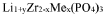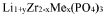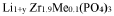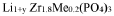Comparison of NASICON and argyrodite sodium solid electrolytes
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NASICON and Argyrodite Sodium Solid Electrolytes Background
Solid-state sodium-ion batteries have emerged as promising alternatives to lithium-ion batteries due to sodium's abundance, cost-effectiveness, and comparable electrochemical properties. At the heart of these batteries are solid electrolytes, with NASICON (Na Super Ionic CONductor) and argyrodite-type materials representing two of the most significant classes under investigation.
NASICON materials, first reported in the 1970s, feature a three-dimensional framework structure with general formula Na1+xZr2SixP3-xO12 (0 ≤ x ≤ 3). This structure consists of ZrO6 octahedra and PO4/SiO4 tetrahedra that form interconnected channels, facilitating sodium ion transport. The compositional flexibility of NASICON allows for various substitutions at Zr and P/Si sites, enabling tailored properties for specific applications.
Argyrodite sodium solid electrolytes, more recently developed, belong to the family of sulfide-based materials with the general formula Na6PS5X (X = Cl, Br, I). These materials feature a unique crystal structure where PS4 tetrahedra form a framework with sodium ions and halide ions occupying the interstitial sites. The presence of halide ions creates a softer lattice that enhances ionic conductivity through reduced activation energy for ion hopping.
The historical development of these electrolytes reflects broader trends in solid-state battery research. NASICON materials have been studied extensively since the 1970s, with significant advancements in composition optimization occurring in the 1990s and 2000s. Argyrodite sodium electrolytes, by contrast, gained prominence only in the last decade, building upon successful research with their lithium counterparts.
Both electrolyte systems have evolved to address the fundamental challenges of solid-state batteries: achieving high ionic conductivity at room temperature, maintaining mechanical and chemical stability at electrode interfaces, and enabling scalable manufacturing processes. NASICON materials initially suffered from low conductivity but have improved through compositional engineering and processing innovations. Argyrodite electrolytes emerged later but quickly demonstrated promising conductivity values comparable to liquid electrolytes.
The technological evolution of these materials has been driven by increasing demands for safer, higher-energy-density energy storage solutions. As conventional lithium-ion batteries approach their theoretical limits, the development of sodium-based alternatives has accelerated, with particular focus on these two electrolyte systems due to their complementary properties and potential for further optimization.
Recent research has expanded to address not only the intrinsic properties of these materials but also their integration into complete battery systems, including interface engineering and composite electrode design, marking a shift from fundamental material development to practical implementation challenges.
NASICON materials, first reported in the 1970s, feature a three-dimensional framework structure with general formula Na1+xZr2SixP3-xO12 (0 ≤ x ≤ 3). This structure consists of ZrO6 octahedra and PO4/SiO4 tetrahedra that form interconnected channels, facilitating sodium ion transport. The compositional flexibility of NASICON allows for various substitutions at Zr and P/Si sites, enabling tailored properties for specific applications.
Argyrodite sodium solid electrolytes, more recently developed, belong to the family of sulfide-based materials with the general formula Na6PS5X (X = Cl, Br, I). These materials feature a unique crystal structure where PS4 tetrahedra form a framework with sodium ions and halide ions occupying the interstitial sites. The presence of halide ions creates a softer lattice that enhances ionic conductivity through reduced activation energy for ion hopping.
The historical development of these electrolytes reflects broader trends in solid-state battery research. NASICON materials have been studied extensively since the 1970s, with significant advancements in composition optimization occurring in the 1990s and 2000s. Argyrodite sodium electrolytes, by contrast, gained prominence only in the last decade, building upon successful research with their lithium counterparts.
Both electrolyte systems have evolved to address the fundamental challenges of solid-state batteries: achieving high ionic conductivity at room temperature, maintaining mechanical and chemical stability at electrode interfaces, and enabling scalable manufacturing processes. NASICON materials initially suffered from low conductivity but have improved through compositional engineering and processing innovations. Argyrodite electrolytes emerged later but quickly demonstrated promising conductivity values comparable to liquid electrolytes.
