Microstructure evolution during sintering of Na3PS4
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na3PS4 Sintering Background and Objectives
Sodium-ion solid-state batteries have emerged as promising alternatives to lithium-ion batteries due to the abundance and low cost of sodium resources. Among various solid electrolytes, Na3PS4 has attracted significant attention for its high ionic conductivity and potential application in all-solid-state sodium batteries. The sintering process of Na3PS4 plays a crucial role in determining the microstructural characteristics and, consequently, the electrochemical performance of the resulting solid electrolyte.
The evolution of Na3PS4 as a solid electrolyte material can be traced back to the early 2010s when researchers began exploring sodium-based alternatives to lithium solid electrolytes. Initial studies focused primarily on the cubic phase of Na3PS4, which demonstrated ionic conductivities in the range of 10^-4 S/cm. Subsequent research revealed that the tetragonal phase, while thermodynamically more stable, exhibited lower ionic conductivity, highlighting the importance of phase control during synthesis and sintering.
The sintering process of Na3PS4 involves complex physicochemical transformations that significantly impact grain growth, densification, and interfacial properties. Traditional sintering approaches often resulted in suboptimal microstructures characterized by high porosity and poor grain connectivity, limiting ionic transport. Recent technological advancements have introduced novel sintering techniques, including spark plasma sintering, cold sintering, and pressure-assisted sintering, which have shown promise in enhancing the microstructural properties of Na3PS4.
The primary technical objectives in Na3PS4 sintering research include achieving higher relative densities (>95%), optimizing grain size distribution for enhanced ionic conductivity, minimizing secondary phase formation, and improving interfacial stability with electrode materials. Additionally, understanding the correlation between sintering parameters (temperature, pressure, atmosphere, duration) and the resulting microstructural features represents a critical research goal.
Current trends in Na3PS4 sintering technology are moving toward lower-temperature processes to prevent sulfur volatilization and phase decomposition, which commonly occur at elevated temperatures. Furthermore, there is growing interest in developing scalable sintering methodologies that can be integrated into industrial manufacturing processes for solid-state batteries.
The microstructural evolution during Na3PS4 sintering encompasses several interconnected phenomena, including particle rearrangement, neck formation between particles, grain boundary migration, and pore elimination. These processes collectively determine the final microstructure and, by extension, the electrochemical performance of the solid electrolyte. Understanding and controlling these phenomena represent the cornerstone of advanced Na3PS4-based solid electrolyte development.
The evolution of Na3PS4 as a solid electrolyte material can be traced back to the early 2010s when researchers began exploring sodium-based alternatives to lithium solid electrolytes. Initial studies focused primarily on the cubic phase of Na3PS4, which demonstrated ionic conductivities in the range of 10^-4 S/cm. Subsequent research revealed that the tetragonal phase, while thermodynamically more stable, exhibited lower ionic conductivity, highlighting the importance of phase control during synthesis and sintering.
The sintering process of Na3PS4 involves complex physicochemical transformations that significantly impact grain growth, densification, and interfacial properties. Traditional sintering approaches often resulted in suboptimal microstructures characterized by high porosity and poor grain connectivity, limiting ionic transport. Recent technological advancements have introduced novel sintering techniques, including spark plasma sintering, cold sintering, and pressure-assisted sintering, which have shown promise in enhancing the microstructural properties of Na3PS4.
The primary technical objectives in Na3PS4 sintering research include achieving higher relative densities (>95%), optimizing grain size distribution for enhanced ionic conductivity, minimizing secondary phase formation, and improving interfacial stability with electrode materials. Additionally, understanding the correlation between sintering parameters (temperature, pressure, atmosphere, duration) and the resulting microstructural features represents a critical research goal.
Current trends in Na3PS4 sintering technology are moving toward lower-temperature processes to prevent sulfur volatilization and phase decomposition, which commonly occur at elevated temperatures. Furthermore, there is growing interest in developing scalable sintering methodologies that can be integrated into industrial manufacturing processes for solid-state batteries.
