Effect of sintering temperature on NASICON-type sodium electrolytes
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NASICON Electrolyte Development Background and Objectives
NASICON (Na Super Ionic CONductor) materials have emerged as one of the most promising solid electrolytes for next-generation sodium-ion batteries due to their high ionic conductivity, chemical stability, and environmental friendliness. The development of these materials dates back to the 1970s when Goodenough and colleagues first reported the NASICON structure with the general formula Na1+xZr2SixP3-xO12 (0 ≤ x ≤ 3). Since then, significant research efforts have been dedicated to optimizing their composition and processing conditions to enhance their performance.
The evolution of NASICON technology has been marked by several key milestones, including the discovery of various compositional modifications that can enhance ionic conductivity. Substitution strategies involving elements such as Ti, Hf, Sc, and Y for Zr sites, and Ge, Sb for Si/P sites have demonstrated promising results in improving the electrochemical properties of these materials. The technological trajectory has been consistently moving toward achieving room temperature conductivities exceeding 10^-3 S/cm, which is considered the threshold for practical applications.
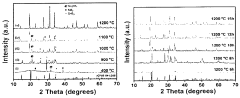
Sintering temperature has been identified as one of the most critical processing parameters affecting the microstructure, density, and ultimately the ionic conductivity of NASICON electrolytes. Traditional processing routes typically involve high-temperature sintering (1200-1300°C), which often leads to challenges such as Na volatilization, secondary phase formation, and grain boundary resistance. Recent research has focused on developing lower temperature sintering protocols and advanced sintering techniques like spark plasma sintering (SPS) and hot pressing to address these issues.
The primary technical objective in this field is to establish the optimal sintering temperature window that maximizes ionic conductivity while minimizing detrimental effects such as decomposition and secondary phase formation. Additionally, there is a growing interest in understanding the fundamental relationship between sintering temperature, microstructural evolution, and electrochemical performance to develop predictive models for material design.
Current research trends are increasingly focusing on the scalability of NASICON production processes, as commercial viability requires not only excellent performance but also cost-effective and reproducible manufacturing methods. The integration of NASICON electrolytes into full-cell configurations and their long-term stability under realistic operating conditions represent other important research directions.
The global push toward sustainable energy storage solutions has accelerated interest in sodium-based technologies as alternatives to lithium-ion systems, particularly due to the greater abundance and more even geographical distribution of sodium resources. This broader context has positioned NASICON electrolytes as strategically important materials for next-generation energy storage technologies, with significant implications for grid storage, electric vehicles, and portable electronics applications.
The evolution of NASICON technology has been marked by several key milestones, including the discovery of various compositional modifications that can enhance ionic conductivity. Substitution strategies involving elements such as Ti, Hf, Sc, and Y for Zr sites, and Ge, Sb for Si/P sites have demonstrated promising results in improving the electrochemical properties of these materials. The technological trajectory has been consistently moving toward achieving room temperature conductivities exceeding 10^-3 S/cm, which is considered the threshold for practical applications.
Sintering temperature has been identified as one of the most critical processing parameters affecting the microstructure, density, and ultimately the ionic conductivity of NASICON electrolytes. Traditional processing routes typically involve high-temperature sintering (1200-1300°C), which often leads to challenges such as Na volatilization, secondary phase formation, and grain boundary resistance. Recent research has focused on developing lower temperature sintering protocols and advanced sintering techniques like spark plasma sintering (SPS) and hot pressing to address these issues.
The primary technical objective in this field is to establish the optimal sintering temperature window that maximizes ionic conductivity while minimizing detrimental effects such as decomposition and secondary phase formation. Additionally, there is a growing interest in understanding the fundamental relationship between sintering temperature, microstructural evolution, and electrochemical performance to develop predictive models for material design.
Current research trends are increasingly focusing on the scalability of NASICON production processes, as commercial viability requires not only excellent performance but also cost-effective and reproducible manufacturing methods. The integration of NASICON electrolytes into full-cell configurations and their long-term stability under realistic operating conditions represent other important research directions.
