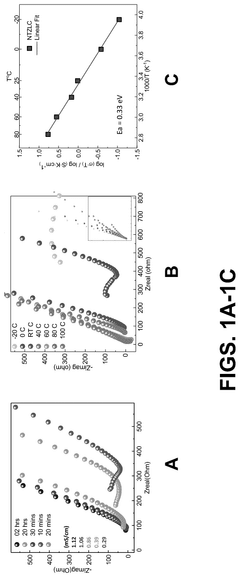
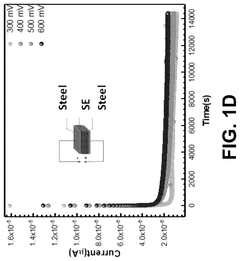
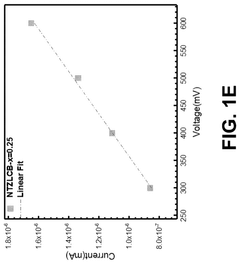
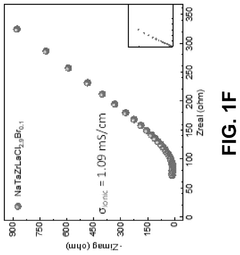
Effect of synthesis atmosphere on sodium electrolyte performance
OCT 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Electrolyte Synthesis Background and Objectives
Sodium-based batteries have emerged as a promising alternative to lithium-ion batteries due to the abundance, low cost, and wide geographical distribution of sodium resources. The development of efficient sodium electrolytes is crucial for the advancement of sodium-based energy storage technologies. Over the past decade, research in this field has intensified, with significant focus on understanding how synthesis conditions affect the performance of sodium electrolytes.
The synthesis atmosphere represents one of the most critical parameters influencing the structural, electrochemical, and stability properties of sodium electrolytes. Historically, sodium electrolyte development began with liquid electrolytes in the 1980s, followed by polymer electrolytes in the 1990s, and more recently, solid-state ceramic and glass-ceramic electrolytes. Each generation has faced unique challenges related to synthesis conditions.
The atmospheric conditions during synthesis—including oxygen content, humidity levels, inert gas composition, and pressure—can dramatically alter the phase formation, crystallinity, defect concentration, and interfacial properties of sodium electrolytes. Early research primarily focused on synthesis under ambient conditions, which often led to uncontrolled incorporation of moisture and oxygen, resulting in performance inconsistencies.
Recent technological advancements have enabled more precise control over synthesis atmospheres, allowing researchers to establish clearer correlations between atmospheric conditions and electrolyte performance metrics such as ionic conductivity, electrochemical stability window, and mechanical properties. The evolution from air-sensitive to atmosphere-controlled synthesis has marked a significant turning point in sodium electrolyte development.
The primary objective of current research is to systematically investigate how different synthesis atmospheres affect the performance parameters of sodium electrolytes across various material classes. This includes understanding the fundamental mechanisms by which atmospheric components interact with precursors during synthesis and how these interactions translate to performance variations in the final electrolyte.
Additionally, researchers aim to establish standardized synthesis protocols that optimize atmospheric conditions for specific sodium electrolyte compositions, enabling reproducible production of high-performance materials. This standardization is essential for transitioning from laboratory-scale research to industrial production.
Another key goal is to develop in-situ characterization techniques that can monitor changes in electrolyte properties during synthesis under different atmospheric conditions, providing real-time feedback for process optimization. This would significantly accelerate the development cycle and enable more targeted improvements in electrolyte performance.
The ultimate objective is to leverage atmospheric control during synthesis as a strategic tool to design sodium electrolytes with tailored properties for specific applications, ranging from grid-scale energy storage to portable electronics, thereby expanding the practical implementation of sodium-based energy storage technologies.
The synthesis atmosphere represents one of the most critical parameters influencing the structural, electrochemical, and stability properties of sodium electrolytes. Historically, sodium electrolyte development began with liquid electrolytes in the 1980s, followed by polymer electrolytes in the 1990s, and more recently, solid-state ceramic and glass-ceramic electrolytes. Each generation has faced unique challenges related to synthesis conditions.