The microstructural evolution during Na3PS4 sintering encompasses several interconnected phenomena, including particle rearrangement, neck formation between particles, grain boundary migration, and pore elimination. These processes collectively determine the final microstructure and, by extension, the electrochemical performance of the solid electrolyte. Understanding and controlling these phenomena represent the cornerstone of advanced Na3PS4-based solid electrolyte development.
Market Analysis for Solid-State Electrolytes
The global solid-state electrolyte market is experiencing unprecedented growth, driven primarily by the increasing demand for safer and higher energy density batteries. Current market valuations place the solid-state battery market at approximately $500 million in 2023, with projections indicating a compound annual growth rate (CAGR) of 34.2% through 2030, potentially reaching a market value of $3.4 billion. Within this broader market, sulfide-based solid electrolytes like Na3PS4 represent a significant and rapidly expanding segment due to their superior ionic conductivity compared to oxide-based alternatives.
The demand for Na3PS4 and similar sodium-based solid electrolytes is being fueled by several market factors. First, the electric vehicle (EV) industry's explosive growth requires battery technologies that offer improved safety profiles and energy density. Solid-state batteries using materials like Na3PS4 can potentially deliver 2-3 times the energy density of conventional lithium-ion batteries while eliminating flammable liquid electrolytes.
Second, the strategic importance of sodium as a more abundant and geographically distributed resource compared to lithium is driving interest in Na-ion battery technologies. Countries seeking to reduce dependence on lithium supply chains are investing heavily in sodium-based alternatives, creating a favorable market environment for Na3PS4 development.
The consumer electronics sector represents another significant market driver, with manufacturers seeking batteries that offer faster charging capabilities and longer lifespans. Na3PS4-based solid-state batteries could potentially deliver charging times reduced by 50% compared to conventional technologies, addressing a key consumer pain point.
Market segmentation analysis reveals that Asia-Pacific currently dominates the solid-state electrolyte market, accounting for approximately 45% of global demand, with Japan and South Korea leading in patents related to Na3PS4 sintering processes. North America and Europe follow with growing research investments in this technology.
Key market challenges include manufacturing scalability and cost considerations. Current production methods for high-quality Na3PS4 electrolytes with optimized microstructures remain expensive, with material costs estimated at 5-8 times those of conventional liquid electrolytes. However, industry analysts project that economies of scale and manufacturing innovations could reduce this cost premium to 2-3 times by 2028.
The regulatory landscape is increasingly favorable for solid-state battery technologies, with several countries implementing safety standards that indirectly benefit solid electrolytes. This regulatory push, combined with substantial government funding initiatives for advanced battery research, is expected to accelerate market adoption of Na3PS4 and similar materials over the next decade.
The demand for Na3PS4 and similar sodium-based solid electrolytes is being fueled by several market factors. First, the electric vehicle (EV) industry's explosive growth requires battery technologies that offer improved safety profiles and energy density. Solid-state batteries using materials like Na3PS4 can potentially deliver 2-3 times the energy density of conventional lithium-ion batteries while eliminating flammable liquid electrolytes.
Second, the strategic importance of sodium as a more abundant and geographically distributed resource compared to lithium is driving interest in Na-ion battery technologies. Countries seeking to reduce dependence on lithium supply chains are investing heavily in sodium-based alternatives, creating a favorable market environment for Na3PS4 development.
The consumer electronics sector represents another significant market driver, with manufacturers seeking batteries that offer faster charging capabilities and longer lifespans. Na3PS4-based solid-state batteries could potentially deliver charging times reduced by 50% compared to conventional technologies, addressing a key consumer pain point.
Market segmentation analysis reveals that Asia-Pacific currently dominates the solid-state electrolyte market, accounting for approximately 45% of global demand, with Japan and South Korea leading in patents related to Na3PS4 sintering processes. North America and Europe follow with growing research investments in this technology.
Key market challenges include manufacturing scalability and cost considerations. Current production methods for high-quality Na3PS4 electrolytes with optimized microstructures remain expensive, with material costs estimated at 5-8 times those of conventional liquid electrolytes. However, industry analysts project that economies of scale and manufacturing innovations could reduce this cost premium to 2-3 times by 2028.
