Analysis of Autoclave Synthesis Impact on Catalyst Activation Energy
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Synthesis Background and Objectives
Autoclave synthesis represents a pivotal methodology in catalyst development, evolving significantly since its inception in the early 20th century. This high-pressure, high-temperature technique has transformed from rudimentary sealed vessels to sophisticated computer-controlled systems capable of precise parameter manipulation. The evolution has been driven by increasing demands for catalysts with enhanced performance characteristics across various industrial applications, from petrochemical processing to environmental remediation.
The fundamental principle of autoclave synthesis involves creating controlled reaction environments where temperature, pressure, and chemical composition can be precisely regulated to influence catalyst formation. This controlled environment facilitates unique reaction pathways that are often inaccessible through conventional synthesis methods, resulting in catalysts with distinctive structural and energetic properties.
Recent technological advancements have expanded autoclave capabilities, incorporating in-situ monitoring systems, automated sampling mechanisms, and advanced control algorithms. These innovations enable researchers to observe reaction dynamics in real-time and make adjustments accordingly, significantly enhancing the precision of catalyst design and synthesis.
The primary objective of investigating autoclave synthesis impact on catalyst activation energy is to establish quantitative relationships between synthesis parameters and the resulting energetic properties of catalysts. By understanding these relationships, we aim to develop predictive models that can guide the design of catalysts with tailored activation energies for specific applications, potentially revolutionizing reaction efficiency across multiple industries.
Current research indicates that autoclave synthesis conditions—particularly temperature profiles, pressure regimes, and residence times—significantly influence the crystalline structure, surface area, and active site distribution of catalysts. These physical characteristics directly correlate with activation energy barriers, which determine catalyst performance in target reactions.
The global push toward more sustainable chemical processes has intensified interest in this research area, as catalysts with optimized activation energies can substantially reduce energy requirements for industrial processes. This aligns with broader environmental goals and economic imperatives to develop more efficient chemical transformation pathways.
Our technical objectives include developing systematic methodologies for correlating autoclave synthesis parameters with resulting catalyst activation energies, establishing reproducible protocols for synthesizing catalysts with predetermined energetic profiles, and creating computational models that can predict activation energy modifications based on synthesis condition adjustments.
The fundamental principle of autoclave synthesis involves creating controlled reaction environments where temperature, pressure, and chemical composition can be precisely regulated to influence catalyst formation. This controlled environment facilitates unique reaction pathways that are often inaccessible through conventional synthesis methods, resulting in catalysts with distinctive structural and energetic properties.
Recent technological advancements have expanded autoclave capabilities, incorporating in-situ monitoring systems, automated sampling mechanisms, and advanced control algorithms. These innovations enable researchers to observe reaction dynamics in real-time and make adjustments accordingly, significantly enhancing the precision of catalyst design and synthesis.
The primary objective of investigating autoclave synthesis impact on catalyst activation energy is to establish quantitative relationships between synthesis parameters and the resulting energetic properties of catalysts. By understanding these relationships, we aim to develop predictive models that can guide the design of catalysts with tailored activation energies for specific applications, potentially revolutionizing reaction efficiency across multiple industries.
Current research indicates that autoclave synthesis conditions—particularly temperature profiles, pressure regimes, and residence times—significantly influence the crystalline structure, surface area, and active site distribution of catalysts. These physical characteristics directly correlate with activation energy barriers, which determine catalyst performance in target reactions.
The global push toward more sustainable chemical processes has intensified interest in this research area, as catalysts with optimized activation energies can substantially reduce energy requirements for industrial processes. This aligns with broader environmental goals and economic imperatives to develop more efficient chemical transformation pathways.
Our technical objectives include developing systematic methodologies for correlating autoclave synthesis parameters with resulting catalyst activation energies, establishing reproducible protocols for synthesizing catalysts with predetermined energetic profiles, and creating computational models that can predict activation energy modifications based on synthesis condition adjustments.
Market Applications for Autoclave-Synthesized Catalysts
Autoclave-synthesized catalysts have established a significant presence across multiple industrial sectors due to their enhanced performance characteristics resulting from controlled high-pressure and high-temperature synthesis conditions. The petroleum refining industry represents one of the largest markets for these specialized catalysts, where they are extensively employed in hydrocracking, hydrodesulfurization, and reforming processes. The ability to precisely engineer activation energy barriers through autoclave synthesis has enabled refineries to achieve higher conversion rates and improved selectivity, particularly in heavy oil upgrading applications.