The technological evolution of these materials has been driven by increasing demands for safer, higher-energy-density energy storage solutions. As conventional lithium-ion batteries approach their theoretical limits, the development of sodium-based alternatives has accelerated, with particular focus on these two electrolyte systems due to their complementary properties and potential for further optimization.
Recent research has expanded to address not only the intrinsic properties of these materials but also their integration into complete battery systems, including interface engineering and composite electrode design, marking a shift from fundamental material development to practical implementation challenges.
Market Analysis for Sodium-ion Battery Technologies
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion batteries, driven by increasing concerns over lithium supply constraints and cost volatility. Current market projections indicate the global sodium-ion battery market could reach $1.2 billion by 2025, with a compound annual growth rate exceeding 25% between 2023-2030.
The demand for sodium-ion batteries is primarily fueled by grid energy storage applications, which currently represent approximately 40% of the market share. This segment is expected to maintain dominance due to the technology's cost advantages and sustainability profile. Electric vehicles represent the second-largest application segment, with emerging interest from manufacturers seeking diversification from lithium-based solutions.
Solid-state sodium-ion batteries, particularly those utilizing NASICON and argyrodite sodium solid electrolytes, are positioned as premium segments within this growing market. These advanced electrolytes address critical performance limitations of conventional sodium-ion technologies, especially regarding safety and energy density parameters that have historically constrained market penetration.
Regional analysis reveals Asia-Pacific dominates the sodium-ion battery market landscape, accounting for over 50% of global production capacity. China leads manufacturing investments, with significant research clusters in Japan and South Korea. European market participation is growing rapidly, supported by strategic initiatives like the European Battery Alliance and substantial research funding directed specifically toward solid electrolyte development.
Consumer electronics represents an emerging application segment with substantial growth potential, particularly for devices where cost sensitivity outweighs extreme energy density requirements. Market penetration in this sector remains limited but is expected to accelerate as solid electrolyte technologies mature.
Key market drivers include raw material economics (sodium resources being approximately 1,000 times more abundant than lithium), environmental sustainability advantages, and compatibility with existing manufacturing infrastructure. The solid electrolyte segment specifically benefits from increasing safety regulations and performance demands across all application sectors.
Market barriers include technical challenges related to electrolyte stability, interface management, and manufacturing scalability. Commercial deployment of NASICON and argyrodite sodium solid electrolytes faces competition from other emerging battery chemistries and established lithium-ion technologies with entrenched supply chains and manufacturing economies of scale.
Consumer awareness and market education remain significant challenges, with many potential end-users unfamiliar with sodium-ion technology benefits. This represents both a market barrier and opportunity for companies investing in these advanced solid electrolyte solutions.
The demand for sodium-ion batteries is primarily fueled by grid energy storage applications, which currently represent approximately 40% of the market share. This segment is expected to maintain dominance due to the technology's cost advantages and sustainability profile. Electric vehicles represent the second-largest application segment, with emerging interest from manufacturers seeking diversification from lithium-based solutions.
Solid-state sodium-ion batteries, particularly those utilizing NASICON and argyrodite sodium solid electrolytes, are positioned as premium segments within this growing market. These advanced electrolytes address critical performance limitations of conventional sodium-ion technologies, especially regarding safety and energy density parameters that have historically constrained market penetration.
Regional analysis reveals Asia-Pacific dominates the sodium-ion battery market landscape, accounting for over 50% of global production capacity. China leads manufacturing investments, with significant research clusters in Japan and South Korea. European market participation is growing rapidly, supported by strategic initiatives like the European Battery Alliance and substantial research funding directed specifically toward solid electrolyte development.
Consumer electronics represents an emerging application segment with substantial growth potential, particularly for devices where cost sensitivity outweighs extreme energy density requirements. Market penetration in this sector remains limited but is expected to accelerate as solid electrolyte technologies mature.