The regulatory landscape is increasingly favorable for solid-state battery technologies, with several countries implementing safety standards that indirectly benefit solid electrolytes. This regulatory push, combined with substantial government funding initiatives for advanced battery research, is expected to accelerate market adoption of Na3PS4 and similar materials over the next decade.
Current Challenges in Na3PS4 Microstructure Control
Despite significant advancements in Na3PS4 solid electrolyte development, controlling its microstructure during sintering remains a formidable challenge. The primary obstacle lies in the delicate balance between achieving high ionic conductivity and maintaining mechanical integrity. Current sintering processes often result in non-uniform grain growth, leading to inconsistent performance across the electrolyte material. This heterogeneity creates preferential pathways for sodium dendrite formation during battery cycling, ultimately compromising long-term stability and safety.
Temperature management presents another critical challenge. Na3PS4 exhibits polymorphic behavior with phase transitions occurring at specific temperature thresholds. The α-phase (high-temperature) and β-phase (room-temperature) possess different conductivity properties, with the α-phase demonstrating superior ionic transport. However, stabilizing the high-conductivity phase during cooling remains problematic, as rapid cooling can introduce microcracks while slow cooling may allow undesirable phase transformations.
Porosity control represents a significant hurdle in Na3PS4 processing. Conventional sintering approaches struggle to eliminate closed pores without inducing excessive grain growth or decomposition. These residual pores act as stress concentrators, reducing mechanical strength and creating potential failure points during battery operation. Additionally, the interface between pores and the solid electrolyte matrix often harbors higher resistivity, impeding overall ionic conductivity.
The high sensitivity of Na3PS4 to moisture and oxygen further complicates microstructure optimization. Even trace amounts of contamination during sintering can trigger side reactions, forming insulating layers at grain boundaries or promoting decomposition into less conductive phases. This necessitates extremely controlled processing environments, which presents significant manufacturing challenges for large-scale production.
Grain boundary engineering remains underdeveloped for Na3PS4 systems. Current sintering protocols provide limited control over grain boundary composition and structure, which significantly influence overall ionic conductivity. The presence of impurities or secondary phases at these boundaries can create resistive interfaces that dominate the material's performance limitations.
Scalability of microstructure control techniques presents perhaps the most pressing challenge for commercial implementation. Laboratory-scale methods that achieve desirable microstructures often rely on precise conditions difficult to maintain in industrial settings. The translation of fundamental understanding to manufacturing processes requires bridging significant gaps in process control, particularly for maintaining consistent microstructural features across large-format solid-state batteries.
Temperature management presents another critical challenge. Na3PS4 exhibits polymorphic behavior with phase transitions occurring at specific temperature thresholds. The α-phase (high-temperature) and β-phase (room-temperature) possess different conductivity properties, with the α-phase demonstrating superior ionic transport. However, stabilizing the high-conductivity phase during cooling remains problematic, as rapid cooling can introduce microcracks while slow cooling may allow undesirable phase transformations.
Porosity control represents a significant hurdle in Na3PS4 processing. Conventional sintering approaches struggle to eliminate closed pores without inducing excessive grain growth or decomposition. These residual pores act as stress concentrators, reducing mechanical strength and creating potential failure points during battery operation. Additionally, the interface between pores and the solid electrolyte matrix often harbors higher resistivity, impeding overall ionic conductivity.
The high sensitivity of Na3PS4 to moisture and oxygen further complicates microstructure optimization. Even trace amounts of contamination during sintering can trigger side reactions, forming insulating layers at grain boundaries or promoting decomposition into less conductive phases. This necessitates extremely controlled processing environments, which presents significant manufacturing challenges for large-scale production.
Grain boundary engineering remains underdeveloped for Na3PS4 systems. Current sintering protocols provide limited control over grain boundary composition and structure, which significantly influence overall ionic conductivity. The presence of impurities or secondary phases at these boundaries can create resistive interfaces that dominate the material's performance limitations.
