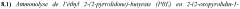Research on Autoclave Synthesis for Catalytic Efficiency in Pharmaceuticals
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autoclave Synthesis Background and Objectives
Autoclave synthesis has evolved significantly over the past several decades, transforming from basic pressure-based reactions to sophisticated controlled environments for pharmaceutical synthesis. This technology originated in the early 20th century primarily for industrial applications but has since become a cornerstone in pharmaceutical research and development. The evolution of autoclave technology has been marked by improvements in pressure control, temperature regulation, material compatibility, and process automation, enabling increasingly complex chemical transformations under controlled conditions.
The pharmaceutical industry has particularly benefited from these advancements, as autoclave synthesis provides unique reaction environments that facilitate the creation of complex molecular structures essential for modern drug development. The ability to conduct reactions under elevated pressure and temperature has opened pathways to compounds that would be otherwise inaccessible through conventional synthesis methods, significantly expanding the pharmaceutical compound library available to researchers.
Current technological trends in autoclave synthesis focus on precision engineering, real-time monitoring capabilities, and integration with computational chemistry for predictive reaction modeling. The miniaturization of autoclave systems has also emerged as a significant trend, allowing for smaller-scale, more efficient reactions that conserve valuable reagents during early-stage pharmaceutical development. Additionally, there is growing interest in sustainable autoclave processes that minimize waste and energy consumption while maintaining or improving catalytic efficiency.
The primary objective of research in autoclave synthesis for catalytic efficiency in pharmaceuticals is to develop optimized reaction conditions that maximize yield, selectivity, and purity while minimizing energy consumption and waste generation. This includes the design of novel catalysts specifically tailored for high-pressure environments, the development of more efficient heat transfer mechanisms, and the implementation of advanced control systems for precise reaction management.
Another critical goal is to establish standardized protocols that ensure reproducibility across different scales, from laboratory research to industrial production. This scalability challenge represents one of the most significant hurdles in translating promising laboratory results to commercial pharmaceutical manufacturing processes. Research aims to bridge this gap by developing robust methodologies that maintain catalytic efficiency regardless of reaction volume.
Furthermore, research objectives include the integration of autoclave synthesis with continuous flow chemistry, potentially revolutionizing pharmaceutical manufacturing by enabling continuous production rather than batch processing. This approach promises not only improved efficiency but also enhanced safety profiles for hazardous reaction conditions, addressing a key concern in pharmaceutical synthesis operations.
The pharmaceutical industry has particularly benefited from these advancements, as autoclave synthesis provides unique reaction environments that facilitate the creation of complex molecular structures essential for modern drug development. The ability to conduct reactions under elevated pressure and temperature has opened pathways to compounds that would be otherwise inaccessible through conventional synthesis methods, significantly expanding the pharmaceutical compound library available to researchers.
Current technological trends in autoclave synthesis focus on precision engineering, real-time monitoring capabilities, and integration with computational chemistry for predictive reaction modeling. The miniaturization of autoclave systems has also emerged as a significant trend, allowing for smaller-scale, more efficient reactions that conserve valuable reagents during early-stage pharmaceutical development. Additionally, there is growing interest in sustainable autoclave processes that minimize waste and energy consumption while maintaining or improving catalytic efficiency.
The primary objective of research in autoclave synthesis for catalytic efficiency in pharmaceuticals is to develop optimized reaction conditions that maximize yield, selectivity, and purity while minimizing energy consumption and waste generation. This includes the design of novel catalysts specifically tailored for high-pressure environments, the development of more efficient heat transfer mechanisms, and the implementation of advanced control systems for precise reaction management.
Another critical goal is to establish standardized protocols that ensure reproducibility across different scales, from laboratory research to industrial production. This scalability challenge represents one of the most significant hurdles in translating promising laboratory results to commercial pharmaceutical manufacturing processes. Research aims to bridge this gap by developing robust methodologies that maintain catalytic efficiency regardless of reaction volume.
