Processing–microstructure–property relationships in Na conductors
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na Conductor Development Background and Objectives
Sodium ion conductors have emerged as a promising alternative to lithium-based technologies in energy storage systems due to the abundance and low cost of sodium resources. The development of these materials has gained significant momentum over the past decade, driven by increasing concerns about the long-term sustainability of lithium supplies and the growing demand for large-scale energy storage solutions.
The evolution of sodium conductor technology can be traced back to the 1970s with the discovery of Na-β-alumina, which demonstrated the feasibility of sodium ion conduction in solid materials. However, research in this field remained relatively dormant until the early 2000s when renewed interest in alternative energy storage technologies sparked a resurgence in sodium-based research. This revival has been characterized by significant advancements in understanding the fundamental mechanisms governing sodium ion transport in various material systems.
Current research trends are focused on establishing clear correlations between processing methods, resulting microstructures, and the ultimate electrochemical properties of sodium conductors. This processing-microstructure-property relationship is crucial for optimizing material performance and enabling practical applications. Researchers are exploring various synthesis techniques, including solid-state reactions, sol-gel methods, and mechanochemical approaches, to control grain size, porosity, and interfacial properties.
The primary technical objectives in this field include achieving room-temperature sodium ionic conductivity exceeding 10^-3 S/cm, enhancing electrochemical stability against electrode materials, improving mechanical properties to prevent dendrite formation, and developing scalable manufacturing processes suitable for industrial production. These objectives are driven by the need to develop sodium conductors that can compete with or surpass the performance of lithium-based systems in specific applications.
Beyond performance metrics, researchers aim to establish comprehensive models that can predict how specific processing parameters influence microstructural features and how these features, in turn, affect ionic conductivity, mechanical stability, and cycling performance. Such predictive capabilities would significantly accelerate material development and optimization processes.
The ultimate goal of sodium conductor research is to enable next-generation energy storage technologies that are not only high-performing but also sustainable, safe, and economically viable. This includes applications in grid-scale energy storage, where cost considerations often outweigh energy density requirements, as well as in specialized applications where the unique properties of sodium-based systems offer distinct advantages over lithium alternatives.
The evolution of sodium conductor technology can be traced back to the 1970s with the discovery of Na-β-alumina, which demonstrated the feasibility of sodium ion conduction in solid materials. However, research in this field remained relatively dormant until the early 2000s when renewed interest in alternative energy storage technologies sparked a resurgence in sodium-based research. This revival has been characterized by significant advancements in understanding the fundamental mechanisms governing sodium ion transport in various material systems.
Current research trends are focused on establishing clear correlations between processing methods, resulting microstructures, and the ultimate electrochemical properties of sodium conductors. This processing-microstructure-property relationship is crucial for optimizing material performance and enabling practical applications. Researchers are exploring various synthesis techniques, including solid-state reactions, sol-gel methods, and mechanochemical approaches, to control grain size, porosity, and interfacial properties.
The primary technical objectives in this field include achieving room-temperature sodium ionic conductivity exceeding 10^-3 S/cm, enhancing electrochemical stability against electrode materials, improving mechanical properties to prevent dendrite formation, and developing scalable manufacturing processes suitable for industrial production. These objectives are driven by the need to develop sodium conductors that can compete with or surpass the performance of lithium-based systems in specific applications.
Beyond performance metrics, researchers aim to establish comprehensive models that can predict how specific processing parameters influence microstructural features and how these features, in turn, affect ionic conductivity, mechanical stability, and cycling performance. Such predictive capabilities would significantly accelerate material development and optimization processes.
The ultimate goal of sodium conductor research is to enable next-generation energy storage technologies that are not only high-performing but also sustainable, safe, and economically viable. This includes applications in grid-scale energy storage, where cost considerations often outweigh energy density requirements, as well as in specialized applications where the unique properties of sodium-based systems offer distinct advantages over lithium alternatives.