Key market drivers include raw material economics (sodium resources being approximately 1,000 times more abundant than lithium), environmental sustainability advantages, and compatibility with existing manufacturing infrastructure. The solid electrolyte segment specifically benefits from increasing safety regulations and performance demands across all application sectors.
Market barriers include technical challenges related to electrolyte stability, interface management, and manufacturing scalability. Commercial deployment of NASICON and argyrodite sodium solid electrolytes faces competition from other emerging battery chemistries and established lithium-ion technologies with entrenched supply chains and manufacturing economies of scale.
Consumer awareness and market education remain significant challenges, with many potential end-users unfamiliar with sodium-ion technology benefits. This represents both a market barrier and opportunity for companies investing in these advanced solid electrolyte solutions.
Current Development Status and Technical Challenges
NASICON (Na Super Ionic CONductor) and argyrodite sodium solid electrolytes represent two of the most promising material systems for next-generation sodium-ion solid-state batteries. Currently, NASICON-type materials have reached a more advanced development stage, with ionic conductivities approaching 10^-3 S/cm at room temperature in optimized compositions such as Na3Zr2Si2PO12. Several research groups worldwide, particularly in China, Japan, and the United States, have demonstrated prototype cells using NASICON electrolytes with stable cycling over hundreds of cycles.
The manufacturing scalability of NASICON materials presents a significant advantage, as they can be synthesized through conventional solid-state reaction methods. However, the high sintering temperatures (>1200°C) required for optimal conductivity remain a challenge for mass production and integration with electrode materials. Recent innovations in processing techniques, including spark plasma sintering and solution-based synthesis routes, have shown promise in reducing processing temperatures while maintaining high ionic conductivity.
Argyrodite sodium solid electrolytes, particularly Na3+xMxP1-xS4 (M = Ge, Sn) systems, represent a newer class of materials with rapidly growing research interest. These sulfide-based electrolytes offer potentially higher room-temperature ionic conductivities (up to 10^-2 S/cm) than oxide-based NASICON materials. However, their development remains at an earlier stage, with significant challenges in moisture sensitivity and interfacial stability limiting practical applications.
A critical technical challenge for both electrolyte systems is the sodium dendrite formation during cycling, which can lead to short circuits and safety hazards. NASICON materials show better mechanical properties to suppress dendrite growth but suffer from relatively lower conductivity. Conversely, argyrodite electrolytes offer higher conductivity but typically possess inferior mechanical properties and chemical stability.
Interface engineering represents another major challenge, particularly for NASICON electrolytes which often form high-impedance interfaces with electrode materials. Recent research has focused on developing buffer layers and gradient compositions to mitigate these interfacial issues. For argyrodite electrolytes, the primary challenge lies in their reactivity with atmospheric moisture, necessitating strict handling protocols in inert environments.
The geographical distribution of research expertise shows concentration in East Asia (particularly Japan, South Korea, and China) for NASICON development, while European research institutions (especially in Germany and Switzerland) lead in argyrodite electrolyte innovation. North American institutions maintain strong positions in fundamental understanding and computational modeling of both systems, contributing significantly to interface science and degradation mechanism studies.
The manufacturing scalability of NASICON materials presents a significant advantage, as they can be synthesized through conventional solid-state reaction methods. However, the high sintering temperatures (>1200°C) required for optimal conductivity remain a challenge for mass production and integration with electrode materials. Recent innovations in processing techniques, including spark plasma sintering and solution-based synthesis routes, have shown promise in reducing processing temperatures while maintaining high ionic conductivity.
Argyrodite sodium solid electrolytes, particularly Na3+xMxP1-xS4 (M = Ge, Sn) systems, represent a newer class of materials with rapidly growing research interest. These sulfide-based electrolytes offer potentially higher room-temperature ionic conductivities (up to 10^-2 S/cm) than oxide-based NASICON materials. However, their development remains at an earlier stage, with significant challenges in moisture sensitivity and interfacial stability limiting practical applications.