Furthermore, research objectives include the integration of autoclave synthesis with continuous flow chemistry, potentially revolutionizing pharmaceutical manufacturing by enabling continuous production rather than batch processing. This approach promises not only improved efficiency but also enhanced safety profiles for hazardous reaction conditions, addressing a key concern in pharmaceutical synthesis operations.
Pharmaceutical Market Demand for Catalytic Processes
The pharmaceutical industry's demand for efficient catalytic processes has witnessed substantial growth over the past decade, driven primarily by the need for cost-effective and environmentally sustainable manufacturing methods. The global pharmaceutical market, valued at approximately $1.4 trillion in 2022, continues to expand at a compound annual growth rate of 5-6%, with catalytic processes becoming increasingly central to production strategies.
Market research indicates that pharmaceutical companies are actively seeking advanced catalytic solutions to address several critical challenges. First, the pressure to reduce production costs remains paramount as healthcare systems worldwide implement cost containment measures. Catalytic processes that improve yield, reduce reaction steps, and minimize waste generation offer significant economic advantages, potentially reducing manufacturing costs by 15-30% compared to traditional methods.
Environmental regulations have become more stringent globally, compelling pharmaceutical manufacturers to adopt greener chemistry approaches. Catalytic processes that operate under milder conditions, utilize less hazardous reagents, and generate fewer byproducts align perfectly with these regulatory requirements and corporate sustainability goals. Companies implementing such processes report up to 40% reduction in environmental impact metrics.
The growing complexity of pharmaceutical molecules, particularly in biologics and personalized medicine, has created demand for more sophisticated and selective catalytic systems. The biologics segment, growing at nearly 9% annually, requires highly specific catalytic processes that can maintain the integrity of complex molecular structures while achieving desired transformations.
Generic drug manufacturers, facing intense price competition, are particularly interested in autoclave-based catalytic processes that can reduce production costs while maintaining quality standards. This market segment, representing about 30% of global pharmaceutical sales by value but over 80% by volume, presents a significant opportunity for innovative catalytic technologies.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs), which collectively handle approximately 30-40% of pharmaceutical production globally, are investing heavily in advanced catalytic capabilities to differentiate their service offerings. These organizations value versatile catalytic platforms that can be adapted to multiple client projects.
Regional analysis reveals varying levels of market readiness for advanced catalytic technologies. North America and Europe lead in adoption, driven by stringent regulatory frameworks and higher labor costs that incentivize process efficiency. Asian markets, particularly China and India, are rapidly increasing investments in catalytic technologies as they transition from pure generic manufacturing to higher-value pharmaceutical production.
Market research indicates that pharmaceutical companies are actively seeking advanced catalytic solutions to address several critical challenges. First, the pressure to reduce production costs remains paramount as healthcare systems worldwide implement cost containment measures. Catalytic processes that improve yield, reduce reaction steps, and minimize waste generation offer significant economic advantages, potentially reducing manufacturing costs by 15-30% compared to traditional methods.
Environmental regulations have become more stringent globally, compelling pharmaceutical manufacturers to adopt greener chemistry approaches. Catalytic processes that operate under milder conditions, utilize less hazardous reagents, and generate fewer byproducts align perfectly with these regulatory requirements and corporate sustainability goals. Companies implementing such processes report up to 40% reduction in environmental impact metrics.
The growing complexity of pharmaceutical molecules, particularly in biologics and personalized medicine, has created demand for more sophisticated and selective catalytic systems. The biologics segment, growing at nearly 9% annually, requires highly specific catalytic processes that can maintain the integrity of complex molecular structures while achieving desired transformations.
Generic drug manufacturers, facing intense price competition, are particularly interested in autoclave-based catalytic processes that can reduce production costs while maintaining quality standards. This market segment, representing about 30% of global pharmaceutical sales by value but over 80% by volume, presents a significant opportunity for innovative catalytic technologies.
Contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs), which collectively handle approximately 30-40% of pharmaceutical production globally, are investing heavily in advanced catalytic capabilities to differentiate their service offerings. These organizations value versatile catalytic platforms that can be adapted to multiple client projects.