Market Analysis for Na-based Energy Storage Solutions
The sodium-ion battery market is experiencing significant growth, projected to reach $1.2 billion by 2025 with a compound annual growth rate of 23.9% from 2020. This surge is primarily driven by the increasing demand for cost-effective energy storage solutions and concerns about lithium resource limitations. Sodium's abundance—approximately 2.8% of the Earth's crust compared to lithium's 0.006%—translates to substantially lower raw material costs, positioning Na-based technologies as economically competitive alternatives.
The electric vehicle sector represents the largest potential market for Na-based conductors, with forecasts suggesting that sodium-ion batteries could capture up to 18% of the EV battery market by 2030, particularly in applications where energy density requirements are less stringent than premium long-range vehicles. Grid-scale energy storage constitutes another substantial market segment, valued at $4.7 billion in 2020 and expected to grow at 20.1% annually through 2027, where cost considerations often outweigh energy density limitations.
Consumer electronics manufacturers are increasingly exploring sodium-ion technologies for low-cost devices, especially in emerging markets where price sensitivity is high. Market research indicates that 65% of consumer electronics manufacturers are currently investigating sodium-based alternatives to lithium-ion batteries for specific product categories.
Geographically, China leads sodium technology commercialization, with companies like CATL and HiNa Battery Technology making significant investments. The European market shows strong growth potential, supported by EU initiatives promoting sustainable battery technologies, while North America is experiencing accelerated research activity but remains behind in commercial deployment.
Key market challenges include competition from established lithium-ion technologies, which benefit from decades of optimization and manufacturing scale. The performance gap—particularly in energy density where sodium-ion currently achieves 160 Wh/kg compared to lithium-ion's 250-300 Wh/kg—remains a significant barrier to widespread adoption in premium applications.
Customer adoption surveys indicate that 72% of potential industrial users cite performance consistency and cycle life as critical factors in considering sodium-based alternatives, highlighting the importance of microstructure-property relationships in developing commercially viable products. The market increasingly demands sodium conductors with optimized microstructures that can deliver consistent performance across varied operating conditions.
The electric vehicle sector represents the largest potential market for Na-based conductors, with forecasts suggesting that sodium-ion batteries could capture up to 18% of the EV battery market by 2030, particularly in applications where energy density requirements are less stringent than premium long-range vehicles. Grid-scale energy storage constitutes another substantial market segment, valued at $4.7 billion in 2020 and expected to grow at 20.1% annually through 2027, where cost considerations often outweigh energy density limitations.
Consumer electronics manufacturers are increasingly exploring sodium-ion technologies for low-cost devices, especially in emerging markets where price sensitivity is high. Market research indicates that 65% of consumer electronics manufacturers are currently investigating sodium-based alternatives to lithium-ion batteries for specific product categories.
Geographically, China leads sodium technology commercialization, with companies like CATL and HiNa Battery Technology making significant investments. The European market shows strong growth potential, supported by EU initiatives promoting sustainable battery technologies, while North America is experiencing accelerated research activity but remains behind in commercial deployment.
Key market challenges include competition from established lithium-ion technologies, which benefit from decades of optimization and manufacturing scale. The performance gap—particularly in energy density where sodium-ion currently achieves 160 Wh/kg compared to lithium-ion's 250-300 Wh/kg—remains a significant barrier to widespread adoption in premium applications.
Customer adoption surveys indicate that 72% of potential industrial users cite performance consistency and cycle life as critical factors in considering sodium-based alternatives, highlighting the importance of microstructure-property relationships in developing commercially viable products. The market increasingly demands sodium conductors with optimized microstructures that can deliver consistent performance across varied operating conditions.
Current Challenges in Na Conductor Microstructure Engineering
Despite significant advancements in sodium conductor technology, several critical challenges persist in microstructure engineering that impede the widespread commercialization of these materials. The primary challenge lies in achieving precise control over grain boundary properties, which significantly influence ionic conductivity. Current processing techniques struggle to consistently produce optimized grain boundary structures that facilitate rather than hinder Na+ ion transport. The heterogeneous nature of grain boundaries creates localized resistance points that can dramatically reduce overall conductivity performance.
