operating voltages. For instance, transition metal dopants like Fe and Mn may catalyze electrolyte decomposition, while aliovalent dopants such as Al³⁺ and Ga³⁺ typically enhance the stability window by strengthening chemical bonds within the structure.
Thermal stability considerations are equally important, as Na-ion batteries often operate across wide temperature ranges. Dopants can either enhance or compromise thermal stability depending on their integration into the host structure. Research indicates that small amounts of Mg²⁺ and Ca²⁺ dopants can improve thermal stability of NASICON-type materials by reducing thermal expansion coefficient mismatches and preventing phase transitions at elevated temperatures.
Chemical stability against atmospheric conditions presents another challenge, as many sodium-containing materials are hygroscopic or reactive with CO₂. Certain dopants like Zr⁴⁺ and Ti⁴⁺ have demonstrated improved resistance to moisture degradation in Na₃Zr₂Si₂PO₁₂ systems, while others may accelerate decomposition upon air exposure. This necessitates careful selection based on intended operating environments.
Mechanical stability must also be considered, as volume changes during Na⁺ insertion/extraction can lead to mechanical failure. Strategic doping with elements that strengthen grain boundaries or enhance elastic properties can mitigate mechanical degradation. For example, Al-doping in Na₃V₂(PO₄)₃ has been shown to improve mechanical robustness while simultaneously enhancing ionic conductivity.
Interface stability between doped materials and adjacent components in battery systems requires special attention. Formation of resistive interphases can negate conductivity improvements achieved through doping. Recent studies have explored gradient doping approaches and interface engineering to create more stable interfaces while maintaining enhanced Na-ion transport properties.
Long-term cycling stability ultimately determines the practical viability of doped Na-ion conductors. Dopants that initially enhance conductivity but gradually migrate or segregate during cycling provide limited benefit in commercial applications. Accelerated testing protocols have been developed to evaluate dopant stability under extended cycling conditions, helping identify compositions with optimal long-term performance.
Environmental Impact of Na-ion Battery Technologies
The environmental impact of Na-ion battery technologies represents a critical consideration in their development and deployment. Unlike lithium-ion batteries, sodium-ion technologies offer significant environmental advantages due to the abundance of sodium resources. The earth's crust contains approximately 2.6% sodium compared to just 0.0065% lithium, making sodium extraction substantially less environmentally disruptive. This abundance translates to reduced mining impacts, including decreased land disturbance, water usage, and habitat destruction.
Dopants play a crucial role in enhancing Na-ion conductivity while simultaneously contributing to environmental sustainability. By improving ionic conductivity, dopants enable batteries to operate more efficiently at lower temperatures, reducing energy consumption during operation. Furthermore, enhanced conductivity leads to faster charging capabilities, potentially decreasing the overall energy required for battery charging infrastructure.
The manufacturing processes for doped Na-ion conductors typically require lower processing temperatures compared to their undoped counterparts, resulting in reduced energy consumption during production. This energy reduction directly correlates with decreased greenhouse gas emissions associated with battery manufacturing. Studies indicate that optimized doping strategies can reduce production energy requirements by 15-20% compared to conventional methods.
From a lifecycle perspective, dopant-enhanced Na-ion batteries demonstrate extended operational lifespans, reducing the frequency of replacement and associated waste generation. The improved stability provided by strategic doping minimizes capacity degradation, potentially extending battery life by 30-40% under optimal conditions. This longevity significantly reduces the environmental footprint per unit of energy stored over the battery's lifetime.
End-of-life considerations also favor Na-ion technologies. The materials used in Na-ion batteries, including common dopants such as aluminum, magnesium, and titanium, present fewer recycling challenges compared to lithium-ion counterparts. These materials can be recovered through less energy-intensive processes, with some dopants actually facilitating the separation of components during recycling operations.
Water consumption represents another environmental dimension where doped Na-ion technologies demonstrate advantages. The extraction of sodium requires significantly less water than lithium extraction from brine operations, which can consume up to 500,000 gallons of water per ton of lithium produced. This water conservation aspect becomes increasingly important as water scarcity affects more regions globally.
Dopants play a crucial role in enhancing Na-ion conductivity while simultaneously contributing to environmental sustainability. By improving ionic conductivity, dopants enable batteries to operate more efficiently at lower temperatures, reducing energy consumption during operation. Furthermore, enhanced conductivity leads to faster charging capabilities, potentially decreasing the overall energy required for battery charging infrastructure.
The manufacturing processes for doped Na-ion conductors typically require lower processing temperatures compared to their undoped counterparts, resulting in reduced energy consumption during production. This energy reduction directly correlates with decreased greenhouse gas emissions associated with battery manufacturing. Studies indicate that optimized doping strategies can reduce production energy requirements by 15-20% compared to conventional methods.
From a lifecycle perspective, dopant-enhanced Na-ion batteries demonstrate extended operational lifespans, reducing the frequency of replacement and associated waste generation. The improved stability provided by strategic doping minimizes capacity degradation, potentially extending battery life by 30-40% under optimal conditions. This longevity significantly reduces the environmental footprint per unit of energy stored over the battery's lifetime.
End-of-life considerations also favor Na-ion technologies. The materials used in Na-ion batteries, including common dopants such as aluminum, magnesium, and titanium, present fewer recycling challenges compared to lithium-ion counterparts. These materials can be recovered through less energy-intensive processes, with some dopants actually facilitating the separation of components during recycling operations.
Water consumption represents another environmental dimension where doped Na-ion technologies demonstrate advantages. The extraction of sodium requires significantly less water than lithium extraction from brine operations, which can consume up to 500,000 gallons of water per ton of lithium produced. This water conservation aspect becomes increasingly important as water scarcity affects more regions globally.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!