The chemical manufacturing sector constitutes another substantial market, with autoclave-synthesized catalysts finding applications in various processes including ammonia synthesis, methanol production, and Fischer-Tropsch synthesis. These catalysts demonstrate superior stability under extreme reaction conditions, translating to extended operational lifetimes and reduced replacement frequency—a critical economic factor for large-scale chemical operations.
Environmental technologies represent a rapidly expanding application domain, particularly in emission control systems. Autoclave-synthesized catalysts with tailored activation energies have proven highly effective in selective catalytic reduction (SCR) of nitrogen oxides and volatile organic compound (VOC) abatement. The automotive industry has increasingly adopted these catalysts in advanced catalytic converters to meet stringent emission standards across global markets.
The pharmaceutical industry utilizes these specialized catalysts for asymmetric synthesis and complex molecule formation, where precise control of activation energy parameters enables higher stereoselectivity and yield in drug manufacturing processes. This application segment is experiencing accelerated growth as pharmaceutical companies seek more efficient and environmentally sustainable production methods.
Emerging applications include renewable energy technologies, where autoclave-synthesized catalysts are being developed for hydrogen production, fuel cells, and energy storage systems. The ability to optimize activation energy profiles makes these catalysts particularly valuable for enhancing energy conversion efficiency in next-generation clean energy applications.
The electronics industry has begun incorporating these catalysts in semiconductor manufacturing processes and advanced materials synthesis, where precisely controlled activation energies facilitate the formation of specialized materials with unique electronic properties. This represents a high-value niche market with significant growth potential as electronics continue to miniaturize and increase in complexity.
Market distribution shows regional variations, with North America and Europe leading in high-value applications such as specialty chemicals and pharmaceuticals, while Asia-Pacific dominates in volume applications including petroleum refining and basic chemical manufacturing. The global market trajectory indicates continued expansion as industries increasingly prioritize process efficiency and sustainability metrics that can be addressed through advanced catalyst technology.
The chemical manufacturing sector constitutes another substantial market, with autoclave-synthesized catalysts finding applications in various processes including ammonia synthesis, methanol production, and Fischer-Tropsch synthesis. These catalysts demonstrate superior stability under extreme reaction conditions, translating to extended operational lifetimes and reduced replacement frequency—a critical economic factor for large-scale chemical operations.
Environmental technologies represent a rapidly expanding application domain, particularly in emission control systems. Autoclave-synthesized catalysts with tailored activation energies have proven highly effective in selective catalytic reduction (SCR) of nitrogen oxides and volatile organic compound (VOC) abatement. The automotive industry has increasingly adopted these catalysts in advanced catalytic converters to meet stringent emission standards across global markets.
The pharmaceutical industry utilizes these specialized catalysts for asymmetric synthesis and complex molecule formation, where precise control of activation energy parameters enables higher stereoselectivity and yield in drug manufacturing processes. This application segment is experiencing accelerated growth as pharmaceutical companies seek more efficient and environmentally sustainable production methods.
Emerging applications include renewable energy technologies, where autoclave-synthesized catalysts are being developed for hydrogen production, fuel cells, and energy storage systems. The ability to optimize activation energy profiles makes these catalysts particularly valuable for enhancing energy conversion efficiency in next-generation clean energy applications.
The electronics industry has begun incorporating these catalysts in semiconductor manufacturing processes and advanced materials synthesis, where precisely controlled activation energies facilitate the formation of specialized materials with unique electronic properties. This represents a high-value niche market with significant growth potential as electronics continue to miniaturize and increase in complexity.
Market distribution shows regional variations, with North America and Europe leading in high-value applications such as specialty chemicals and pharmaceuticals, while Asia-Pacific dominates in volume applications including petroleum refining and basic chemical manufacturing. The global market trajectory indicates continued expansion as industries increasingly prioritize process efficiency and sustainability metrics that can be addressed through advanced catalyst technology.
Current Challenges in Catalyst Activation Energy Reduction
Despite significant advancements in catalyst technology, the reduction of activation energy remains a formidable challenge in the field of catalysis. Current catalytic systems often require substantial energy inputs to initiate and sustain reactions, limiting their industrial applicability and economic viability. The autoclave synthesis method, while effective for certain catalyst preparations, presents several critical challenges when optimizing for lower activation energies.