Another significant challenge is the inherent trade-off between mechanical stability and ionic conductivity. Processing conditions that enhance conductivity often compromise mechanical integrity, creating materials that perform well electrochemically but fail under operational stresses. This dichotomy is particularly problematic for solid-state battery applications where both properties are essential for long-term performance and safety.
Microstructural evolution during cycling represents a third major challenge. Sodium conductors frequently experience undesirable microstructural changes during repeated charge-discharge cycles, including grain growth, void formation, and interfacial degradation. These changes progressively deteriorate performance and ultimately lead to premature failure. Current processing methodologies have not adequately addressed this dynamic aspect of microstructure engineering.
The scalability of laboratory-optimized processing techniques presents another formidable obstacle. Many promising microstructural engineering approaches that yield excellent results at small scales encounter significant challenges when translated to industrial production volumes. Issues such as compositional homogeneity, defect control, and reproducibility become increasingly problematic at larger scales.
Environmental sensitivity during processing also poses significant challenges. Many sodium conductors are hygroscopic or reactive with atmospheric components, necessitating stringent environmental controls during fabrication. This sensitivity complicates processing and increases production costs, while potentially introducing microstructural defects that compromise performance.
Finally, there exists a substantial knowledge gap in understanding the complex relationships between processing parameters, resultant microstructures, and functional properties. The multivariable nature of these relationships makes predictive engineering difficult, often resulting in empirical rather than systematic approaches to material optimization. Advanced characterization techniques and computational modeling tools are needed to bridge this gap and enable more rational design of sodium conductor microstructures.
Another significant challenge is the inherent trade-off between mechanical stability and ionic conductivity. Processing conditions that enhance conductivity often compromise mechanical integrity, creating materials that perform well electrochemically but fail under operational stresses. This dichotomy is particularly problematic for solid-state battery applications where both properties are essential for long-term performance and safety.
Microstructural evolution during cycling represents a third major challenge. Sodium conductors frequently experience undesirable microstructural changes during repeated charge-discharge cycles, including grain growth, void formation, and interfacial degradation. These changes progressively deteriorate performance and ultimately lead to premature failure. Current processing methodologies have not adequately addressed this dynamic aspect of microstructure engineering.
The scalability of laboratory-optimized processing techniques presents another formidable obstacle. Many promising microstructural engineering approaches that yield excellent results at small scales encounter significant challenges when translated to industrial production volumes. Issues such as compositional homogeneity, defect control, and reproducibility become increasingly problematic at larger scales.
Environmental sensitivity during processing also poses significant challenges. Many sodium conductors are hygroscopic or reactive with atmospheric components, necessitating stringent environmental controls during fabrication. This sensitivity complicates processing and increases production costs, while potentially introducing microstructural defects that compromise performance.
Finally, there exists a substantial knowledge gap in understanding the complex relationships between processing parameters, resultant microstructures, and functional properties. The multivariable nature of these relationships makes predictive engineering difficult, often resulting in empirical rather than systematic approaches to material optimization. Advanced characterization techniques and computational modeling tools are needed to bridge this gap and enable more rational design of sodium conductor microstructures.
Current Processing Methods for Optimized Na Conductors
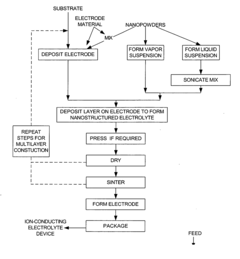
01 Microstructural engineering of Na-ion conductors
Microstructural engineering plays a crucial role in enhancing the performance of sodium ion conductors. By controlling grain size, grain boundary characteristics, and crystallographic orientation, the ionic conductivity can be significantly improved. Techniques such as controlled sintering, doping, and composite formation can create optimized microstructures that facilitate faster Na-ion transport while maintaining mechanical stability. These approaches help reduce interfacial resistance and create more efficient ion transport pathways.- Microstructural engineering for enhanced Na-ion conductivity: Microstructural engineering plays a crucial role in enhancing sodium ion conductivity in solid electrolytes. By controlling grain size, grain boundary density, and crystallographic orientation, the ionic pathways can be optimized. Techniques such as controlled sintering, doping, and composite formation can create favorable microstructures that reduce impedance at interfaces and improve overall conductivity. These approaches help overcome the inherent limitations of sodium ion transport in solid materials.