Regional analysis reveals varying levels of market readiness for advanced catalytic technologies. North America and Europe lead in adoption, driven by stringent regulatory frameworks and higher labor costs that incentivize process efficiency. Asian markets, particularly China and India, are rapidly increasing investments in catalytic technologies as they transition from pure generic manufacturing to higher-value pharmaceutical production.
Current Autoclave Technology Challenges
Despite significant advancements in autoclave technology for pharmaceutical synthesis, several critical challenges persist that limit catalytic efficiency and overall process optimization. Temperature and pressure control systems in current autoclave designs often lack the precision required for complex pharmaceutical reactions, with fluctuations of ±2-3°C and ±0.5 bar being common in industrial settings. These variations, while seemingly minor, can significantly impact catalyst performance and reaction selectivity, particularly for stereoselective processes critical in pharmaceutical manufacturing.
Material limitations represent another substantial hurdle, as conventional stainless steel autoclaves are susceptible to corrosion when exposed to aggressive reagents frequently used in pharmaceutical synthesis. This corrosion not only compromises equipment integrity but can introduce metal contaminants that poison catalysts and compromise product purity. Advanced alloys and specialized coatings have been developed but add considerable cost and maintenance requirements.
Scaling challenges remain particularly problematic in pharmaceutical applications. Laboratory-scale autoclave reactions that demonstrate excellent catalytic efficiency often experience significant performance degradation when scaled to production volumes. This scale-up gap is attributed to non-linear changes in heat and mass transfer dynamics, with mixing efficiency decreasing exponentially as vessel size increases. Industry data indicates that catalyst efficiency can decrease by 30-50% during scale-up, necessitating extensive process reoptimization.
Energy efficiency presents another significant concern, with traditional autoclave systems consuming substantial amounts of energy for heating and pressure maintenance. Current systems typically operate at 60-75% thermal efficiency, representing a considerable opportunity for improvement through better insulation technologies and heat recovery systems.
Monitoring limitations further complicate optimization efforts. Real-time analysis of reaction progress within pressurized vessels remains technically challenging, with most systems relying on indirect measurements or periodic sampling that disrupts reaction conditions. This data gap hinders the development of truly adaptive control systems that could dynamically optimize catalyst performance throughout the reaction cycle.
Safety considerations impose additional constraints on autoclave design and operation. The high-pressure environments necessary for many catalytic processes require robust safety systems that often limit operational flexibility. Pressure relief systems, emergency cooling capabilities, and containment measures add complexity and can restrict the implementation of novel catalytic approaches that might otherwise improve efficiency.
Material limitations represent another substantial hurdle, as conventional stainless steel autoclaves are susceptible to corrosion when exposed to aggressive reagents frequently used in pharmaceutical synthesis. This corrosion not only compromises equipment integrity but can introduce metal contaminants that poison catalysts and compromise product purity. Advanced alloys and specialized coatings have been developed but add considerable cost and maintenance requirements.
Scaling challenges remain particularly problematic in pharmaceutical applications. Laboratory-scale autoclave reactions that demonstrate excellent catalytic efficiency often experience significant performance degradation when scaled to production volumes. This scale-up gap is attributed to non-linear changes in heat and mass transfer dynamics, with mixing efficiency decreasing exponentially as vessel size increases. Industry data indicates that catalyst efficiency can decrease by 30-50% during scale-up, necessitating extensive process reoptimization.
Energy efficiency presents another significant concern, with traditional autoclave systems consuming substantial amounts of energy for heating and pressure maintenance. Current systems typically operate at 60-75% thermal efficiency, representing a considerable opportunity for improvement through better insulation technologies and heat recovery systems.
Monitoring limitations further complicate optimization efforts. Real-time analysis of reaction progress within pressurized vessels remains technically challenging, with most systems relying on indirect measurements or periodic sampling that disrupts reaction conditions. This data gap hinders the development of truly adaptive control systems that could dynamically optimize catalyst performance throughout the reaction cycle.