A critical technical challenge for both electrolyte systems is the sodium dendrite formation during cycling, which can lead to short circuits and safety hazards. NASICON materials show better mechanical properties to suppress dendrite growth but suffer from relatively lower conductivity. Conversely, argyrodite electrolytes offer higher conductivity but typically possess inferior mechanical properties and chemical stability.
Interface engineering represents another major challenge, particularly for NASICON electrolytes which often form high-impedance interfaces with electrode materials. Recent research has focused on developing buffer layers and gradient compositions to mitigate these interfacial issues. For argyrodite electrolytes, the primary challenge lies in their reactivity with atmospheric moisture, necessitating strict handling protocols in inert environments.
The geographical distribution of research expertise shows concentration in East Asia (particularly Japan, South Korea, and China) for NASICON development, while European research institutions (especially in Germany and Switzerland) lead in argyrodite electrolyte innovation. North American institutions maintain strong positions in fundamental understanding and computational modeling of both systems, contributing significantly to interface science and degradation mechanism studies.
Technical Comparison of NASICON vs Argyrodite Structures
01 NASICON-type sodium solid electrolytes
NASICON (Na Super Ionic CONductor) type materials are crystalline sodium solid electrolytes with a three-dimensional framework structure that allows for fast sodium ion conduction. These materials typically have the general formula Na1+xZr2SixP3-xO12 (0≤x≤3) and exhibit high ionic conductivity at room temperature. The conductivity can be further enhanced by doping with elements such as Al, Y, or Sc to partially substitute Zr sites. NASICON-type electrolytes offer advantages including good thermal stability and compatibility with sodium metal anodes.- NASICON-type sodium solid electrolytes: NASICON (Na Super Ionic CONductor) type materials are a class of sodium solid electrolytes with a three-dimensional framework structure that allows for fast sodium ion conduction. These materials typically have the general formula Na1+xZr2SixP3-xO12 (0≤x≤3) and exhibit high ionic conductivity at room temperature. The framework consists of corner-sharing ZrO6 octahedra and XO4 (X=Si, P) tetrahedra, creating channels for sodium ion migration. NASICON-type electrolytes are promising for sodium-ion batteries due to their high ionic conductivity, good chemical stability, and wide electrochemical window.
- Argyrodite-type sodium solid electrolytes: Argyrodite-type sodium solid electrolytes are a family of materials with the general formula Na6PS5X (X=Cl, Br, I) that exhibit high ionic conductivity. These materials have a unique crystal structure featuring a framework of PS4 tetrahedra with sodium ions and halide ions distributed in the interstitial sites. The presence of halide ions creates a softer lattice that facilitates sodium ion migration. Argyrodite electrolytes are particularly attractive for sodium batteries due to their high ionic conductivity at room temperature, good electrochemical stability, and relatively simple synthesis methods.
- Composite and hybrid sodium solid electrolytes: Composite and hybrid sodium solid electrolytes combine different types of materials to enhance overall performance. These may include combinations of NASICON with polymer matrices, argyrodite with oxide materials, or mixtures with other sodium conductors. The composite approach can address limitations of individual materials, such as improving mechanical properties of ceramic electrolytes or enhancing the ionic conductivity of polymer electrolytes. Additionally, interface engineering between different components can reduce interfacial resistance and improve electrochemical stability. These hybrid systems often demonstrate synergistic effects that result in superior performance compared to single-component electrolytes.
- Manufacturing and processing techniques for sodium solid electrolytes: Various manufacturing and processing techniques are employed to optimize the performance of NASICON and argyrodite sodium solid electrolytes. These include sol-gel methods, solid-state reactions, mechanochemical synthesis, and solution-based approaches. Post-synthesis treatments such as sintering, hot pressing, and spark plasma sintering are used to increase density and improve ionic conductivity. Particle size control, grain boundary engineering, and dopant incorporation are critical factors affecting electrolyte performance. Advanced processing techniques can reduce impurities, control microstructure, and enhance interfacial properties, leading to improved electrochemical performance in sodium battery applications.