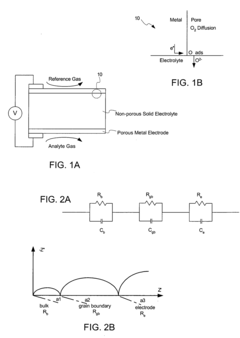
- Grain boundary effects on Na-ion transport: Grain boundaries significantly influence sodium ion transport in solid-state conductors. The composition, structure, and distribution of grain boundaries can either facilitate or impede ion migration. Research shows that certain grain boundary configurations create fast ion conduction pathways, while others act as barriers. Controlling grain boundary chemistry through dopants and processing conditions can minimize resistive effects and enhance overall ionic conductivity in polycrystalline sodium conductors.
- Defect chemistry and Na-ion mobility relationship: Defect chemistry fundamentally determines sodium ion mobility in conductor materials. Point defects, such as vacancies and interstitials, create pathways for ion migration. The concentration, distribution, and mobility of these defects directly correlate with ionic conductivity. By intentionally introducing specific defects through doping, thermal treatments, or mechanical processing, the sodium ion transport properties can be significantly enhanced. Understanding this relationship enables the design of materials with optimized defect structures for superior ionic conductivity.
- Composite structures for improved Na conductivity: Composite structures combining different materials can significantly enhance sodium ion conductivity. By creating heterogeneous interfaces between ceramic, polymer, or glass components, new ion transport pathways emerge. These composites often exhibit conductivity values exceeding those of their individual constituents. The synergistic effects arise from space charge regions, strain effects at interfaces, and the formation of highly conductive interphases. Careful selection of component materials and precise control of their spatial arrangement are essential for optimizing these composite sodium conductors.
- Advanced characterization techniques for Na-ion conductors: Advanced characterization techniques are essential for understanding the microstructure-property relationships in sodium ion conductors. Methods such as impedance spectroscopy, electron microscopy, X-ray tomography, and nuclear magnetic resonance provide critical insights into structural features at multiple length scales. These techniques reveal ion migration pathways, local chemical environments, and structural defects that influence conductivity. Computational modeling combined with experimental characterization creates a powerful approach for correlating microstructural features with macroscopic transport properties in sodium conductor materials.
02 Grain boundary effects on Na-ion conductivity
Grain boundaries significantly influence the overall conductivity of sodium ion conductors. The composition, structure, and distribution of grain boundaries can either enhance or impede Na-ion transport. Research shows that certain grain boundary engineering techniques can reduce resistivity at interfaces, while maintaining mechanical integrity. Controlling grain boundary phases and minimizing impurities at these interfaces are essential strategies for optimizing the performance of solid-state sodium conductors for battery applications.Expand Specific Solutions03 Composite Na-ion conductors with enhanced properties
Composite sodium ion conductors combine different materials to achieve superior performance characteristics. By incorporating secondary phases, such as ceramics, polymers, or nanoparticles, into the primary conductor matrix, both ionic conductivity and mechanical properties can be enhanced. These composites often exhibit synergistic effects where the interface between different materials creates fast ion conduction pathways. The microstructural design of these composites is critical for optimizing the balance between conductivity, stability, and mechanical strength.Expand Specific Solutions04 Defect chemistry and doping strategies
Defect engineering through strategic doping is a powerful approach to enhance sodium ion conductivity. Introducing specific dopants can create beneficial defects in the crystal structure that facilitate Na-ion migration. The type, concentration, and distribution of these defects directly influence the material's transport properties. Understanding the relationship between defect chemistry and microstructure allows for the design of materials with optimized vacancy concentrations and reduced activation energies for ion migration, resulting in superior conductivity performance.Expand Specific Solutions05 Processing-microstructure-property correlations