The global push toward sustainable energy storage solutions has accelerated interest in sodium-based technologies as alternatives to lithium-ion systems, particularly due to the greater abundance and more even geographical distribution of sodium resources. This broader context has positioned NASICON electrolytes as strategically important materials for next-generation energy storage technologies, with significant implications for grid storage, electric vehicles, and portable electronics applications.
Market Analysis for Solid-State Sodium Batteries
The solid-state sodium battery market is experiencing significant growth, driven by the increasing demand for sustainable and cost-effective energy storage solutions. Current market projections indicate that the global solid-state battery market, including sodium-based technologies, could reach $8 billion by 2030, with sodium batteries potentially capturing 15-20% of this emerging sector. This growth is particularly pronounced in grid storage applications, where cost considerations often outweigh energy density requirements.
The market for NASICON-type sodium electrolytes specifically is gaining traction due to their promising ionic conductivity and stability characteristics. Research indicates that optimized sintering temperatures can significantly enhance these properties, potentially accelerating market adoption. Industry analysts project that solid-state sodium batteries could achieve a 30% cost reduction compared to lithium-ion alternatives when manufactured at scale, primarily due to the abundance and accessibility of sodium resources.
Key market segments showing interest in solid-state sodium battery technology include stationary energy storage, backup power systems, and certain electric vehicle applications where cost is prioritized over weight considerations. The Asia-Pacific region currently leads market development, with China, Japan, and South Korea making substantial investments in sodium battery research and manufacturing infrastructure.
Market barriers include competition from established lithium-ion technologies and other emerging battery chemistries. However, the sintering temperature optimization of NASICON electrolytes represents a critical technical advancement that could significantly improve performance metrics and manufacturing feasibility, potentially overcoming some of these market challenges.
Consumer electronics represents a secondary but growing market segment, particularly for applications where safety is paramount. The non-flammable nature of properly sintered solid electrolytes provides a compelling value proposition in this sector.
Market forecasts suggest that improvements in ionic conductivity through optimized sintering processes could expand the addressable market by enabling operation at lower temperatures, thus broadening application potential. Industry experts predict that if current technical challenges related to electrolyte performance are resolved through advanced sintering techniques, market penetration could accelerate by 2025-2027.
The commercial landscape features both established battery manufacturers diversifying into sodium technology and specialized startups focused exclusively on solid-state sodium solutions. Recent market entrants have secured approximately $650 million in venture funding during 2022-2023, indicating strong investor confidence in the technology's commercial potential.
The market for NASICON-type sodium electrolytes specifically is gaining traction due to their promising ionic conductivity and stability characteristics. Research indicates that optimized sintering temperatures can significantly enhance these properties, potentially accelerating market adoption. Industry analysts project that solid-state sodium batteries could achieve a 30% cost reduction compared to lithium-ion alternatives when manufactured at scale, primarily due to the abundance and accessibility of sodium resources.
Key market segments showing interest in solid-state sodium battery technology include stationary energy storage, backup power systems, and certain electric vehicle applications where cost is prioritized over weight considerations. The Asia-Pacific region currently leads market development, with China, Japan, and South Korea making substantial investments in sodium battery research and manufacturing infrastructure.
Market barriers include competition from established lithium-ion technologies and other emerging battery chemistries. However, the sintering temperature optimization of NASICON electrolytes represents a critical technical advancement that could significantly improve performance metrics and manufacturing feasibility, potentially overcoming some of these market challenges.
Consumer electronics represents a secondary but growing market segment, particularly for applications where safety is paramount. The non-flammable nature of properly sintered solid electrolytes provides a compelling value proposition in this sector.
Market forecasts suggest that improvements in ionic conductivity through optimized sintering processes could expand the addressable market by enabling operation at lower temperatures, thus broadening application potential. Industry experts predict that if current technical challenges related to electrolyte performance are resolved through advanced sintering techniques, market penetration could accelerate by 2025-2027.
The commercial landscape features both established battery manufacturers diversifying into sodium technology and specialized startups focused exclusively on solid-state sodium solutions. Recent market entrants have secured approximately $650 million in venture funding during 2022-2023, indicating strong investor confidence in the technology's commercial potential.