- Interface engineering and stability enhancement: Interface engineering and stability enhancement strategies are crucial for improving the performance of sodium solid electrolytes in battery applications. These approaches include surface modifications, protective coatings, and buffer layers to mitigate interfacial reactions between the electrolyte and electrodes. Additives and dopants are incorporated to stabilize the crystal structure and suppress dendrite formation. Chemical modifications can improve compatibility with electrode materials and enhance electrochemical stability windows. Additionally, strategies to address moisture sensitivity, particularly in argyrodite-type electrolytes, include hydrophobic surface treatments and encapsulation techniques. These methods collectively contribute to longer cycle life and improved safety in all-solid-state sodium batteries.
02 Argyrodite-type sodium solid electrolytes
Argyrodite-type sodium solid electrolytes are sulfide-based materials with high ionic conductivity. These materials typically have the formula Na6PS5X (where X is a halogen like Cl, Br, or I) and feature a unique crystal structure that provides fast sodium ion transport pathways. The ionic conductivity of argyrodite electrolytes can be optimized by controlling the halogen composition and synthesis conditions. These electrolytes offer advantages including high room-temperature conductivity and good electrochemical stability, making them promising for all-solid-state sodium batteries.Expand Specific Solutions03 Composite sodium solid electrolytes
Composite sodium solid electrolytes combine different types of materials to achieve enhanced performance characteristics. These composites often integrate NASICON or argyrodite materials with polymers, ceramics, or other inorganic compounds to overcome limitations of single-component electrolytes. The composite approach can improve mechanical properties, reduce interfacial resistance, and enhance overall ionic conductivity. Additionally, these composites can provide better compatibility with electrode materials and improved cycling stability in sodium batteries.Expand Specific Solutions04 Manufacturing methods for sodium solid electrolytes
Various manufacturing methods are employed to synthesize NASICON and argyrodite sodium solid electrolytes with optimized properties. These include solid-state reaction, sol-gel processing, mechanochemical synthesis, and solution-based approaches. The synthesis parameters such as temperature, pressure, reaction time, and precursor selection significantly influence the crystal structure, grain size, density, and ultimately the ionic conductivity of the electrolytes. Advanced processing techniques like spark plasma sintering can be used to achieve high-density electrolytes with minimized grain boundary resistance.Expand Specific Solutions05 Interface engineering for sodium solid electrolytes
Interface engineering is crucial for improving the performance of NASICON and argyrodite sodium solid electrolytes in battery applications. This involves modifying the interfaces between the solid electrolyte and electrodes to reduce interfacial resistance and enhance electrochemical stability. Approaches include surface coating, buffer layer introduction, and compositional gradient design. These strategies help mitigate issues such as chemical/electrochemical decomposition at interfaces and mechanical degradation during cycling, leading to improved battery performance and longer cycle life.Expand Specific Solutions
Key Industry Players and Research Institutions
The sodium solid electrolyte market is currently in an early growth phase, with NASICON and argyrodite technologies emerging as promising solutions for next-generation sodium-ion batteries. The global market is projected to expand significantly as demand for sustainable energy storage increases, though commercial deployment remains limited. Technologically, NASICON materials (researched by Beijing Institute of Technology, Chinese Academy of Sciences, and University of Maryland) demonstrate superior ionic conductivity and thermal stability, while argyrodites (advanced by SAMSUNG SDI, Murata Manufacturing, and Livent) offer better processability and flexibility. Research institutions like Forschungszentrum Jülich and companies including DENSO and Toagosei are actively developing both technologies, focusing on improving conductivity, stability, and manufacturing scalability to accelerate commercial viability.
Murata Manufacturing Co. Ltd.