The processing methods used to synthesize sodium ion conductors have profound effects on their microstructure and resulting properties. Parameters such as sintering temperature, pressure, atmosphere, and cooling rates directly influence grain growth, densification, and phase formation. Advanced characterization techniques help establish correlations between processing conditions, microstructural features, and ionic conductivity. This understanding enables the development of optimized manufacturing protocols that consistently produce materials with desired microstructural characteristics and enhanced sodium ion transport properties.Expand Specific Solutions
Leading Research Groups and Industrial Players
The sodium conductor technology landscape is currently in a growth phase, characterized by increasing research intensity and emerging commercial applications. The market for Na conductors is expanding due to their potential in energy storage, particularly as alternatives to lithium-ion technologies. While the technology remains in mid-maturity stages, significant advancements are being driven by diverse players across academia and industry. Research institutions like MIT, University of Chicago, and Indian Institute of Science are establishing fundamental processing-microstructure-property relationships, while companies including Samsung Electronics, IBM, and TSMC are developing practical applications. GlobalFoundries and Intel are leveraging their semiconductor expertise to enhance manufacturing processes for these materials, creating a competitive ecosystem that spans basic research to commercial implementation.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed advanced sodium-ion battery technology focusing on optimized microstructure-property relationships in Na conductors. Their approach involves engineering NASICON-type (Na Super Ionic CONductor) materials with controlled grain boundaries and crystalline structures to enhance ionic conductivity. Samsung's research has achieved solid-state Na conductors with conductivities exceeding 1 mS/cm at room temperature through precise control of synthesis parameters including temperature profiles, pressure conditions, and dopant concentrations. They've implemented innovative processing techniques such as cold sintering and spark plasma sintering to create dense ceramic electrolytes with minimized interfacial resistance. Samsung has also developed composite electrolytes combining ceramic Na conductors with polymers to improve mechanical properties while maintaining high ionic conductivity.
Strengths: Extensive manufacturing infrastructure allows for scalable production; strong integration capabilities with existing battery technologies; comprehensive materials characterization facilities. Weaknesses: Primary focus remains on lithium-ion technology, with sodium research as secondary; challenges in achieving conductivity values comparable to liquid electrolytes.
Intel Corp.
Technical Solution: Intel has developed specialized materials processing techniques for sodium-ion conductors targeting next-generation computing applications. Their approach focuses on thin-film deposition of Na-based solid electrolytes using atomic layer deposition (ALD) and physical vapor deposition (PVD) methods to create precisely controlled microstructures. Intel's research has demonstrated that nanoscale engineering of interfaces between Na conductors and electrodes significantly reduces interfacial resistance, enabling faster ion transport. Their proprietary processing methods create amorphous-crystalline composite structures with enhanced stability against environmental degradation. Intel has particularly focused on Na3Zr2Si2PO12 and related compositions, developing methods to control grain size distribution and crystallographic orientation to optimize conduction pathways. Their techniques allow for integration of Na conductors into semiconductor manufacturing processes.
Strengths: Exceptional thin-film deposition capabilities; advanced characterization techniques for nanoscale analysis; expertise in integrating novel materials into existing manufacturing processes. Weaknesses: Limited experience with bulk sodium conductor materials; research primarily focused on specialized computing applications rather than energy storage.
Key Microstructural Factors Affecting Na Ion Conductivity
Products comprising nano-precision engineered electronic components
PatentInactiveUS20040218345A1
Innovation
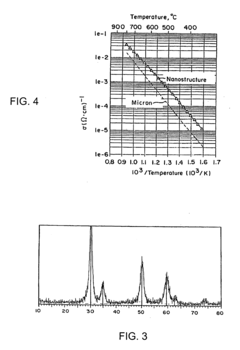
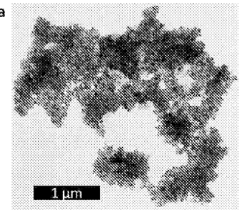
- The use of nanoscale powders to create nanostructured solid electrolytes with grain sizes less than 100 nanometers, enhancing ion conductivity and triple point concentrations, thereby reducing impedance and improving device performance at lower temperatures.