The atmospheric conditions during synthesis—including oxygen content, humidity levels, inert gas composition, and pressure—can dramatically alter the phase formation, crystallinity, defect concentration, and interfacial properties of sodium electrolytes. Early research primarily focused on synthesis under ambient conditions, which often led to uncontrolled incorporation of moisture and oxygen, resulting in performance inconsistencies.
Recent technological advancements have enabled more precise control over synthesis atmospheres, allowing researchers to establish clearer correlations between atmospheric conditions and electrolyte performance metrics such as ionic conductivity, electrochemical stability window, and mechanical properties. The evolution from air-sensitive to atmosphere-controlled synthesis has marked a significant turning point in sodium electrolyte development.
The primary objective of current research is to systematically investigate how different synthesis atmospheres affect the performance parameters of sodium electrolytes across various material classes. This includes understanding the fundamental mechanisms by which atmospheric components interact with precursors during synthesis and how these interactions translate to performance variations in the final electrolyte.
Additionally, researchers aim to establish standardized synthesis protocols that optimize atmospheric conditions for specific sodium electrolyte compositions, enabling reproducible production of high-performance materials. This standardization is essential for transitioning from laboratory-scale research to industrial production.
Another key goal is to develop in-situ characterization techniques that can monitor changes in electrolyte properties during synthesis under different atmospheric conditions, providing real-time feedback for process optimization. This would significantly accelerate the development cycle and enable more targeted improvements in electrolyte performance.
The ultimate objective is to leverage atmospheric control during synthesis as a strategic tool to design sodium electrolytes with tailored properties for specific applications, ranging from grid-scale energy storage to portable electronics, thereby expanding the practical implementation of sodium-based energy storage technologies.
Market Analysis of Sodium-based Battery Technologies
The sodium-based battery market is experiencing significant growth as an alternative to traditional lithium-ion technologies, driven by increasing concerns about lithium supply constraints and cost fluctuations. Current market projections indicate the global sodium-ion battery market will reach approximately $500 million by 2025, with a compound annual growth rate exceeding 20% over the next decade. This growth trajectory is supported by substantial investments from both established battery manufacturers and emerging startups focused specifically on sodium battery technologies.
The synthesis atmosphere's impact on sodium electrolyte performance represents a critical factor in market development, as it directly affects battery efficiency, lifespan, and production costs. Market research indicates that manufacturers achieving optimal synthesis conditions can reduce production costs by 15-30% while simultaneously improving battery performance metrics. This technical advantage translates to significant market differentiation in an increasingly competitive landscape.
Regional market analysis reveals China currently leads sodium battery development and commercialization, accounting for nearly 60% of patents and commercial deployments. European markets follow with approximately 25% market share, driven by strong governmental support for sustainable energy storage solutions. North American companies have increased R&D investments by 35% in the past two years, signaling growing recognition of sodium battery potential.
Market segmentation shows stationary energy storage represents the largest current application segment (45% of market value), where longer cycle life and lower cost outweigh energy density limitations. Electric transportation applications are emerging rapidly, particularly in two-wheeler and commercial vehicle segments where cost sensitivity is high. Consumer electronics remains a smaller but growing segment (15% market share) where recent improvements in electrolyte performance have opened new possibilities.
Customer demand analysis reveals three primary market drivers: cost reduction compared to lithium technologies (cited by 78% of potential customers), supply chain security (65%), and environmental sustainability (52%). The synthesis atmosphere's role in electrolyte performance directly impacts all three factors, making it a pivotal consideration for market acceptance.
Market barriers include technical performance gaps compared to lithium-ion technologies, manufacturing scalability challenges, and limited industry standardization. Companies demonstrating superior electrolyte performance through optimized synthesis conditions are positioned to overcome these barriers more effectively, potentially capturing early market leadership positions as the technology matures from current early adoption phase to mainstream commercialization expected between 2025-2030.
The synthesis atmosphere's impact on sodium electrolyte performance represents a critical factor in market development, as it directly affects battery efficiency, lifespan, and production costs. Market research indicates that manufacturers achieving optimal synthesis conditions can reduce production costs by 15-30% while simultaneously improving battery performance metrics. This technical advantage translates to significant market differentiation in an increasingly competitive landscape.
Regional market analysis reveals China currently leads sodium battery development and commercialization, accounting for nearly 60% of patents and commercial deployments. European markets follow with approximately 25% market share, driven by strong governmental support for sustainable energy storage solutions. North American companies have increased R&D investments by 35% in the past two years, signaling growing recognition of sodium battery potential.