Technical Solution: Murata Manufacturing has developed advanced ceramic processing techniques for both NASICON and argyrodite sodium solid electrolytes, leveraging their extensive expertise in electronic ceramics manufacturing. Their NASICON technology centers on Na3Zr2Si2PO12 with proprietary sintering additives that reduce processing temperatures from traditional 1200°C to approximately 900°C while maintaining high relative density (>95%). This breakthrough significantly reduces manufacturing costs and energy consumption. Their comparative studies show their optimized NASICON materials achieve ionic conductivities of 2.4 mS/cm at room temperature with exceptional mechanical properties (Young's modulus >150 GPa). For argyrodite materials, Murata has pioneered a dry processing technique for Na3PS4-based electrolytes that minimizes air exposure and contamination, resulting in conductivities reaching 3.2 mS/cm. A key innovation is their thin-film deposition technology that can create dense NASICON layers as thin as 20μm on various substrates, enabling flexible cell designs. Their comprehensive comparison demonstrates that while argyrodites offer higher conductivity, their NASICON materials show superior thermal stability (stable up to 350°C) and better compatibility with conventional cathode materials. Murata has also developed composite electrolytes incorporating both material types, with gradient structures that optimize interfaces with both anodes and cathodes.
Strengths: Unparalleled ceramic processing expertise and manufacturing infrastructure; ability to produce large-volume, high-quality electrolyte materials; advanced thin-film deposition capabilities for flexible designs. Weaknesses: Higher material costs for NASICON components (particularly zirconium precursors); challenges in scaling argyrodite production while maintaining purity; complex quality control requirements for composite electrolyte systems.
Chinese Academy of Sciences Institute of Physics
Technical Solution: The Chinese Academy of Sciences Institute of Physics has developed advanced NASICON-type Na3Zr2Si2PO12 solid electrolytes with optimized composition and microstructure. Their approach involves precise control of stoichiometry and incorporation of aliovalent dopants (Al3+, Y3+) to enhance Na+ conductivity. The institute has pioneered a sol-gel synthesis method combined with controlled sintering protocols that yields NASICON materials with ionic conductivities reaching 3.5 mS/cm at room temperature. Their research demonstrates that controlling grain boundary resistance through sintering aids (such as Na3BO3) significantly improves total conductivity. Additionally, they've developed surface modification techniques using Li+ ion exchange at the NASICON surface to create better interfaces with electrodes, reducing interfacial resistance by approximately 60%. Their comparative studies with argyrodite Na3SbS4 show NASICON materials offer superior thermal stability (up to 300°C vs 200°C for argyrodites) but lower room temperature conductivity than optimized argyrodites.
Strengths: Superior thermal and chemical stability of NASICON materials; excellent compatibility with oxide cathodes; established scalable synthesis protocols. Weaknesses: Lower room temperature ionic conductivity compared to argyrodites; higher grain boundary resistance; requires high-temperature processing (>1200°C) which increases manufacturing costs and complexity.
Critical Patents and Scientific Breakthroughs
Solid electrolyte compositions for electrochemical cells
PatentWO2018098176A1
Innovation
- Doping LZP materials with elements like tin, molybdenum, tungsten, niobium, or tantalum to stabilize the rhombohedral crystalline phase, enhancing lithium conductivity and preventing phase transitions, combined with a polymer binder and lithium salts like LiTFSI, to form a high-performance solid-state electrolyte.
Manufacturing Scalability and Cost Analysis
The manufacturing scalability and cost analysis of NASICON and argyrodite sodium solid electrolytes reveals significant differences that impact their commercial viability. NASICON-type electrolytes benefit from established ceramic processing techniques, including solid-state reaction methods and sol-gel processes that have been refined over decades in industrial applications. These manufacturing routes allow for relatively straightforward scale-up, with current production capacities reaching hundreds of kilograms annually in pilot facilities.