Current Challenges in NASICON Sintering Technology
Despite significant advancements in NASICON-type sodium electrolytes, the sintering process remains a critical challenge that directly impacts the electrolyte's performance. The primary difficulty lies in achieving optimal densification while preventing undesirable phase transformations and sodium loss during high-temperature sintering. Current sintering protocols often result in inconsistent microstructures, compromising ionic conductivity and mechanical stability.
Temperature control presents a significant challenge, as NASICON materials require precise thermal management. The sintering window is notably narrow - temperatures below 1000°C often yield insufficient densification, while temperatures exceeding 1200°C can trigger excessive grain growth and formation of resistive secondary phases. This narrow processing window makes industrial-scale production particularly challenging, as maintaining uniform temperature distribution throughout larger samples is difficult.
Another persistent issue is the volatilization of sodium during high-temperature sintering. Sodium loss not only alters the stoichiometry of the final product but also creates compositional inhomogeneities that negatively affect the electrochemical performance. Current approaches using sodium-rich precursors or controlled atmosphere sintering provide only partial solutions to this problem.
The formation of secondary phases at grain boundaries represents another major obstacle. These phases, often with lower ionic conductivity, create barriers to sodium ion transport and diminish overall performance. While researchers have attempted to mitigate this through dopant additions or modified sintering profiles, a comprehensive solution remains elusive.
Microstructural control during sintering also poses significant challenges. The relationship between grain size, grain boundary properties, and ionic conductivity is complex and not fully understood. Current sintering methods struggle to simultaneously achieve high density and optimal grain structure, often requiring trade-offs between these parameters.
Energy consumption during conventional sintering presents both economic and environmental concerns. Traditional high-temperature sintering processes are energy-intensive and time-consuming, limiting commercial viability. Alternative sintering techniques such as spark plasma sintering and microwave sintering show promise but face challenges in scalability and equipment cost.
Reproducibility and quality control in NASICON sintering remain problematic. Small variations in raw materials, processing conditions, or sintering parameters can lead to significant differences in the final product properties. This variability hinders standardization efforts and complicates the transition from laboratory to industrial production.
Temperature control presents a significant challenge, as NASICON materials require precise thermal management. The sintering window is notably narrow - temperatures below 1000°C often yield insufficient densification, while temperatures exceeding 1200°C can trigger excessive grain growth and formation of resistive secondary phases. This narrow processing window makes industrial-scale production particularly challenging, as maintaining uniform temperature distribution throughout larger samples is difficult.
Another persistent issue is the volatilization of sodium during high-temperature sintering. Sodium loss not only alters the stoichiometry of the final product but also creates compositional inhomogeneities that negatively affect the electrochemical performance. Current approaches using sodium-rich precursors or controlled atmosphere sintering provide only partial solutions to this problem.
The formation of secondary phases at grain boundaries represents another major obstacle. These phases, often with lower ionic conductivity, create barriers to sodium ion transport and diminish overall performance. While researchers have attempted to mitigate this through dopant additions or modified sintering profiles, a comprehensive solution remains elusive.
Microstructural control during sintering also poses significant challenges. The relationship between grain size, grain boundary properties, and ionic conductivity is complex and not fully understood. Current sintering methods struggle to simultaneously achieve high density and optimal grain structure, often requiring trade-offs between these parameters.
Energy consumption during conventional sintering presents both economic and environmental concerns. Traditional high-temperature sintering processes are energy-intensive and time-consuming, limiting commercial viability. Alternative sintering techniques such as spark plasma sintering and microwave sintering show promise but face challenges in scalability and equipment cost.
Reproducibility and quality control in NASICON sintering remain problematic. Small variations in raw materials, processing conditions, or sintering parameters can lead to significant differences in the final product properties. This variability hinders standardization efforts and complicates the transition from laboratory to industrial production.