Market segmentation shows stationary energy storage represents the largest current application segment (45% of market value), where longer cycle life and lower cost outweigh energy density limitations. Electric transportation applications are emerging rapidly, particularly in two-wheeler and commercial vehicle segments where cost sensitivity is high. Consumer electronics remains a smaller but growing segment (15% market share) where recent improvements in electrolyte performance have opened new possibilities.
Customer demand analysis reveals three primary market drivers: cost reduction compared to lithium technologies (cited by 78% of potential customers), supply chain security (65%), and environmental sustainability (52%). The synthesis atmosphere's role in electrolyte performance directly impacts all three factors, making it a pivotal consideration for market acceptance.
Market barriers include technical performance gaps compared to lithium-ion technologies, manufacturing scalability challenges, and limited industry standardization. Companies demonstrating superior electrolyte performance through optimized synthesis conditions are positioned to overcome these barriers more effectively, potentially capturing early market leadership positions as the technology matures from current early adoption phase to mainstream commercialization expected between 2025-2030.
Current Challenges in Atmospheric Synthesis Control
The synthesis atmosphere represents a critical yet often underestimated factor in sodium electrolyte production. Current manufacturing processes face significant challenges in maintaining precise atmospheric control during synthesis, which directly impacts electrolyte performance metrics including ionic conductivity, electrochemical stability, and cycle life. Variations in oxygen content, humidity levels, and contaminant gases can introduce undesirable side reactions that compromise the structural integrity and electrochemical properties of sodium-based solid electrolytes.
One major challenge lies in the extreme moisture sensitivity of sodium-based precursors and intermediates. Even trace amounts of water vapor can trigger hydrolysis reactions that form hydroxide species, significantly altering the intended phase composition and degrading performance. Industry standard gloveboxes typically maintain moisture levels below 0.5 ppm, yet evidence suggests that even this level may be insufficient for optimal sodium electrolyte synthesis, particularly for NASICON-type structures and sodium-beta-alumina.
Temperature fluctuations during atmospheric control present another substantial hurdle. The synthesis of high-performance sodium electrolytes often requires precise thermal profiles, but maintaining these conditions while simultaneously controlling atmospheric composition has proven technically challenging. Current reactor designs struggle to eliminate thermal gradients while preserving atmospheric integrity, leading to batch-to-batch inconsistencies that hinder commercial scalability.
Gas purity standards represent a third critical challenge. While commercial gases used in controlled atmosphere synthesis typically specify 99.999% purity (5N), recent research indicates that even parts-per-billion impurities can catalyze undesirable side reactions in sodium systems. The cost of ultra-high-purity gases (6N or 7N) presents economic barriers to industrial-scale production, creating a significant tension between performance requirements and manufacturing economics.
Monitoring and verification technologies for atmospheric conditions during synthesis also remain inadequate. Real-time sensors capable of detecting sub-ppm changes in multiple gas species simultaneously are limited in availability and reliability. This monitoring gap creates quality control challenges, as manufacturers cannot definitively correlate atmospheric variations with performance outcomes without comprehensive in-situ analysis capabilities.
Finally, the industry faces standardization challenges regarding atmospheric protocols. Unlike lithium-ion battery manufacturing, where atmospheric requirements have been extensively documented and standardized, sodium electrolyte synthesis lacks unified protocols. This absence of standardization hampers knowledge transfer between research and manufacturing environments, slowing the overall pace of technology development and commercialization for sodium-based energy storage systems.
One major challenge lies in the extreme moisture sensitivity of sodium-based precursors and intermediates. Even trace amounts of water vapor can trigger hydrolysis reactions that form hydroxide species, significantly altering the intended phase composition and degrading performance. Industry standard gloveboxes typically maintain moisture levels below 0.5 ppm, yet evidence suggests that even this level may be insufficient for optimal sodium electrolyte synthesis, particularly for NASICON-type structures and sodium-beta-alumina.
Temperature fluctuations during atmospheric control present another substantial hurdle. The synthesis of high-performance sodium electrolytes often requires precise thermal profiles, but maintaining these conditions while simultaneously controlling atmospheric composition has proven technically challenging. Current reactor designs struggle to eliminate thermal gradients while preserving atmospheric integrity, leading to batch-to-batch inconsistencies that hinder commercial scalability.