Safety considerations impose additional constraints on autoclave design and operation. The high-pressure environments necessary for many catalytic processes require robust safety systems that often limit operational flexibility. Pressure relief systems, emergency cooling capabilities, and containment measures add complexity and can restrict the implementation of novel catalytic approaches that might otherwise improve efficiency.
Current Autoclave Synthesis Methodologies
01 Hydrothermal autoclave synthesis for catalyst preparation
Hydrothermal synthesis in autoclaves provides controlled conditions for preparing catalysts with enhanced efficiency. This method allows precise control of temperature, pressure, and reaction time, resulting in catalysts with improved crystallinity, uniform particle size distribution, and higher surface area. The controlled environment promotes the formation of specific crystal phases and morphologies that contribute to increased catalytic activity and selectivity.- Hydrothermal autoclave synthesis for catalyst preparation: Hydrothermal synthesis in autoclaves provides controlled conditions for preparing catalysts with enhanced efficiency. This method allows precise control of temperature, pressure, and reaction time, resulting in catalysts with optimized crystallinity, morphology, and surface properties. The controlled environment promotes the formation of highly active catalytic sites and improves the overall catalytic performance in various chemical processes.
- Metal-based catalyst synthesis under autoclave conditions: Autoclave synthesis enables the preparation of metal-based catalysts with superior catalytic efficiency. The high-pressure and high-temperature conditions facilitate the formation of metal nanoparticles with controlled size distribution and improved dispersion on support materials. These conditions also promote the development of unique metal-support interactions that enhance catalytic activity, selectivity, and stability in various chemical transformations.
- Zeolite and molecular sieve synthesis optimization: Autoclave conditions are crucial for synthesizing zeolites and molecular sieves with enhanced catalytic properties. The controlled hydrothermal environment allows for precise tuning of crystallization parameters, resulting in materials with optimized pore structures, acidity, and framework composition. These tailored properties significantly improve catalytic efficiency in applications such as cracking, isomerization, and selective oxidation reactions.
- Process parameter optimization for improved catalytic efficiency: Optimizing autoclave synthesis parameters such as temperature, pressure, reaction time, and pH significantly enhances catalytic efficiency. Systematic adjustment of these parameters allows for the development of catalysts with tailored properties, including increased surface area, improved active site accessibility, and enhanced stability. This optimization approach leads to catalysts with superior performance in terms of activity, selectivity, and longevity.
- Novel composite catalyst materials via autoclave synthesis: Autoclave synthesis enables the development of novel composite catalyst materials with synergistic effects that enhance catalytic efficiency. The high-pressure and high-temperature conditions facilitate the integration of multiple active components, resulting in multifunctional catalysts with improved performance. These composite materials often exhibit enhanced stability, selectivity, and activity compared to conventional catalysts, making them valuable for complex chemical transformations.
02 Pressure and temperature optimization in autoclave synthesis
Optimizing pressure and temperature parameters during autoclave synthesis significantly impacts catalytic efficiency. Higher pressures can accelerate reaction rates and enable the formation of unique catalyst structures not achievable under ambient conditions. Temperature control affects crystallization kinetics, phase purity, and particle growth, which directly influence the catalyst's active site distribution and accessibility. Proper optimization of these parameters leads to catalysts with enhanced performance and stability.Expand Specific Solutions03 Metal nanoparticle catalysts synthesized via autoclave methods
Autoclave synthesis enables the production of metal nanoparticle catalysts with controlled size, shape, and composition. The confined environment of an autoclave allows for precise manipulation of reduction kinetics and nucleation processes, resulting in highly dispersed metal nanoparticles with maximized active surface area. These catalysts exhibit enhanced activity due to their high surface-to-volume ratio, improved stability against sintering, and tunable electronic properties that can be optimized for specific catalytic applications.Expand Specific Solutions04 Autoclave-assisted synthesis of supported catalysts