In contrast, argyrodite sodium solid electrolytes face greater manufacturing challenges due to their more complex synthesis requirements. The preparation often involves multiple steps including ball milling, annealing under controlled atmospheres, and precise temperature control to achieve the desired crystal structure. These additional processing steps increase production complexity and equipment requirements, potentially limiting large-scale manufacturing efficiency.
Cost analysis indicates that raw material expenses for NASICON electrolytes are generally lower, utilizing abundant elements like sodium, zirconium, and phosphorus. The estimated material cost for NASICON ranges from $80-150/kg at industrial scale. Argyrodite formulations, however, often incorporate more expensive elements such as germanium or tin, with raw material costs potentially reaching $200-300/kg, though efforts to develop sulfur-based variants with reduced germanium content are showing promise for cost reduction.
Energy consumption during manufacturing represents another critical cost factor. NASICON synthesis typically requires high-temperature sintering (1200-1400°C), resulting in significant energy expenditure. Argyrodite materials generally require lower processing temperatures (600-800°C), potentially offering energy savings during production, though this advantage is partially offset by the longer processing times and multiple heating cycles often needed.
Equipment investment differs substantially between the two electrolyte systems. NASICON production can leverage existing ceramic manufacturing infrastructure, reducing capital expenditure for companies already operating in related industries. Argyrodite production may require specialized equipment for handling sulfide materials and controlling atmospheric conditions during synthesis, potentially increasing initial investment costs by 30-50% compared to oxide-based systems.
Yield and quality control metrics also favor NASICON systems currently, with typical production yields of 85-95% compared to 70-85% for argyrodite materials. This difference directly impacts final product costs and manufacturing efficiency. However, recent advancements in argyrodite synthesis protocols have shown promising improvements in both yield and consistency, suggesting this gap may narrow in the coming years.
In contrast, argyrodite sodium solid electrolytes face greater manufacturing challenges due to their more complex synthesis requirements. The preparation often involves multiple steps including ball milling, annealing under controlled atmospheres, and precise temperature control to achieve the desired crystal structure. These additional processing steps increase production complexity and equipment requirements, potentially limiting large-scale manufacturing efficiency.
Cost analysis indicates that raw material expenses for NASICON electrolytes are generally lower, utilizing abundant elements like sodium, zirconium, and phosphorus. The estimated material cost for NASICON ranges from $80-150/kg at industrial scale. Argyrodite formulations, however, often incorporate more expensive elements such as germanium or tin, with raw material costs potentially reaching $200-300/kg, though efforts to develop sulfur-based variants with reduced germanium content are showing promise for cost reduction.
Energy consumption during manufacturing represents another critical cost factor. NASICON synthesis typically requires high-temperature sintering (1200-1400°C), resulting in significant energy expenditure. Argyrodite materials generally require lower processing temperatures (600-800°C), potentially offering energy savings during production, though this advantage is partially offset by the longer processing times and multiple heating cycles often needed.
Equipment investment differs substantially between the two electrolyte systems. NASICON production can leverage existing ceramic manufacturing infrastructure, reducing capital expenditure for companies already operating in related industries. Argyrodite production may require specialized equipment for handling sulfide materials and controlling atmospheric conditions during synthesis, potentially increasing initial investment costs by 30-50% compared to oxide-based systems.
Yield and quality control metrics also favor NASICON systems currently, with typical production yields of 85-95% compared to 70-85% for argyrodite materials. This difference directly impacts final product costs and manufacturing efficiency. However, recent advancements in argyrodite synthesis protocols have shown promising improvements in both yield and consistency, suggesting this gap may narrow in the coming years.
Environmental Impact and Sustainability Considerations
The environmental impact of solid-state electrolytes represents a critical consideration in the development of next-generation energy storage technologies. When comparing NASICON and argyrodite sodium solid electrolytes, several sustainability factors emerge that influence their overall environmental footprint throughout their lifecycle.