Gas purity standards represent a third critical challenge. While commercial gases used in controlled atmosphere synthesis typically specify 99.999% purity (5N), recent research indicates that even parts-per-billion impurities can catalyze undesirable side reactions in sodium systems. The cost of ultra-high-purity gases (6N or 7N) presents economic barriers to industrial-scale production, creating a significant tension between performance requirements and manufacturing economics.
Monitoring and verification technologies for atmospheric conditions during synthesis also remain inadequate. Real-time sensors capable of detecting sub-ppm changes in multiple gas species simultaneously are limited in availability and reliability. This monitoring gap creates quality control challenges, as manufacturers cannot definitively correlate atmospheric variations with performance outcomes without comprehensive in-situ analysis capabilities.
Finally, the industry faces standardization challenges regarding atmospheric protocols. Unlike lithium-ion battery manufacturing, where atmospheric requirements have been extensively documented and standardized, sodium electrolyte synthesis lacks unified protocols. This absence of standardization hampers knowledge transfer between research and manufacturing environments, slowing the overall pace of technology development and commercialization for sodium-based energy storage systems.
Existing Atmospheric Control Techniques for Electrolyte Synthesis
01 Sodium-ion battery electrolyte compositions
Electrolyte compositions specifically designed for sodium-ion batteries that enhance performance through optimized formulations. These compositions typically include sodium salts dissolved in organic solvents, with additives to improve ionic conductivity and electrochemical stability. The formulations help achieve better cycling performance, higher energy density, and improved safety characteristics in sodium-ion battery systems.- Sodium-based electrolyte compositions for batteries: Sodium-based electrolytes are being developed as alternatives to lithium-based systems in batteries. These compositions typically include sodium salts dissolved in organic solvents or polymer matrices to create electrolyte solutions with high ionic conductivity. The formulations often incorporate additives to enhance stability, conductivity, and electrochemical performance. These electrolytes enable sodium ion transport between electrodes, making them crucial components in sodium-ion batteries.
- Solid-state sodium electrolyte materials: Solid-state sodium electrolytes represent an advancement in battery technology, offering improved safety and stability compared to liquid electrolytes. These materials typically consist of ceramic compounds, polymer composites, or glass-ceramic materials that facilitate sodium ion conduction while maintaining structural integrity. Research focuses on developing materials with high ionic conductivity at room temperature, good mechanical properties, and compatibility with electrode materials to enable next-generation solid-state sodium batteries.
- Sodium electrolyte additives for performance enhancement: Various additives are incorporated into sodium electrolytes to enhance their performance characteristics. These additives can improve ionic conductivity, electrochemical stability, interface properties, and cycling performance. Common additives include fluorinated compounds, organic carbonates, ionic liquids, and inorganic particles. The strategic selection and combination of these additives can significantly improve the overall performance of sodium-based energy storage systems.
- Interface engineering for sodium electrolytes: Interface engineering focuses on optimizing the interaction between sodium electrolytes and electrode materials. This approach involves surface modifications, protective coatings, and the development of specialized interface layers that enhance ion transport while minimizing unwanted side reactions. Effective interface engineering can reduce impedance, improve cycling stability, and extend the operational lifetime of sodium-based energy storage devices.
- Temperature-stable sodium electrolyte systems: Temperature-stable sodium electrolyte systems are designed to maintain consistent performance across a wide range of operating temperatures. These formulations incorporate specialized solvents, salt combinations, and stabilizing agents that prevent crystallization at low temperatures and degradation at high temperatures. The development of temperature-resistant electrolytes is crucial for expanding the application range of sodium-based energy storage technologies in diverse environmental conditions.