Low resistance nanostructured electrical conductor
PatentActiveIN202147055567A
Innovation
- A nanostructured electrical conductor is created by dispersing metal nanoparticles with diameters between 0.3 to 2.5 nanometers in a second set of metal nanoparticles, forming a low-resistance conductor with an encapsulation layer to protect against environmental factors, achieving resistivity three decades lower than conventional metals and maintaining low resistance up to 1000 K.
Sustainability and Resource Considerations
The sustainability aspects of sodium conductors represent a significant advantage over lithium-based technologies. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, making it a substantially more sustainable resource for large-scale energy storage applications. This abundance translates directly to reduced extraction impacts and more stable supply chains, which are critical considerations as global demand for energy storage continues to exponentially increase.
Environmental impact assessments of sodium conductor manufacturing processes reveal considerably lower carbon footprints compared to lithium alternatives. The extraction of sodium compounds typically requires less energy and produces fewer harmful byproducts. Additionally, the geographical distribution of sodium resources is more equitable globally, reducing geopolitical tensions associated with resource concentration that currently plague lithium supply chains.
Processing techniques for sodium conductors can be further optimized for sustainability. Recent research indicates that water-based processing methods can replace toxic organic solvents traditionally used in electrolyte preparation, significantly reducing environmental hazards and processing costs. These green chemistry approaches align with circular economy principles and increasingly stringent environmental regulations worldwide.
The relationship between microstructure and recyclability presents another important sustainability dimension. Sodium conductors with simpler, more homogeneous microstructures generally facilitate more efficient end-of-life recovery and recycling. Studies demonstrate that up to 90% of sodium compounds can be recovered from spent conductors using optimized hydrometallurgical processes, compared to only 50-70% recovery rates for complex lithium systems.
Resource efficiency in sodium conductor manufacturing can be enhanced through precise microstructural control. By engineering grain boundaries and interfaces at the nanoscale, researchers have achieved higher ionic conductivity while using less raw material. This microstructure-property optimization reduces resource intensity while maintaining or improving performance metrics.
Life cycle analyses indicate that sodium conductors with optimized microstructures can achieve energy payback periods approximately 30% shorter than comparable lithium technologies. This advantage stems from both the reduced energy requirements during material extraction and the lower processing temperatures needed to achieve desired microstructural characteristics in many sodium conductor systems.
Future research directions should focus on developing processing techniques that simultaneously optimize microstructure for performance and minimize environmental impact. Particular attention should be given to water-based synthesis routes, low-temperature processing methods, and designing microstructures specifically engineered for eventual recyclability without compromising electrochemical performance.
Environmental impact assessments of sodium conductor manufacturing processes reveal considerably lower carbon footprints compared to lithium alternatives. The extraction of sodium compounds typically requires less energy and produces fewer harmful byproducts. Additionally, the geographical distribution of sodium resources is more equitable globally, reducing geopolitical tensions associated with resource concentration that currently plague lithium supply chains.
Processing techniques for sodium conductors can be further optimized for sustainability. Recent research indicates that water-based processing methods can replace toxic organic solvents traditionally used in electrolyte preparation, significantly reducing environmental hazards and processing costs. These green chemistry approaches align with circular economy principles and increasingly stringent environmental regulations worldwide.
The relationship between microstructure and recyclability presents another important sustainability dimension. Sodium conductors with simpler, more homogeneous microstructures generally facilitate more efficient end-of-life recovery and recycling. Studies demonstrate that up to 90% of sodium compounds can be recovered from spent conductors using optimized hydrometallurgical processes, compared to only 50-70% recovery rates for complex lithium systems.
Resource efficiency in sodium conductor manufacturing can be enhanced through precise microstructural control. By engineering grain boundaries and interfaces at the nanoscale, researchers have achieved higher ionic conductivity while using less raw material. This microstructure-property optimization reduces resource intensity while maintaining or improving performance metrics.
Life cycle analyses indicate that sodium conductors with optimized microstructures can achieve energy payback periods approximately 30% shorter than comparable lithium technologies. This advantage stems from both the reduced energy requirements during material extraction and the lower processing temperatures needed to achieve desired microstructural characteristics in many sodium conductor systems.