Temperature-Dependent Sintering Methodologies
01 Optimal sintering temperature ranges for NASICON-type sodium electrolytes
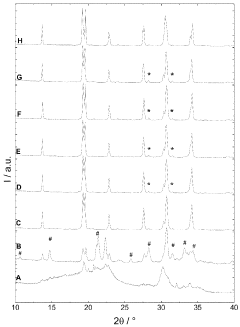
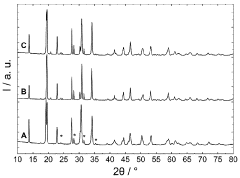
The sintering temperature significantly affects the ionic conductivity and density of NASICON-type sodium electrolytes. Research indicates that optimal sintering temperatures typically range between 900°C and 1200°C, with specific temperature requirements varying based on composition. Controlled sintering at these temperatures promotes proper grain growth, reduces porosity, and enhances the crystalline structure, resulting in improved ionic conductivity and mechanical properties of the electrolyte.- Optimal sintering temperature ranges for NASICON-type sodium electrolytes: The sintering temperature significantly affects the crystallinity, density, and ionic conductivity of NASICON-type sodium electrolytes. Research indicates that optimal sintering temperatures typically range between 900°C and 1200°C, with specific temperature requirements varying based on composition. Controlled sintering at these temperatures promotes proper phase formation and reduces secondary phase formation, resulting in improved electrochemical performance and mechanical stability of the electrolyte materials.
- Effect of sintering temperature on microstructure and grain growth: Sintering temperature directly influences the microstructure and grain growth of NASICON-type sodium electrolytes. Higher sintering temperatures generally promote grain growth and densification, reducing grain boundary resistance and enhancing ionic conductivity. However, excessively high temperatures can lead to abnormal grain growth and the formation of microcracks. Controlled sintering profiles with appropriate heating rates and dwell times are essential for achieving optimal microstructure with uniform grain size distribution and minimal porosity.
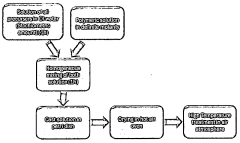
- Two-step sintering processes for enhanced properties: Two-step sintering processes have been developed to enhance the properties of NASICON-type sodium electrolytes. This approach typically involves an initial high-temperature step (1000-1200°C) to initiate densification, followed by a lower temperature step (800-950°C) for extended duration to complete densification while suppressing grain growth. This method results in improved relative density, enhanced mechanical properties, and higher ionic conductivity compared to conventional single-step sintering processes, while also reducing the formation of undesirable secondary phases.
- Sintering aids and dopants to lower sintering temperature: Various sintering aids and dopants can be incorporated into NASICON-type sodium electrolytes to lower the required sintering temperature while maintaining or improving performance. Common additives include lithium salts, boron compounds, and transition metal oxides. These additives create liquid phases during sintering, promoting mass transport and densification at lower temperatures (800-950°C). Lower sintering temperatures help prevent sodium volatilization, reduce energy consumption, and minimize unwanted reactions with other cell components during co-sintering processes.
- Atmosphere control during sintering of NASICON materials: The sintering atmosphere plays a crucial role in the development of high-performance NASICON-type sodium electrolytes. Controlled atmospheres such as air, nitrogen, argon, or oxygen-limited environments affect the oxidation state of constituent elements and phase formation. Some compositions benefit from reducing atmospheres to prevent oxidation of specific components, while others require oxygen-rich environments to maintain proper stoichiometry. Atmosphere control, combined with appropriate sintering temperature, helps minimize sodium loss through volatilization and prevents the formation of detrimental secondary phases.