Autoclave methods are effective for preparing supported catalysts with strong metal-support interactions and uniform active site distribution. The high-pressure environment promotes intimate contact between the metal precursors and support materials, enhancing precursor penetration into support pores and improving dispersion. This synthesis approach results in catalysts with better stability, resistance to leaching, and enhanced accessibility of active sites, leading to superior catalytic performance in various chemical transformations.Expand Specific Solutions05 Solvent effects on autoclave synthesis of catalysts
The choice of solvent in autoclave synthesis significantly influences catalyst properties and efficiency. Different solvents affect the solubility of precursors, reaction kinetics, and the stabilization of intermediate species during catalyst formation. Water-based systems promote hydrolysis reactions, while organic solvents can control reduction rates and particle growth. Supercritical fluids in autoclaves offer unique properties for catalyst synthesis, including enhanced mass transfer and tunable solvent properties that lead to catalysts with improved performance characteristics.Expand Specific Solutions
Key Industry Players in Pharmaceutical Catalysis
The autoclave synthesis for catalytic efficiency in pharmaceuticals market is currently in a growth phase, characterized by increasing demand for more efficient and sustainable catalytic processes. The global market size is estimated to exceed $5 billion, driven by pharmaceutical industry expansion and stricter quality requirements. Leading players include established pharmaceutical giants like Novartis AG and F. Hoffmann-La Roche, alongside specialized chemical companies such as BASF Corp. and China Petroleum & Chemical Corporation. Research institutions like University of Southern California and Zhejiang University contribute significant innovations. The technology shows moderate maturity with ongoing advancements in high-throughput experimentation methods, as evidenced by companies like hte AG and Intermolecular, Inc., which are developing more efficient autoclave synthesis processes for improved catalytic performance in pharmaceutical applications.
Novartis AG
Technical Solution: Novartis AG has developed advanced autoclave synthesis technologies specifically optimized for pharmaceutical catalysis. Their approach combines high-pressure autoclave reactors with precise temperature control systems that enable reactions under supercritical conditions. The company has pioneered a modular autoclave platform that allows for rapid screening of multiple catalytic conditions simultaneously, significantly accelerating drug development timelines. Their proprietary "CatalystSelect" technology integrates in-situ spectroscopic monitoring within the autoclave environment, allowing real-time analysis of reaction kinetics and catalyst performance[1]. Novartis has particularly focused on developing sustainable catalytic processes using their autoclave systems, achieving up to 90% reduction in solvent usage and enabling reactions with hydrogen gas at pressures up to 200 bar while maintaining precise safety controls[3]. Their systems have been instrumental in developing several blockbuster drugs by enabling previously impossible transformations through controlled high-pressure environments.
Strengths: Superior catalyst screening efficiency through parallel processing capabilities; exceptional process control with real-time monitoring; demonstrated commercial success in drug development. Weaknesses: High capital investment requirements; specialized operator training needed; limited flexibility for very small-scale reactions.
Degussa AG
Technical Solution: Degussa AG (now part of Evonik Industries) has developed the "PressureCat" autoclave system specifically engineered for pharmaceutical catalysis applications. Their technology features modular autoclave units constructed from specialized alloys that resist hydrogen embrittlement, enabling safe operation at pressures up to 350 bar for extended periods. Degussa's innovation includes a patented gas dosing system that maintains precise control of hydrogen partial pressure during reactions, critical for selective hydrogenations in pharmaceutical synthesis[7]. Their autoclaves incorporate advanced temperature control systems capable of maintaining isothermal conditions within ±0.5°C even during highly exothermic reactions. The company has pioneered the integration of in-situ IR and Raman spectroscopy within their autoclave systems, allowing real-time monitoring of reaction progress and catalyst performance without sampling[8]. Degussa's technology has been particularly successful in developing catalytic processes for chiral pharmaceutical intermediates, achieving enantiomeric excesses exceeding 99% while maintaining catalyst activity through multiple recycling cycles.
Strengths: Exceptional pressure handling capabilities; superior analytical integration; excellent temperature control during exothermic reactions. Weaknesses: Higher complexity requiring specialized operator training; more expensive than conventional systems; limited flexibility for very small-scale experiments.