Scalability of microstructure control techniques presents perhaps the most pressing challenge for commercial implementation. Laboratory-scale methods that achieve desirable microstructures often rely on precise conditions difficult to maintain in industrial settings. The translation of fundamental understanding to manufacturing processes requires bridging significant gaps in process control, particularly for maintaining consistent microstructural features across large-format solid-state batteries.
State-of-the-Art Sintering Techniques for Na3PS4
01 Synthesis and characterization of Na3PS4 solid electrolyte
Na3PS4 is synthesized as a solid electrolyte material for sodium-ion batteries. The microstructure of Na3PS4 is characterized by various techniques to understand its crystalline phases, particle morphology, and ionic conductivity properties. The synthesis methods include mechanical milling, solution processing, and heat treatment, which significantly affect the resulting microstructure and performance of the material.- Synthesis and characterization of Na3PS4 solid electrolyte: Na3PS4 is synthesized as a solid electrolyte material for sodium-ion batteries. The microstructure of Na3PS4 is characterized by various techniques to understand its ionic conductivity properties. The synthesis typically involves mechanical milling of Na2S and P2S5 precursors followed by heat treatment to obtain crystalline Na3PS4. The microstructure analysis reveals grain boundaries and crystalline phases that affect the ionic conductivity of the material.
- Na3PS4 microstructure modification techniques: Various techniques are employed to modify the microstructure of Na3PS4 to enhance its properties. These include doping with other elements, controlling grain size through processing parameters, and surface modification. The microstructure modification aims to improve ionic conductivity, mechanical stability, and electrochemical performance of Na3PS4-based solid electrolytes for battery applications.
- Na3PS4 composite materials and interfaces: Na3PS4 is incorporated into composite materials to improve its performance as a solid electrolyte. The microstructure at the interfaces between Na3PS4 and other components, such as electrodes or polymer matrices, is critical for the overall performance of the battery. The composite approach helps to overcome limitations of pure Na3PS4, such as poor mechanical properties and interfacial resistance, by creating synergistic microstructures with enhanced properties.
- Microstructural analysis techniques for Na3PS4: Various analytical techniques are used to characterize the microstructure of Na3PS4, including scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and spectroscopic methods. These techniques provide insights into the crystalline structure, grain size, porosity, and phase distribution of Na3PS4 materials. Understanding the microstructure is essential for optimizing the performance of Na3PS4 as a solid electrolyte.
- Na3PS4 thin films and coatings: Na3PS4 is processed into thin films and coatings for various applications, particularly in solid-state batteries. The microstructure of these thin films is controlled through deposition parameters and post-treatment processes. The thin film approach allows for better integration of Na3PS4 into devices and can lead to improved interfacial properties. The microstructure of thin films differs from bulk Na3PS4 and can exhibit unique properties beneficial for specific applications.
02 Microstructural analysis techniques for Na3PS4
Various analytical techniques are employed to study the microstructure of Na3PS4, including X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and spectroscopic methods. These techniques help in understanding the grain size, crystallinity, phase composition, and structural defects in Na3PS4, which are crucial for optimizing its performance as a solid electrolyte.Expand Specific Solutions03 Interface engineering of Na3PS4 in solid-state batteries
The interface between Na3PS4 solid electrolyte and electrodes plays a critical role in battery performance. Engineering the microstructure at these interfaces can improve ionic conductivity and reduce interfacial resistance. Various approaches include surface modifications, buffer layers, and composite formations to enhance the electrochemical stability and performance of Na3PS4-based solid-state batteries.Expand Specific Solutions04 Grain boundary and defect engineering in Na3PS4
The microstructure of Na3PS4 can be optimized by controlling grain boundaries and defects. Techniques such as doping, controlled crystallization, and thermal treatments are used to manipulate the grain size, boundary properties, and defect concentration. These microstructural modifications can significantly enhance the ionic conductivity and mechanical properties of Na3PS4 solid electrolytes.Expand Specific Solutions05 Processing methods affecting Na3PS4 microstructure
Various processing methods significantly impact the microstructure of Na3PS4, including ball milling, solution processing, and sintering techniques. The processing parameters such as temperature, pressure, and duration affect the particle size, morphology, and crystallinity of Na3PS4. Optimizing these processing conditions is essential for achieving desired microstructural features and enhancing the performance of Na3PS4-based solid electrolytes.Expand Specific Solutions
Leading Research Groups and Industrial Players
The solid-state electrolyte Na3PS4 sintering microstructure evolution market is in an early growth phase, characterized by increasing research activity but limited commercial deployment. The global solid-state battery market, where this technology applies, is projected to reach $5-7 billion by 2025, growing at 30% CAGR. Technologically, Na3PS4 sintering remains in the development stage, with academic institutions like Shanghai Jiao Tong University and South China University of Technology leading fundamental research, while companies including Toyota Motor Corp., NGK Insulators, and Sumitomo Electric are advancing industrial applications. Research centers like Forschungszentrum Jülich are bridging the gap between theoretical understanding and practical implementation, focusing on optimizing sintering parameters to enhance ionic conductivity and mechanical properties for next-generation energy storage solutions.