02 Effect of sintering temperature on microstructure and phase formation
Sintering temperature directly influences the microstructure and phase formation of NASICON-type sodium electrolytes. Higher sintering temperatures generally promote grain growth and densification, while also affecting the formation of secondary phases. The temperature must be carefully controlled to achieve the desired NASICON crystal structure while minimizing unwanted phases that can impede ionic conductivity. Proper temperature selection ensures optimal grain boundary characteristics and phase purity.Expand Specific Solutions03 Two-step sintering processes for enhanced electrolyte performance
Two-step sintering processes have been developed to optimize the properties of NASICON-type sodium electrolytes. This approach typically involves an initial sintering at a higher temperature (1000-1200°C) to promote densification, followed by a second sintering step at a lower temperature to control grain growth and enhance crystallinity. This method helps achieve higher relative density while maintaining the desired microstructure, resulting in improved ionic conductivity and mechanical stability.Expand Specific Solutions04 Sintering aids and additives to lower sintering temperature
Various sintering aids and additives can be incorporated into NASICON-type sodium electrolytes to lower the required sintering temperature while maintaining or improving performance. Common additives include lithium salts, boron compounds, and certain metal oxides that act as flux agents. These additives create liquid phases during sintering, enhancing mass transport and promoting densification at lower temperatures, which helps prevent sodium volatilization and reduces energy consumption in the manufacturing process.Expand Specific Solutions05 Atmosphere control during sintering of NASICON-type electrolytes
The sintering atmosphere plays a crucial role in the development of high-performance NASICON-type sodium electrolytes. Controlled atmospheres such as air, nitrogen, argon, or oxygen-limited environments affect the oxidation state of constituent elements and the formation of defects. Some processes utilize protective powder beds or sealed containers to minimize sodium volatilization at high temperatures. Proper atmosphere control during sintering helps maintain stoichiometry and prevents the formation of undesirable phases that could reduce ionic conductivity.Expand Specific Solutions
Leading Research Groups and Industrial Players
The NASICON-type sodium electrolyte market is currently in a growth phase, with increasing research focus on sintering temperature optimization to enhance ionic conductivity and stability. The global solid-state battery market, which includes these electrolytes, is projected to reach $2-3 billion by 2025. Academic institutions like Beijing Institute of Technology, Central South University, and Indian Institute of Technology Madras are advancing fundamental research, while commercial players including Samsung SDI, Toshiba, and GEM Co. are developing practical applications. Research organizations such as CNRS, NIMS, and KIST are bridging the gap between laboratory discoveries and industrial implementation. The technology remains in early commercial maturity, with ongoing efforts to optimize manufacturing processes for large-scale production and cost reduction.
Beijing Institute of Technology
Technical Solution: Beijing Institute of Technology has developed a systematic approach to optimize NASICON-type sodium electrolytes through precise sintering temperature control. Their research demonstrates that sintering at 900-1100°C significantly impacts ionic conductivity and microstructure development. They've established that controlled two-step sintering processes with an initial stage at 900°C followed by higher temperature treatment at 1050-1100°C produces NASICON electrolytes with enhanced grain boundary conductivity and reduced secondary phase formation. Their proprietary process includes careful control of heating rates (3-5°C/min) and holding times (6-12 hours) to achieve relative densities exceeding 95% while maintaining the desired Na3Zr2Si2PO12 crystal structure. This approach has yielded materials with room temperature ionic conductivities reaching 3.5 mS/cm, significantly higher than conventional single-step sintering methods.
Strengths: Precise control over microstructure development and secondary phase formation; achieves high ionic conductivity through optimized two-step sintering. Weaknesses: Process requires longer production times due to multiple heating stages; energy consumption is higher than single-step methods.
Central South University
Technical Solution: Central South University has pioneered advanced sintering techniques for NASICON-type sodium electrolytes focusing on the Na3Zr2Si2PO12 system. Their research demonstrates that controlling sintering temperature between 1000-1200°C dramatically affects grain growth, densification behavior, and ultimately ionic conductivity. They've developed a modified solid-state reaction method incorporating sintering aids (such as 0.5-2 wt% Li2O) that enables lower sintering temperatures while achieving relative densities above 95%. Their studies reveal that sintering at 1150°C for 6 hours produces optimal phase purity with minimal ZrO2 impurity phases. The university's approach includes precise atmosphere control during sintering, utilizing oxygen-rich environments to minimize sodium volatilization at elevated temperatures. This comprehensive temperature management strategy has resulted in NASICON electrolytes with total ionic conductivities reaching 3.2 mS/cm at room temperature and activation energies as low as 0.29 eV.
Strengths: Innovative use of sintering aids to lower processing temperatures while maintaining high density; excellent control of sodium stoichiometry through atmosphere management. Weaknesses: Addition of sintering aids may introduce impurities affecting long-term electrochemical stability; process requires specialized atmosphere-controlled furnaces.