One primary obstacle is the precise control of temperature and pressure conditions within the autoclave environment. Minor fluctuations can significantly alter the morphology, crystallinity, and surface properties of catalysts, directly impacting their activation energy profiles. Research indicates that even variations of 5-10°C during synthesis can lead to activation energy differences of up to 15-20 kJ/mol in the resulting catalysts.
The heterogeneity of nucleation and growth processes during autoclave synthesis further complicates efforts to achieve consistent catalyst performance. Current methodologies struggle to ensure uniform distribution of active sites, leading to catalysts with varying activation energies across their surfaces. This non-uniformity necessitates higher overall energy inputs to ensure complete reaction conversion.
Another significant challenge lies in the scalability of optimized autoclave synthesis protocols. Laboratory-scale successes in reducing activation energy often fail to translate to industrial production scales due to heat and mass transfer limitations in larger autoclaves. The industry currently lacks robust scale-up models that accurately predict how synthesis parameters affect activation energy across different production volumes.
The selection and ratio of precursors in autoclave synthesis present additional complications. Current research indicates that precursor interactions during hydrothermal or solvothermal processes can form intermediate complexes that significantly influence the final catalyst's energy landscape. However, these interactions remain poorly understood, particularly under the high-temperature, high-pressure conditions typical of autoclave environments.
Material limitations also pose constraints on innovation. Autoclave vessels capable of withstanding extreme conditions necessary for certain novel catalyst syntheses are either prohibitively expensive or technically unfeasible with current materials science capabilities. This restricts exploration of potentially promising synthesis pathways that might yield catalysts with dramatically lower activation energies.
Furthermore, real-time monitoring and characterization of catalysts during autoclave synthesis remain technically challenging. The inability to observe formation processes in situ limits researchers' capacity to identify critical formation stages that determine activation energy properties, resulting in largely empirical rather than mechanistically-driven optimization approaches.
One primary obstacle is the precise control of temperature and pressure conditions within the autoclave environment. Minor fluctuations can significantly alter the morphology, crystallinity, and surface properties of catalysts, directly impacting their activation energy profiles. Research indicates that even variations of 5-10°C during synthesis can lead to activation energy differences of up to 15-20 kJ/mol in the resulting catalysts.
The heterogeneity of nucleation and growth processes during autoclave synthesis further complicates efforts to achieve consistent catalyst performance. Current methodologies struggle to ensure uniform distribution of active sites, leading to catalysts with varying activation energies across their surfaces. This non-uniformity necessitates higher overall energy inputs to ensure complete reaction conversion.
Another significant challenge lies in the scalability of optimized autoclave synthesis protocols. Laboratory-scale successes in reducing activation energy often fail to translate to industrial production scales due to heat and mass transfer limitations in larger autoclaves. The industry currently lacks robust scale-up models that accurately predict how synthesis parameters affect activation energy across different production volumes.
The selection and ratio of precursors in autoclave synthesis present additional complications. Current research indicates that precursor interactions during hydrothermal or solvothermal processes can form intermediate complexes that significantly influence the final catalyst's energy landscape. However, these interactions remain poorly understood, particularly under the high-temperature, high-pressure conditions typical of autoclave environments.
Material limitations also pose constraints on innovation. Autoclave vessels capable of withstanding extreme conditions necessary for certain novel catalyst syntheses are either prohibitively expensive or technically unfeasible with current materials science capabilities. This restricts exploration of potentially promising synthesis pathways that might yield catalysts with dramatically lower activation energies.
Furthermore, real-time monitoring and characterization of catalysts during autoclave synthesis remain technically challenging. The inability to observe formation processes in situ limits researchers' capacity to identify critical formation stages that determine activation energy properties, resulting in largely empirical rather than mechanistically-driven optimization approaches.