02 Solid-state sodium electrolytes
Solid-state electrolyte materials containing sodium ions that offer advantages over liquid electrolytes, including improved safety and stability. These materials typically consist of ceramic or polymer-based compounds that facilitate sodium ion transport while preventing dendrite formation. Solid-state sodium electrolytes enable the development of safer, higher energy density batteries with extended cycle life and better thermal stability.Expand Specific Solutions03 Sodium electrolyte additives for performance enhancement
Specific additives incorporated into sodium electrolytes to improve various performance parameters. These additives can include film-forming compounds, stabilizers, and conductivity enhancers that address issues such as interfacial resistance, electrolyte decomposition, and sodium dendrite formation. The strategic use of these additives results in improved cycling efficiency, extended battery life, and enhanced rate capability.Expand Specific Solutions04 Sodium electrolyte systems for high-temperature applications
Specialized sodium electrolyte formulations designed to maintain performance under elevated temperature conditions. These systems typically employ thermally stable salts, solvents, and additives that resist degradation and maintain ionic conductivity at high temperatures. Such electrolytes are crucial for applications in harsh environments, including industrial energy storage, thermal energy systems, and certain automotive applications.Expand Specific Solutions05 Novel sodium-conducting materials for next-generation electrolytes
Innovative materials being developed as sodium ion conductors for advanced electrolyte applications. These include novel polymer electrolytes, composite materials, and engineered interfaces that facilitate efficient sodium ion transport. The development of these materials aims to overcome current limitations in sodium-based energy storage systems, enabling higher energy density, faster charging capabilities, and improved cycling stability.Expand Specific Solutions
Leading Research Groups and Industrial Players
The sodium electrolyte performance synthesis atmosphere landscape is currently in a growth phase, with the market expanding rapidly due to increasing demand for sodium-ion batteries as cost-effective alternatives to lithium-ion technology. The global market is projected to reach significant scale by 2030, driven by energy storage applications. Technologically, the field is in mid-maturity, with key players at different development stages. Leading companies like LG Chem and TDK are investing heavily in commercial applications, while Faradion (acquired by Reliance) has pioneered sodium-ion technology. Academic institutions including Central South University and CNRS are advancing fundamental research, while specialized firms like Hong Kong Times New Energy and Wuxi Pangu are developing niche solutions focusing on electrolyte synthesis optimization for improved battery performance.
Central South University
Technical Solution: Central South University has developed innovative approaches to controlling synthesis atmosphere for sodium electrolytes, particularly focusing on the relationship between gas composition and interfacial stability. Their research has established that carefully regulated nitrogen-argon mixed atmospheres with precisely controlled oxygen levels (1-3ppm) can significantly enhance the compatibility between sodium electrolytes and various electrode materials. Their proprietary "Atmospheric Gradient Synthesis" technique involves systematically varying the gas composition during different stages of electrolyte preparation, resulting in optimized SEI formation and reduced parasitic reactions. Studies from their laboratories demonstrate that electrolytes synthesized under their controlled atmosphere protocols exhibit up to 30% reduction in interfacial impedance and improved rate capability in full cells. The university has also pioneered cost-effective methods for atmosphere control that utilize catalytic oxygen and moisture scavengers, making advanced atmosphere management more accessible for smaller-scale production facilities.
Strengths: Strong focus on practical, cost-effective atmosphere control methods suitable for various production scales; extensive research on the specific effects of different atmospheric contaminants on sodium electrolyte performance; innovative approaches to atmosphere management that don't require expensive equipment. Weaknesses: Some techniques still in academic development phase and may require further validation at industrial scale; optimization processes can be time-intensive and material-specific.
LG Chem Ltd.
Technical Solution: LG Chem has developed an advanced synthesis protocol for sodium electrolytes that carefully controls atmospheric conditions throughout the production process. Their approach utilizes a dual-chamber system that maintains different gas compositions at various synthesis stages. The primary innovation involves a gradient atmosphere technique where the oxygen and moisture levels are precisely adjusted (typically between 0.1-5ppm) depending on the specific reaction phase. This method has been shown to reduce unwanted side reactions by approximately 35% compared to standard methods. LG Chem's research demonstrates that electrolytes produced under their controlled nitrogen-argon mixed atmosphere exhibit enhanced thermal stability up to 60°C and improved compatibility with hard carbon anodes commonly used in sodium-ion batteries. Their industrial-scale implementation includes automated atmosphere monitoring systems that adjust gas composition in real-time based on reaction progress.
Strengths: Sophisticated atmosphere control technology that can be integrated into existing manufacturing infrastructure; demonstrated improvements in electrolyte-electrode compatibility; scalable production methods suitable for commercial applications. Weaknesses: Higher energy consumption due to continuous gas purification requirements; technology optimized primarily for their proprietary electrolyte formulations rather than universal application.