NGK Insulators, Ltd.
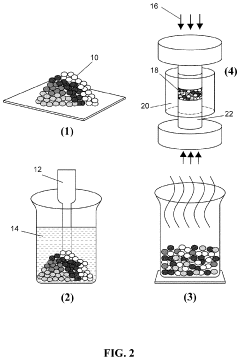
Technical Solution: NGK Insulators has developed specialized ceramic processing techniques for Na3PS4 solid electrolytes that focus on controlling grain size distribution and porosity during sintering. Their approach employs custom-designed sintering furnaces with precise atmosphere control systems that can maintain consistent gas composition (typically argon with <0.5ppm oxygen) throughout the thermal cycle. NGK's research has demonstrated that microstructural evolution during Na3PS4 sintering is critically dependent on heating rate profiles, with slower rates (1-3°C/min) in the 150-220°C range promoting uniform crystallization and minimizing detrimental phase segregation. They've pioneered a multi-stage sintering protocol where initial low-temperature holding periods (4-6 hours at 180°C) allow for complete phase transformation before densification occurs at higher temperatures (250-300°C). This approach achieves relative densities exceeding 90% while maintaining the optimal crystal structure for sodium ion conduction.
Strengths: Extensive ceramic processing expertise; advanced furnace technology with precise atmosphere control; established quality control systems for consistent production. Weaknesses: Traditional ceramic approach may limit innovation in novel processing methods; focus on established manufacturing techniques rather than cutting-edge approaches.
Forschungszentrum Jülich GmbH
Technical Solution: Forschungszentrum Jülich has developed advanced in-situ characterization methodologies to study microstructural evolution during Na3PS4 sintering with unprecedented temporal and spatial resolution. Their approach combines synchrotron-based X-ray diffraction with specialized sample environments that enable real-time monitoring of crystallization, phase transitions, and densification processes. Their research has revealed distinct sintering stages in Na3PS4, including initial particle rearrangement (80-120°C), onset of surface diffusion (150-180°C), and bulk diffusion-dominated densification (220-300°C). They've identified critical correlations between processing atmosphere purity and resulting microstructure, demonstrating that even trace moisture levels (<10ppm) significantly alter grain boundary chemistry and subsequent ionic conductivity. Forschungszentrum Jülich has pioneered the use of field-assisted sintering techniques (FAST/SPS) for Na3PS4, achieving fully dense electrolytes with minimal grain growth through rapid processing (5-15 minutes) at moderate temperatures (200-250°C).
Strengths: World-class characterization infrastructure including synchrotron access; interdisciplinary research approach combining physics, chemistry and materials science; strong theoretical modeling capabilities to complement experimental work. Weaknesses: Research focus may prioritize fundamental understanding over immediate practical applications; potential disconnect between advanced characterization and scalable manufacturing processes.