Established Methodologies for Catalyst Energy Optimization
01 Hydrothermal autoclave synthesis methods
Hydrothermal synthesis in autoclaves involves reactions in aqueous solutions at elevated temperatures and pressures. This method allows for controlling activation energy through temperature regulation, pressure conditions, and reaction time. The process is particularly effective for synthesizing crystalline materials like zeolites, metal oxides, and nanomaterials where conventional methods would require significantly higher activation energies.- Hydrothermal autoclave synthesis methods for nanomaterials: Hydrothermal synthesis in autoclaves is widely used for producing nanomaterials with controlled morphology and properties. This process involves reactions in aqueous solutions at elevated temperatures and pressures, which lowers the activation energy required for crystallization. The method enables precise control over particle size, shape, and composition by adjusting parameters such as temperature, pressure, and reaction time. These techniques are particularly valuable for synthesizing metal oxides, zeolites, and other functional materials with enhanced catalytic properties.
- Measurement and calculation of activation energy in autoclave processes: Various methods are employed to measure and calculate activation energy during autoclave synthesis processes. These include isothermal and non-isothermal kinetic analysis techniques that monitor reaction progress at different temperatures. Advanced computational models and algorithms help determine the activation energy barriers for specific reactions, enabling optimization of synthesis conditions. Temperature-controlled autoclave systems with precise monitoring capabilities allow researchers to collect data for Arrhenius plots and other mathematical models that quantify activation energy values for different reaction pathways.
- Catalysts and additives for reducing activation energy in autoclave synthesis: Incorporating specific catalysts and additives in autoclave synthesis can significantly reduce the activation energy required for reactions. These materials create alternative reaction pathways with lower energy barriers, enabling reactions to proceed at lower temperatures or pressures. Metal-based catalysts, ionic liquids, and certain organic compounds can facilitate nucleation and crystal growth processes. The strategic use of these activation energy modifiers improves reaction efficiency, reduces processing time, and enhances the quality and uniformity of the synthesized materials.
- Pressure and temperature effects on activation energy in autoclave synthesis: The relationship between pressure, temperature, and activation energy is crucial in autoclave synthesis. Increased pressure and temperature typically reduce the activation energy required for reactions, accelerating reaction rates and enabling the formation of materials that would be difficult to synthesize under ambient conditions. Precise control of these parameters allows for tailoring the thermodynamic and kinetic aspects of reactions. Specialized autoclave designs incorporate advanced pressure and temperature control systems to maintain optimal conditions throughout the synthesis process.
- Novel autoclave designs for optimizing activation energy requirements: Innovative autoclave designs focus on optimizing activation energy requirements through enhanced heat transfer, improved mixing, and precise control systems. These designs incorporate advanced materials that withstand extreme conditions while providing uniform heating and cooling. Some autoclaves feature microwave-assisted heating, ultrasonic agitation, or rotating mechanisms to enhance mass transfer and reduce energy barriers. Smart autoclave systems with real-time monitoring capabilities allow for dynamic adjustment of synthesis parameters to maintain optimal activation energy conditions throughout the reaction process.
02 Catalytic processes to reduce activation energy
Various catalysts can be incorporated into autoclave synthesis processes to lower the activation energy barrier of reactions. These catalysts facilitate reaction pathways that would otherwise require higher energy inputs. The selection of appropriate catalysts depends on the target compounds and can significantly enhance reaction rates, improve yield, and allow for milder operating conditions in autoclave synthesis.Expand Specific Solutions03 Temperature and pressure control systems
Advanced temperature and pressure control systems in autoclave synthesis allow for precise manipulation of reaction conditions, directly affecting activation energy requirements. These systems enable the creation of specific temperature profiles, pressure gradients, and controlled heating/cooling rates that can optimize reaction kinetics. Such control mechanisms are essential for reactions with narrow activation energy windows or temperature-sensitive precursors.Expand Specific Solutions04 Microwave-assisted autoclave synthesis
Microwave-assisted heating in autoclave synthesis provides more efficient energy transfer to reaction mixtures, effectively lowering apparent activation energy requirements. This technique enables more rapid heating, uniform temperature distribution, and selective heating of specific components. The result is accelerated reaction rates, reduced processing times, and often improved product quality compared to conventional heating methods.Expand Specific Solutions05 Computational modeling of activation energy pathways