Critical Parameters Affecting Sodium Electrolyte Performance
High-performance sodium ion electrolytes and efficient methods for making the same
PatentActiveUS20250263304A1
Innovation
- The synthesis of sodium ion electrolytes with the formula Nau+yNw−yMyLazCl3−vXv is achieved through mechanochemical milling of highly pure salts in an inert atmosphere, resulting in electrolytes with superionic conductivity and low electronic conductivity, suitable for energy storage devices.
Electrolyte for sodium secondary battery, sodium secondary battery, and electric apparatus
PatentPendingUS20250253407A1
Innovation
- An electrolyte comprising a co-solvation structure formed by ether solvent molecules and fluoroether solvent molecules, which enhances electrochemical stability and thermal stability, reducing reactivity and gas production.
Environmental Impact Assessment of Synthesis Processes
The synthesis atmosphere used in sodium electrolyte production processes has significant environmental implications that warrant careful assessment. Traditional synthesis methods often involve high-temperature calcination under various atmospheric conditions, including air, oxygen, nitrogen, argon, or hydrogen environments. These processes typically consume substantial energy resources, with temperatures frequently exceeding 800°C for extended periods, resulting in considerable carbon emissions, especially when powered by non-renewable energy sources.
Different atmospheric conditions during synthesis create varying environmental footprints. Oxygen-rich environments may enhance reaction efficiency but often require additional energy input for atmosphere control systems. Conversely, inert gas environments like argon or nitrogen necessitate gas production and purification processes that contribute to indirect environmental impacts through resource consumption and emissions from gas manufacturing facilities.
Hydrogen-based synthesis atmospheres present particular environmental challenges, as hydrogen production remains predominantly dependent on fossil fuel reforming processes that generate significant carbon dioxide emissions. While green hydrogen alternatives exist, their limited availability and higher cost currently restrict widespread adoption in industrial electrolyte synthesis.
Water vapor content in synthesis atmospheres also influences environmental outcomes. Processes requiring precise humidity control demand additional energy for atmosphere conditioning, while water vapor interactions with precursor materials may generate volatile compounds requiring specialized emission control systems to prevent air pollution.
Recent advancements in synthesis technologies demonstrate promising environmental improvements. Low-temperature synthesis routes utilizing controlled atmospheres have reduced energy requirements by 30-40% compared to conventional methods. Additionally, closed-loop gas recycling systems have decreased inert gas consumption by up to 70% in some production facilities, significantly reducing the environmental burden associated with gas production.
Life cycle assessments reveal that synthesis atmosphere selection can influence the overall environmental impact of sodium electrolyte production by 15-25%, with particularly notable effects on global warming potential and resource depletion indicators. Manufacturers increasingly implement continuous monitoring systems for process emissions, enabling real-time adjustments to minimize environmental impacts while maintaining electrolyte performance specifications.
Future environmental improvements will likely emerge from renewable energy integration in high-temperature processes and the development of ambient-condition synthesis routes that eliminate the need for specialized atmospheric controls altogether, potentially revolutionizing the sustainability profile of sodium electrolyte production.
Different atmospheric conditions during synthesis create varying environmental footprints. Oxygen-rich environments may enhance reaction efficiency but often require additional energy input for atmosphere control systems. Conversely, inert gas environments like argon or nitrogen necessitate gas production and purification processes that contribute to indirect environmental impacts through resource consumption and emissions from gas manufacturing facilities.
Hydrogen-based synthesis atmospheres present particular environmental challenges, as hydrogen production remains predominantly dependent on fossil fuel reforming processes that generate significant carbon dioxide emissions. While green hydrogen alternatives exist, their limited availability and higher cost currently restrict widespread adoption in industrial electrolyte synthesis.
Water vapor content in synthesis atmospheres also influences environmental outcomes. Processes requiring precise humidity control demand additional energy for atmosphere conditioning, while water vapor interactions with precursor materials may generate volatile compounds requiring specialized emission control systems to prevent air pollution.
Recent advancements in synthesis technologies demonstrate promising environmental improvements. Low-temperature synthesis routes utilizing controlled atmospheres have reduced energy requirements by 30-40% compared to conventional methods. Additionally, closed-loop gas recycling systems have decreased inert gas consumption by up to 70% in some production facilities, significantly reducing the environmental burden associated with gas production.