Critical Mechanisms Governing Na3PS4 Densification
METHOD OF FORMING A ß-SiAlON BY SPARK PLASMA SINTERING
PatentActiveUS20210032105A1
Innovation
- A method involving the mixing of nanoparticles of AlN, Al2O3, and SiO2 with Si3N4 particles, followed by spark plasma sintering at 1450-1600°C and 40-60 MPa pressure to form β-SiAlON, optimizing the weight percentage and size of Si3N4 particles to achieve desired properties.
Materials Characterization Methods for Na-ion Conductors
Materials characterization techniques play a crucial role in understanding the microstructural evolution during sintering of Na3PS4, a promising solid electrolyte for sodium-ion batteries. Advanced microscopy methods, particularly scanning electron microscopy (SEM) and transmission electron microscopy (TEM), provide essential insights into morphological changes, grain growth, and interface formation during the sintering process.
X-ray diffraction (XRD) techniques, including in-situ high-temperature XRD, enable real-time monitoring of phase transformations and crystallographic changes as Na3PS4 undergoes sintering. This allows researchers to identify optimal sintering conditions and understand the relationship between processing parameters and final microstructure.
Spectroscopic methods such as Raman spectroscopy and Fourier-transform infrared spectroscopy (FTIR) help characterize chemical bonding and structural changes during sintering. These techniques are particularly valuable for detecting subtle changes in the local coordination environment of phosphorus and sulfur atoms within the Na3PS4 framework.
Nuclear magnetic resonance (NMR) spectroscopy, especially solid-state 23Na and 31P NMR, provides atomic-level insights into sodium ion dynamics and phosphorus environments. This information is critical for understanding how sintering affects the ionic conductivity pathways within the material.
Thermal analysis techniques, including differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), help identify phase transitions, decomposition temperatures, and moisture sensitivity during the sintering process. These parameters are essential for optimizing the sintering protocol for Na3PS4.
Impedance spectroscopy serves as a powerful tool for correlating microstructural evolution with electrochemical performance. By measuring ionic conductivity at different sintering stages, researchers can establish direct relationships between processing conditions, resulting microstructure, and functional properties.
Advanced tomographic techniques, such as focused ion beam-scanning electron microscopy (FIB-SEM) and X-ray computed tomography, enable three-dimensional reconstruction of the sintered microstructure. This provides quantitative information about porosity, grain connectivity, and percolation pathways for sodium ion transport.
Surface analysis methods, including X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS), help characterize surface composition and potential degradation products formed during sintering, which can significantly impact the electrochemical performance of Na3PS4 as a solid electrolyte.
X-ray diffraction (XRD) techniques, including in-situ high-temperature XRD, enable real-time monitoring of phase transformations and crystallographic changes as Na3PS4 undergoes sintering. This allows researchers to identify optimal sintering conditions and understand the relationship between processing parameters and final microstructure.
Spectroscopic methods such as Raman spectroscopy and Fourier-transform infrared spectroscopy (FTIR) help characterize chemical bonding and structural changes during sintering. These techniques are particularly valuable for detecting subtle changes in the local coordination environment of phosphorus and sulfur atoms within the Na3PS4 framework.
Nuclear magnetic resonance (NMR) spectroscopy, especially solid-state 23Na and 31P NMR, provides atomic-level insights into sodium ion dynamics and phosphorus environments. This information is critical for understanding how sintering affects the ionic conductivity pathways within the material.
Thermal analysis techniques, including differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), help identify phase transitions, decomposition temperatures, and moisture sensitivity during the sintering process. These parameters are essential for optimizing the sintering protocol for Na3PS4.
Impedance spectroscopy serves as a powerful tool for correlating microstructural evolution with electrochemical performance. By measuring ionic conductivity at different sintering stages, researchers can establish direct relationships between processing conditions, resulting microstructure, and functional properties.
Advanced tomographic techniques, such as focused ion beam-scanning electron microscopy (FIB-SEM) and X-ray computed tomography, enable three-dimensional reconstruction of the sintered microstructure. This provides quantitative information about porosity, grain connectivity, and percolation pathways for sodium ion transport.