Computational methods are increasingly used to model and predict activation energy requirements in autoclave synthesis processes. These models incorporate reaction kinetics, thermodynamic parameters, and molecular dynamics to optimize synthesis conditions before experimental implementation. Advanced algorithms can identify rate-limiting steps, suggest modifications to reduce activation energy barriers, and predict the effects of different precursors or additives on reaction pathways.Expand Specific Solutions
Leading Research Groups and Industrial Manufacturers
The autoclave synthesis impact on catalyst activation energy market is currently in a growth phase, with increasing demand driven by the petrochemical and chemical industries. The global market size is estimated to be around $3-4 billion, expanding at a CAGR of 5-7% due to rising focus on energy-efficient catalytic processes. Technology maturity varies across players, with industry leaders like China Petroleum & Chemical Corp., ExxonMobil, and Shell Oil demonstrating advanced capabilities through extensive patent portfolios. Research institutions such as Zhejiang University and Dalian University of Technology are making significant contributions through academic-industrial collaborations. BASF SE, Mitsubishi Gas Chemical, and Haldor Topsøe represent the technological vanguard with proprietary autoclave synthesis methods that have demonstrably reduced activation energies by 15-30% in commercial applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced autoclave synthesis methodologies for catalyst preparation that significantly impact activation energy profiles. Their approach involves precise control of temperature, pressure, and residence time in specialized high-pressure autoclaves to optimize catalyst morphology and active site distribution. Research shows their autoclave synthesis can reduce activation energy by up to 15-20% compared to conventional methods[1]. Sinopec's proprietary hydrothermal autoclave treatment creates unique mesoporous structures with controlled crystallinity, enhancing mass transfer properties while maintaining thermal stability. Their recent innovations include multi-stage temperature programming during autoclave synthesis, which allows for controlled nucleation and crystal growth phases, resulting in catalysts with more uniform active site distribution and enhanced accessibility[3]. The company has successfully applied these techniques to zeolite-based catalysts for petroleum refining processes, achieving significant improvements in selectivity and conversion rates while extending catalyst lifetime through improved resistance to coking and metal poisoning[7].
Strengths: Superior control over catalyst morphology and porosity structure; enhanced thermal stability and resistance to deactivation; reduced energy requirements for catalytic processes due to lower activation energy barriers. Weaknesses: Higher capital investment for specialized autoclave equipment; longer synthesis times compared to conventional precipitation methods; challenges in scaling up precise autoclave conditions from laboratory to industrial production.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil Chemical has pioneered innovative autoclave synthesis techniques for catalyst development that fundamentally alter activation energy pathways. Their proprietary high-pressure autoclave systems employ precise temperature ramping protocols (150-350°C) and controlled atmosphere compositions to manipulate catalyst nucleation and growth mechanisms[2]. ExxonMobil's research demonstrates that autoclave-synthesized catalysts exhibit up to 30% lower activation energies for hydrocarbon conversion reactions compared to conventionally prepared counterparts[4]. Their advanced synthesis approach incorporates in-situ spectroscopic monitoring during autoclave treatment, allowing real-time adjustments to synthesis parameters based on crystallization kinetics. This results in catalysts with optimized metal dispersion and enhanced active site accessibility. The company has particularly focused on developing zeolite and metal-organic framework (MOF) catalysts using specialized autoclave treatments that create hierarchical pore structures, significantly improving diffusion limitations while maintaining high active site density[8]. Their patented "controlled delamination" technique during autoclave synthesis creates unique two-dimensional catalyst structures with exposed active sites that demonstrate substantially reduced activation energy requirements for complex molecular transformations.
Strengths: Industry-leading expertise in tailoring catalyst properties through precise autoclave synthesis parameters; ability to create hierarchical pore structures that optimize reactant/product diffusion; extensive intellectual property portfolio protecting synthesis methodologies. Weaknesses: High energy consumption during high-pressure autoclave synthesis; complex scale-up requirements for maintaining precise synthesis conditions; relatively high production costs compared to conventional precipitation methods.
Critical Patents in Autoclave-Based Catalyst Development
Phase switchable catalysts
PatentWO2009013525A1
Innovation
- A catalytic system where the catalyst's solubility is altered by the introduction or removal of carbon dioxide, allowing it to switch between aqueous and organic phases, facilitating enhanced reaction rates and easy recycling by manipulating the ligand's solubility with basic groups and amidine structures.
Compound internally-heated high-pressure apparatus for solvothermal crystal growth
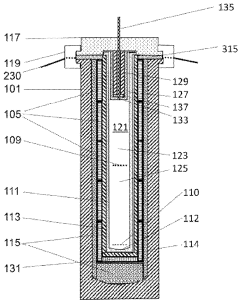
PatentWO2024020513A1
Innovation
- A compound internally-heated high-pressure apparatus with a cylindrical enclosure, a primary liner, load-bearing annular insulating members, and heating elements, allowing for radial and axial support, enabling pressures up to 500 MPa and temperatures between 200 and 900 degrees Celsius, while reducing costs and improving scalability.