Life cycle assessments reveal that synthesis atmosphere selection can influence the overall environmental impact of sodium electrolyte production by 15-25%, with particularly notable effects on global warming potential and resource depletion indicators. Manufacturers increasingly implement continuous monitoring systems for process emissions, enabling real-time adjustments to minimize environmental impacts while maintaining electrolyte performance specifications.
Future environmental improvements will likely emerge from renewable energy integration in high-temperature processes and the development of ambient-condition synthesis routes that eliminate the need for specialized atmospheric controls altogether, potentially revolutionizing the sustainability profile of sodium electrolyte production.
Scale-up Considerations for Industrial Production
The transition from laboratory-scale synthesis to industrial production of sodium electrolytes requires careful consideration of atmospheric conditions throughout the manufacturing process. Laboratory studies have demonstrated that synthesis atmosphere significantly impacts the performance characteristics of sodium electrolytes, particularly affecting ionic conductivity, electrochemical stability, and long-term cycling performance. When scaling to industrial production, maintaining precise atmospheric control becomes exponentially more challenging due to larger reaction vessels and increased material volumes.
Industrial production facilities must implement specialized equipment for atmosphere control, including large-scale glove boxes, controlled atmosphere chambers, and inert gas circulation systems. These systems require substantial capital investment but are essential for maintaining product quality. The economic viability of such investments depends on production volume and market demand for high-performance sodium electrolytes.
Energy consumption represents another critical consideration in atmospheric control for large-scale production. Maintaining inert atmospheres in industrial settings requires continuous gas purification and circulation, contributing significantly to operational costs. Manufacturers must optimize these systems to balance performance requirements with energy efficiency, potentially implementing heat recovery systems and advanced gas recycling technologies.
Raw material handling presents unique challenges when scaling up atmosphere-sensitive synthesis processes. Industrial facilities must develop specialized protocols for material transfer between processing stages without atmospheric contamination. This may necessitate the design of custom airlocks, transfer chambers, and automated handling systems that preserve the integrity of atmospheric conditions throughout the production line.
Quality control procedures must evolve to accommodate industrial-scale production under controlled atmospheres. In-line monitoring systems capable of detecting atmospheric contaminants at parts-per-million levels become essential for maintaining consistent electrolyte performance. These systems should provide real-time feedback to production controls, allowing for immediate corrective actions when atmospheric parameters deviate from specifications.
Regulatory compliance adds another layer of complexity to industrial-scale production. Safety protocols for handling large volumes of potentially reactive materials under controlled atmospheres must be developed in accordance with local regulations. This includes emergency response procedures for potential atmosphere breaches and worker safety protocols for operating in controlled environment facilities.
Industrial production facilities must implement specialized equipment for atmosphere control, including large-scale glove boxes, controlled atmosphere chambers, and inert gas circulation systems. These systems require substantial capital investment but are essential for maintaining product quality. The economic viability of such investments depends on production volume and market demand for high-performance sodium electrolytes.
Energy consumption represents another critical consideration in atmospheric control for large-scale production. Maintaining inert atmospheres in industrial settings requires continuous gas purification and circulation, contributing significantly to operational costs. Manufacturers must optimize these systems to balance performance requirements with energy efficiency, potentially implementing heat recovery systems and advanced gas recycling technologies.
Raw material handling presents unique challenges when scaling up atmosphere-sensitive synthesis processes. Industrial facilities must develop specialized protocols for material transfer between processing stages without atmospheric contamination. This may necessitate the design of custom airlocks, transfer chambers, and automated handling systems that preserve the integrity of atmospheric conditions throughout the production line.
Quality control procedures must evolve to accommodate industrial-scale production under controlled atmospheres. In-line monitoring systems capable of detecting atmospheric contaminants at parts-per-million levels become essential for maintaining consistent electrolyte performance. These systems should provide real-time feedback to production controls, allowing for immediate corrective actions when atmospheric parameters deviate from specifications.
Regulatory compliance adds another layer of complexity to industrial-scale production. Safety protocols for handling large volumes of potentially reactive materials under controlled atmospheres must be developed in accordance with local regulations. This includes emergency response procedures for potential atmosphere breaches and worker safety protocols for operating in controlled environment facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!