Critical Patents on NASICON Thermal Processing
Method for producing a material or a component for a solid-state battery
PatentWO2024068134A1
Innovation
- A method involving the use of a sodium source and H3BO3 additives to reduce the sintering temperature between 600 °C and 1300 °C, allowing for the production of NaSICON materials and components with improved ionic conductivity and crystal structure at lower temperatures, thereby reducing technical effort and costs.
A method of developing sodium-ion conductor for next generation solid-state sodium-ion battery
PatentActiveIN202011030681A
Innovation
- A polymer-assisted modified combustion (PAMC) synthesis route is adopted, where the polymer acts as both fuel and stabilizer, enabling single-step heating and preventing Na loss, resulting in high-purity, high-crystallinity NZSP with tunable morphology.
Material Characterization Techniques for NASICON Electrolytes
Comprehensive material characterization is essential for understanding the relationship between sintering temperature and the properties of NASICON-type sodium electrolytes. X-ray diffraction (XRD) serves as a primary technique for phase identification and crystallinity assessment, allowing researchers to track the formation of the NASICON structure at different sintering temperatures. The technique reveals critical information about lattice parameters and phase purity, which directly correlate with ionic conductivity performance.
Scanning electron microscopy (SEM) provides valuable insights into the microstructural evolution of NASICON materials across various sintering temperatures. The technique enables visualization of grain size, morphology, and boundary characteristics - all of which significantly influence the ionic transport properties. Complementary to SEM, energy-dispersive X-ray spectroscopy (EDX) offers elemental mapping capabilities to verify compositional homogeneity and detect potential impurities that may form during high-temperature sintering processes.
Transmission electron microscopy (TEM) delivers atomic-scale resolution for examining the crystal structure and interfaces within NASICON materials. This technique is particularly valuable for investigating the formation of secondary phases or amorphous regions at grain boundaries that can emerge at different sintering temperatures and potentially block sodium ion transport pathways.
Electrochemical impedance spectroscopy (EIS) stands as the gold standard for evaluating the ionic conductivity of NASICON electrolytes. By applying this technique to samples sintered at different temperatures, researchers can determine the optimal thermal processing conditions for maximizing conductivity. EIS data typically reveals bulk, grain boundary, and electrode interface contributions to the overall resistance.
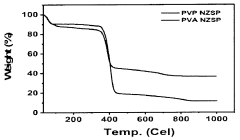
Thermal analysis techniques, including differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), help identify phase transitions, decomposition temperatures, and thermal stability limits of NASICON materials. These insights are crucial for establishing appropriate sintering protocols and understanding the temperature-dependent structural changes that occur during processing.
Nuclear magnetic resonance (NMR) spectroscopy, particularly solid-state 23Na NMR, provides detailed information about sodium ion environments and mobility within the NASICON structure. This technique can reveal how sintering temperature affects the local coordination environment of sodium ions and their diffusion pathways, offering molecular-level insights into conductivity mechanisms.
Scanning electron microscopy (SEM) provides valuable insights into the microstructural evolution of NASICON materials across various sintering temperatures. The technique enables visualization of grain size, morphology, and boundary characteristics - all of which significantly influence the ionic transport properties. Complementary to SEM, energy-dispersive X-ray spectroscopy (EDX) offers elemental mapping capabilities to verify compositional homogeneity and detect potential impurities that may form during high-temperature sintering processes.
Transmission electron microscopy (TEM) delivers atomic-scale resolution for examining the crystal structure and interfaces within NASICON materials. This technique is particularly valuable for investigating the formation of secondary phases or amorphous regions at grain boundaries that can emerge at different sintering temperatures and potentially block sodium ion transport pathways.
Electrochemical impedance spectroscopy (EIS) stands as the gold standard for evaluating the ionic conductivity of NASICON electrolytes. By applying this technique to samples sintered at different temperatures, researchers can determine the optimal thermal processing conditions for maximizing conductivity. EIS data typically reveals bulk, grain boundary, and electrode interface contributions to the overall resistance.