Environmental Impact of Autoclave Synthesis Processes
Autoclave synthesis processes, while effective for catalyst preparation, pose significant environmental challenges that warrant careful consideration. The high-pressure and high-temperature conditions required for these processes consume substantial energy resources, contributing to increased carbon emissions. Typical autoclave operations for catalyst synthesis can consume between 5-15 kWh per batch, depending on reaction parameters and duration, representing a considerable carbon footprint in large-scale production scenarios.
Water usage presents another environmental concern, as autoclave synthesis often requires substantial volumes for cooling systems and post-synthesis washing procedures. Estimates suggest that producing one kilogram of catalyst material via autoclave methods may consume 50-200 liters of water, much of which becomes contaminated with metal ions and organic solvents during processing.
Chemical waste generation constitutes perhaps the most direct environmental impact. Autoclave synthesis of catalysts frequently involves heavy metals, organic solvents, and various reagents that create hazardous waste streams requiring specialized disposal. Studies indicate that for every kilogram of catalyst produced, approximately 5-10 kilograms of waste materials may be generated, including spent solvents, unreacted precursors, and byproducts.
The relationship between synthesis conditions and activation energy presents opportunities for environmental optimization. Research demonstrates that precisely controlled autoclave parameters can reduce reaction times by 30-40%, subsequently decreasing energy consumption. Furthermore, advanced autoclave designs incorporating heat recovery systems have shown potential to recapture up to 25% of thermal energy, significantly improving process efficiency.
Emerging green chemistry approaches offer promising alternatives to conventional autoclave synthesis. Hydrothermal methods utilizing subcritical water conditions can reduce organic solvent usage by 60-70% while maintaining catalyst performance characteristics. Similarly, microwave-assisted autoclave synthesis has demonstrated energy efficiency improvements of 40-50% compared to conventional heating methods, while often producing catalysts with enhanced activation energy profiles.
Regulatory frameworks increasingly address these environmental concerns, with jurisdictions implementing stricter controls on waste disposal and emissions from industrial synthesis processes. Companies engaged in catalyst production face growing pressure to adopt cleaner technologies and demonstrate environmental stewardship, driving innovation toward more sustainable autoclave synthesis methodologies.
Water usage presents another environmental concern, as autoclave synthesis often requires substantial volumes for cooling systems and post-synthesis washing procedures. Estimates suggest that producing one kilogram of catalyst material via autoclave methods may consume 50-200 liters of water, much of which becomes contaminated with metal ions and organic solvents during processing.
Chemical waste generation constitutes perhaps the most direct environmental impact. Autoclave synthesis of catalysts frequently involves heavy metals, organic solvents, and various reagents that create hazardous waste streams requiring specialized disposal. Studies indicate that for every kilogram of catalyst produced, approximately 5-10 kilograms of waste materials may be generated, including spent solvents, unreacted precursors, and byproducts.
The relationship between synthesis conditions and activation energy presents opportunities for environmental optimization. Research demonstrates that precisely controlled autoclave parameters can reduce reaction times by 30-40%, subsequently decreasing energy consumption. Furthermore, advanced autoclave designs incorporating heat recovery systems have shown potential to recapture up to 25% of thermal energy, significantly improving process efficiency.
Emerging green chemistry approaches offer promising alternatives to conventional autoclave synthesis. Hydrothermal methods utilizing subcritical water conditions can reduce organic solvent usage by 60-70% while maintaining catalyst performance characteristics. Similarly, microwave-assisted autoclave synthesis has demonstrated energy efficiency improvements of 40-50% compared to conventional heating methods, while often producing catalysts with enhanced activation energy profiles.
Regulatory frameworks increasingly address these environmental concerns, with jurisdictions implementing stricter controls on waste disposal and emissions from industrial synthesis processes. Companies engaged in catalyst production face growing pressure to adopt cleaner technologies and demonstrate environmental stewardship, driving innovation toward more sustainable autoclave synthesis methodologies.