Surface analysis methods, including X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS), help characterize surface composition and potential degradation products formed during sintering, which can significantly impact the electrochemical performance of Na3PS4 as a solid electrolyte.
Environmental Impact of Na-based Solid Electrolyte Processing
The processing of Na-based solid electrolytes, particularly Na3PS4, raises significant environmental concerns that warrant careful consideration in the development of next-generation energy storage technologies. The extraction of raw materials for these electrolytes, including sodium, phosphorus, and sulfur compounds, involves mining operations that can lead to habitat disruption, soil erosion, and water contamination. Particularly concerning is phosphate mining, which generates substantial waste material and can release phosphogypsum with trace radioactive elements into the environment.
During the synthesis of Na3PS4, high-temperature sintering processes consume considerable energy, contributing to carbon emissions when powered by non-renewable sources. The ball milling techniques commonly employed in Na3PS4 preparation also require electricity and may generate fine particulate matter that poses inhalation hazards if not properly contained. Additionally, the use of organic solvents in certain processing routes creates volatile organic compound (VOC) emissions and hazardous waste streams requiring specialized disposal.
The handling of sulfide-based materials presents unique environmental challenges, as these compounds can react with atmospheric moisture to produce hydrogen sulfide gas—a toxic air pollutant with a characteristic rotten egg odor. This necessitates controlled processing environments that further increase energy consumption and infrastructure requirements.
Waste management throughout the electrolyte production lifecycle represents another environmental concern. Unreacted precursors, off-specification batches, and end-of-life disposal of sodium-based batteries all contribute to potential environmental contamination if not properly managed. The sulfide components in particular may leach into groundwater systems if improperly disposed of, potentially affecting aquatic ecosystems.
Recent life cycle assessments of Na-ion battery technologies indicate that while they offer advantages over lithium-based systems in terms of resource abundance, their overall environmental footprint remains substantial. Efforts to mitigate these impacts include developing lower-temperature synthesis routes, solvent-free processing methods, and closed-loop recycling systems for production waste and end-of-life materials.
The microstructural evolution during sintering directly influences processing efficiency—optimized sintering protocols that achieve desired electrolyte properties at lower temperatures or shorter durations could significantly reduce energy consumption and associated environmental impacts. Research into green chemistry approaches for Na3PS4 synthesis represents a promising direction for environmentally sustainable solid-state battery development.
During the synthesis of Na3PS4, high-temperature sintering processes consume considerable energy, contributing to carbon emissions when powered by non-renewable sources. The ball milling techniques commonly employed in Na3PS4 preparation also require electricity and may generate fine particulate matter that poses inhalation hazards if not properly contained. Additionally, the use of organic solvents in certain processing routes creates volatile organic compound (VOC) emissions and hazardous waste streams requiring specialized disposal.
The handling of sulfide-based materials presents unique environmental challenges, as these compounds can react with atmospheric moisture to produce hydrogen sulfide gas—a toxic air pollutant with a characteristic rotten egg odor. This necessitates controlled processing environments that further increase energy consumption and infrastructure requirements.
Waste management throughout the electrolyte production lifecycle represents another environmental concern. Unreacted precursors, off-specification batches, and end-of-life disposal of sodium-based batteries all contribute to potential environmental contamination if not properly managed. The sulfide components in particular may leach into groundwater systems if improperly disposed of, potentially affecting aquatic ecosystems.
Recent life cycle assessments of Na-ion battery technologies indicate that while they offer advantages over lithium-based systems in terms of resource abundance, their overall environmental footprint remains substantial. Efforts to mitigate these impacts include developing lower-temperature synthesis routes, solvent-free processing methods, and closed-loop recycling systems for production waste and end-of-life materials.
The microstructural evolution during sintering directly influences processing efficiency—optimized sintering protocols that achieve desired electrolyte properties at lower temperatures or shorter durations could significantly reduce energy consumption and associated environmental impacts. Research into green chemistry approaches for Na3PS4 synthesis represents a promising direction for environmentally sustainable solid-state battery development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!