Future research directions should focus on developing processing techniques that simultaneously optimize microstructure for performance and minimize environmental impact. Particular attention should be given to water-based synthesis routes, low-temperature processing methods, and designing microstructures specifically engineered for eventual recyclability without compromising electrochemical performance.
Performance Benchmarking Against Li-based Technologies
When comparing sodium-based conductors with lithium-based technologies, several key performance metrics must be considered. Sodium-ion conductors currently demonstrate ionic conductivities ranging from 10^-4 to 10^-3 S/cm at room temperature, which remains approximately one order of magnitude lower than state-of-the-art lithium solid electrolytes that can reach 10^-2 S/cm. This conductivity gap represents one of the primary challenges for sodium technology adoption.
Electrochemical stability windows also differ significantly between these technologies. While advanced lithium solid electrolytes can maintain stability up to 5V vs. Li/Li+, sodium conductors typically exhibit narrower windows of 3-4V vs. Na/Na+, limiting the potential energy density of resulting devices. This constraint affects the selection of compatible cathode materials and overall system design.
Mechanical properties comparison reveals that sodium conductors often possess lower hardness and elastic moduli compared to their lithium counterparts. This characteristic presents both advantages and disadvantages - improved processability and reduced interfacial resistance, but potentially compromised long-term structural integrity during cycling.
Thermal stability assessments indicate that sodium-based systems generally demonstrate superior performance at elevated temperatures compared to lithium technologies. Many Na conductors maintain structural integrity and conductivity at temperatures exceeding 150°C, whereas some lithium systems experience phase transitions or decomposition at lower temperatures.
Cost analysis strongly favors sodium-based technologies, with raw material costs approximately 30-50% lower than equivalent lithium systems. The greater natural abundance of sodium (2.6% of Earth's crust versus 0.002% for lithium) ensures long-term supply chain stability and reduced geopolitical dependencies.
Environmental impact assessments reveal sodium technologies generate approximately 20-30% lower carbon footprint during production compared to lithium alternatives. This advantage stems from less energy-intensive extraction processes and reduced requirements for harsh chemical treatments during material synthesis and processing.
Cycling performance metrics currently favor lithium technologies, with state-of-the-art lithium cells demonstrating 2000+ cycles at 80% capacity retention, while sodium systems typically achieve 500-1000 cycles before reaching similar degradation thresholds. This performance gap continues to narrow as microstructural engineering of sodium conductors advances.
Electrochemical stability windows also differ significantly between these technologies. While advanced lithium solid electrolytes can maintain stability up to 5V vs. Li/Li+, sodium conductors typically exhibit narrower windows of 3-4V vs. Na/Na+, limiting the potential energy density of resulting devices. This constraint affects the selection of compatible cathode materials and overall system design.
Mechanical properties comparison reveals that sodium conductors often possess lower hardness and elastic moduli compared to their lithium counterparts. This characteristic presents both advantages and disadvantages - improved processability and reduced interfacial resistance, but potentially compromised long-term structural integrity during cycling.
Thermal stability assessments indicate that sodium-based systems generally demonstrate superior performance at elevated temperatures compared to lithium technologies. Many Na conductors maintain structural integrity and conductivity at temperatures exceeding 150°C, whereas some lithium systems experience phase transitions or decomposition at lower temperatures.
Cost analysis strongly favors sodium-based technologies, with raw material costs approximately 30-50% lower than equivalent lithium systems. The greater natural abundance of sodium (2.6% of Earth's crust versus 0.002% for lithium) ensures long-term supply chain stability and reduced geopolitical dependencies.
Environmental impact assessments reveal sodium technologies generate approximately 20-30% lower carbon footprint during production compared to lithium alternatives. This advantage stems from less energy-intensive extraction processes and reduced requirements for harsh chemical treatments during material synthesis and processing.
Cycling performance metrics currently favor lithium technologies, with state-of-the-art lithium cells demonstrating 2000+ cycles at 80% capacity retention, while sodium systems typically achieve 500-1000 cycles before reaching similar degradation thresholds. This performance gap continues to narrow as microstructural engineering of sodium conductors advances.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!