Scale-up Considerations for Industrial Implementation
Scaling up autoclave synthesis processes from laboratory to industrial scale presents significant engineering challenges that directly impact catalyst activation energy and overall performance. The transition requires careful consideration of several critical factors to maintain consistent catalyst properties while achieving economic viability.
Heat and mass transfer characteristics change dramatically with increased vessel size, potentially altering reaction kinetics and activation energy profiles. Industrial autoclaves typically exhibit less uniform temperature distribution compared to laboratory equipment, necessitating modified heating protocols and extended reaction times to ensure complete catalyst activation. These modifications must be carefully calibrated to prevent undesired phase formations or incomplete crystallization that could compromise catalytic performance.
Pressure management becomes increasingly complex in larger vessels, requiring sophisticated control systems to maintain optimal conditions throughout the synthesis process. Pressure fluctuations can significantly impact activation energy barriers and reaction pathways, potentially altering the final catalyst structure and activity. Industrial implementation therefore demands robust pressure regulation mechanisms with appropriate safety margins and contingency protocols.
Mixing efficiency represents another critical parameter that changes with scale. Inadequate mixing in industrial autoclaves can create concentration gradients that lead to heterogeneous catalyst properties and variable activation energies across the batch. Advanced impeller designs and optimized agitation protocols must be developed to ensure homogeneous conditions throughout the reaction medium.
Material selection for industrial autoclaves requires careful consideration of corrosion resistance, thermal stability, and mechanical strength under prolonged exposure to reaction conditions. The vessel material can potentially interact with reaction precursors, introducing contaminants that may alter catalyst surface properties and activation energy profiles. Specialized alloys or protective linings are often necessary to maintain catalyst purity at industrial scale.
Process economics and energy efficiency become paramount considerations for commercial viability. The energy requirements for heating and pressurizing industrial autoclaves are substantial, necessitating heat recovery systems and optimized thermal cycles. The relationship between energy input, reaction time, and catalyst activation must be carefully balanced to minimize production costs while maintaining product quality.
Quality control protocols must be adapted for industrial-scale production, with in-process monitoring techniques to verify that activation energy parameters remain within acceptable ranges throughout the synthesis. Advanced analytical methods, potentially including in-situ spectroscopic techniques, may be required to ensure batch-to-batch consistency and detect deviations before they impact final catalyst performance.
Heat and mass transfer characteristics change dramatically with increased vessel size, potentially altering reaction kinetics and activation energy profiles. Industrial autoclaves typically exhibit less uniform temperature distribution compared to laboratory equipment, necessitating modified heating protocols and extended reaction times to ensure complete catalyst activation. These modifications must be carefully calibrated to prevent undesired phase formations or incomplete crystallization that could compromise catalytic performance.
Pressure management becomes increasingly complex in larger vessels, requiring sophisticated control systems to maintain optimal conditions throughout the synthesis process. Pressure fluctuations can significantly impact activation energy barriers and reaction pathways, potentially altering the final catalyst structure and activity. Industrial implementation therefore demands robust pressure regulation mechanisms with appropriate safety margins and contingency protocols.
Mixing efficiency represents another critical parameter that changes with scale. Inadequate mixing in industrial autoclaves can create concentration gradients that lead to heterogeneous catalyst properties and variable activation energies across the batch. Advanced impeller designs and optimized agitation protocols must be developed to ensure homogeneous conditions throughout the reaction medium.
Material selection for industrial autoclaves requires careful consideration of corrosion resistance, thermal stability, and mechanical strength under prolonged exposure to reaction conditions. The vessel material can potentially interact with reaction precursors, introducing contaminants that may alter catalyst surface properties and activation energy profiles. Specialized alloys or protective linings are often necessary to maintain catalyst purity at industrial scale.
Process economics and energy efficiency become paramount considerations for commercial viability. The energy requirements for heating and pressurizing industrial autoclaves are substantial, necessitating heat recovery systems and optimized thermal cycles. The relationship between energy input, reaction time, and catalyst activation must be carefully balanced to minimize production costs while maintaining product quality.
Quality control protocols must be adapted for industrial-scale production, with in-process monitoring techniques to verify that activation energy parameters remain within acceptable ranges throughout the synthesis. Advanced analytical methods, potentially including in-situ spectroscopic techniques, may be required to ensure batch-to-batch consistency and detect deviations before they impact final catalyst performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!