Thermal analysis techniques, including differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), help identify phase transitions, decomposition temperatures, and thermal stability limits of NASICON materials. These insights are crucial for establishing appropriate sintering protocols and understanding the temperature-dependent structural changes that occur during processing.
Nuclear magnetic resonance (NMR) spectroscopy, particularly solid-state 23Na NMR, provides detailed information about sodium ion environments and mobility within the NASICON structure. This technique can reveal how sintering temperature affects the local coordination environment of sodium ions and their diffusion pathways, offering molecular-level insights into conductivity mechanisms.
Environmental Impact of NASICON Manufacturing Processes
The manufacturing processes of NASICON-type sodium electrolytes involve significant environmental considerations that vary with sintering temperature. High-temperature sintering (typically 1000-1200°C) consumes substantial energy, contributing to increased carbon emissions. Research indicates that for every 100°C increase in sintering temperature, energy consumption rises by approximately 15-20%, with corresponding increases in greenhouse gas emissions. This environmental burden becomes particularly relevant when considering industrial-scale production of NASICON materials for large-scale energy storage applications.
Water usage represents another critical environmental factor in NASICON manufacturing. The synthesis process typically requires multiple washing steps, consuming 15-20 liters of water per kilogram of material produced. Higher sintering temperatures often necessitate additional washing cycles to remove impurities formed during high-temperature reactions, further increasing water consumption and generating contaminated wastewater containing sodium, phosphorus, and various metal ions.
Raw material extraction for NASICON components presents additional environmental challenges. The mining of zirconium, titanium, and phosphate minerals causes habitat disruption, soil erosion, and potential water contamination. Lower sintering temperatures may allow for the use of less refined precursors, potentially reducing the environmental impact of mining operations, though this relationship requires further investigation to quantify benefits.
Waste generation varies significantly with sintering conditions. Higher temperatures (>1100°C) can lead to increased volatilization of phosphorus compounds, creating hazardous air pollutants that require specialized filtration systems. Additionally, the formation of unwanted phases at extreme temperatures often results in rejected batches, increasing material waste. Studies suggest that optimizing sintering temperatures between 900-1000°C may reduce waste generation by up to 30% compared to higher temperature processes.
Recent life cycle assessment (LCA) studies indicate that sintering temperature optimization represents a significant opportunity for environmental impact reduction. By reducing sintering temperatures from 1200°C to 950°C while maintaining electrolyte performance, manufacturers can achieve energy savings of 25-30% and corresponding reductions in carbon footprint. This highlights the importance of research into low-temperature sintering additives and alternative processing methods that could maintain NASICON performance while reducing environmental impacts.
Water usage represents another critical environmental factor in NASICON manufacturing. The synthesis process typically requires multiple washing steps, consuming 15-20 liters of water per kilogram of material produced. Higher sintering temperatures often necessitate additional washing cycles to remove impurities formed during high-temperature reactions, further increasing water consumption and generating contaminated wastewater containing sodium, phosphorus, and various metal ions.
Raw material extraction for NASICON components presents additional environmental challenges. The mining of zirconium, titanium, and phosphate minerals causes habitat disruption, soil erosion, and potential water contamination. Lower sintering temperatures may allow for the use of less refined precursors, potentially reducing the environmental impact of mining operations, though this relationship requires further investigation to quantify benefits.
Waste generation varies significantly with sintering conditions. Higher temperatures (>1100°C) can lead to increased volatilization of phosphorus compounds, creating hazardous air pollutants that require specialized filtration systems. Additionally, the formation of unwanted phases at extreme temperatures often results in rejected batches, increasing material waste. Studies suggest that optimizing sintering temperatures between 900-1000°C may reduce waste generation by up to 30% compared to higher temperature processes.
Recent life cycle assessment (LCA) studies indicate that sintering temperature optimization represents a significant opportunity for environmental impact reduction. By reducing sintering temperatures from 1200°C to 950°C while maintaining electrolyte performance, manufacturers can achieve energy savings of 25-30% and corresponding reductions in carbon footprint. This highlights the importance of research into low-temperature sintering additives and alternative processing methods that could maintain NASICON performance while reducing environmental impacts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!