NASICON-type electrolytes, primarily composed of Na3Zr2Si2PO12 and its derivatives, utilize zirconium as a key component, which presents certain environmental challenges. Zirconium mining and processing are energy-intensive operations that can lead to habitat disruption and generate significant carbon emissions. However, NASICON materials demonstrate exceptional thermal stability and long-term durability, potentially extending battery lifespans and reducing waste generation from frequent replacements.
Argyrodite sodium solid electrolytes, typically based on Na-containing sulfide structures, present a different environmental profile. Their synthesis often requires lower processing temperatures compared to NASICON materials, potentially reducing energy consumption during manufacturing. However, sulfide-based compounds may pose greater environmental risks if improperly handled at end-of-life, as they can potentially release sulfur compounds under certain conditions.
Water sensitivity represents another important environmental consideration. NASICON materials generally exhibit superior stability in humid environments compared to argyrodite electrolytes, which are highly moisture-sensitive. This difference impacts manufacturing requirements, with argyrodite production necessitating stringent moisture-free environments that consume additional energy and resources.
Recycling potential differs significantly between these electrolyte types. NASICON's oxide-based structure allows for more established recycling pathways, while the recovery processes for sulfide-based argyrodite materials remain less developed and potentially more hazardous due to possible H2S formation during processing.
Raw material availability also influences sustainability. NASICON contains zirconium, which has geographically concentrated reserves, while argyrodite electrolytes may utilize more abundant elements depending on their specific composition. This affects supply chain resilience and the environmental impact of material transportation.
Life cycle assessments indicate that both electrolyte types offer significant environmental advantages over liquid electrolytes by enabling safer, longer-lasting batteries with higher energy densities. However, comprehensive cradle-to-grave analyses that account for extraction, processing, use-phase efficiency, and end-of-life management reveal nuanced differences in their overall environmental impact profiles.
As research advances, efforts to reduce critical material content, develop greener synthesis methods, and establish efficient recycling protocols will be essential to enhancing the sustainability credentials of both NASICON and argyrodite sodium solid electrolytes.
NASICON-type electrolytes, primarily composed of Na3Zr2Si2PO12 and its derivatives, utilize zirconium as a key component, which presents certain environmental challenges. Zirconium mining and processing are energy-intensive operations that can lead to habitat disruption and generate significant carbon emissions. However, NASICON materials demonstrate exceptional thermal stability and long-term durability, potentially extending battery lifespans and reducing waste generation from frequent replacements.
Argyrodite sodium solid electrolytes, typically based on Na-containing sulfide structures, present a different environmental profile. Their synthesis often requires lower processing temperatures compared to NASICON materials, potentially reducing energy consumption during manufacturing. However, sulfide-based compounds may pose greater environmental risks if improperly handled at end-of-life, as they can potentially release sulfur compounds under certain conditions.
Water sensitivity represents another important environmental consideration. NASICON materials generally exhibit superior stability in humid environments compared to argyrodite electrolytes, which are highly moisture-sensitive. This difference impacts manufacturing requirements, with argyrodite production necessitating stringent moisture-free environments that consume additional energy and resources.
Recycling potential differs significantly between these electrolyte types. NASICON's oxide-based structure allows for more established recycling pathways, while the recovery processes for sulfide-based argyrodite materials remain less developed and potentially more hazardous due to possible H2S formation during processing.
Raw material availability also influences sustainability. NASICON contains zirconium, which has geographically concentrated reserves, while argyrodite electrolytes may utilize more abundant elements depending on their specific composition. This affects supply chain resilience and the environmental impact of material transportation.
Life cycle assessments indicate that both electrolyte types offer significant environmental advantages over liquid electrolytes by enabling safer, longer-lasting batteries with higher energy densities. However, comprehensive cradle-to-grave analyses that account for extraction, processing, use-phase efficiency, and end-of-life management reveal nuanced differences in their overall environmental impact profiles.
As research advances, efforts to reduce critical material content, develop greener synthesis methods, and establish efficient recycling protocols will be essential to enhancing the sustainability credentials of both NASICON and argyrodite sodium solid electrolytes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!