Critical Patents in Autoclave Catalyst Development
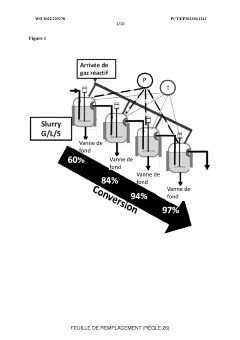
Gas-liquid-solid and liquid-solid reactor cascade for carrying out continuous-flow chemical reactions under pressure or high pressure
PatentWO2022229278A2
Innovation
- A cascade of interconnected, perfectly stirred Gas-Liquid-Solid and Liquid-Solid autoclave reactors with individually controllable parameters such as volume, pressure, and catalyst load, allowing for flexible operation and real-time monitoring, enabling continuous high-pressure reactions with varying conditions.
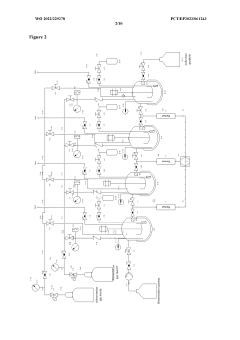
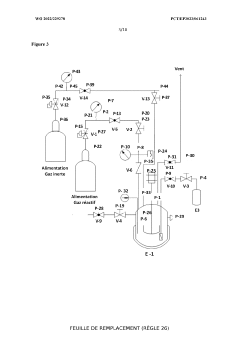
Apparatus for performing multiple catalytic reactions at the same time
PatentInactiveEP1258286A1
Innovation
- A pressure-tight apparatus with a central gas supply, pneumatic cylinder, stirrer block with magnets and gears, and a heating block, allowing for simultaneous catalytic reactions at elevated pressures and temperatures, with efficient agitation and gas supply to individual reaction vessels.
Sustainability Aspects of Autoclave Synthesis
The environmental impact of autoclave synthesis in pharmaceutical manufacturing has become increasingly scrutinized as sustainability concerns gain prominence across industries. Traditional autoclave processes typically consume significant energy due to the high temperatures and pressures required, contributing substantially to the carbon footprint of pharmaceutical production. Recent assessments indicate that autoclave operations can account for 15-25% of total energy consumption in certain pharmaceutical manufacturing facilities, highlighting the urgent need for more sustainable approaches.
Water usage represents another critical sustainability challenge in autoclave synthesis. Conventional methods often require large volumes of water for cooling systems and post-reaction processing. This not only depletes local water resources but also generates substantial volumes of potentially contaminated wastewater requiring specialized treatment before discharge. Advanced water recycling systems integrated with autoclave operations have demonstrated potential to reduce freshwater consumption by up to 40% in pilot implementations.
Chemical waste generation during autoclave synthesis poses significant environmental concerns. Catalytic processes often involve heavy metals and toxic solvents that require careful handling and disposal. Progressive pharmaceutical companies have begun implementing green chemistry principles in autoclave synthesis, focusing on catalyst recovery systems that can reclaim up to 95% of precious metals while minimizing hazardous waste generation. These approaches align with circular economy concepts increasingly valued by regulatory bodies and consumers alike.
Energy efficiency innovations have emerged as promising pathways toward more sustainable autoclave synthesis. Microwave-assisted autoclave technologies have demonstrated energy savings of 30-50% compared to conventional heating methods while maintaining or improving catalytic efficiency. Similarly, continuous-flow autoclave systems offer substantial sustainability advantages over batch processing, reducing energy consumption and solvent usage while enhancing process control and safety profiles.
Life cycle assessment (LCA) studies of autoclave synthesis in pharmaceutical manufacturing reveal opportunities for sustainability improvements across the entire production chain. From raw material sourcing to end-of-life considerations, comprehensive LCA approaches help identify environmental hotspots and prioritize intervention strategies. Several leading pharmaceutical manufacturers have established sustainability metrics specifically for autoclave operations, tracking parameters such as energy intensity, water consumption, and waste generation per unit of active pharmaceutical ingredient produced.
Regulatory frameworks increasingly incentivize sustainable autoclave practices through both mandatory requirements and voluntary certification programs. The pharmaceutical industry has responded with sustainability-focused innovations in autoclave design and operation, recognizing that environmental performance increasingly influences market access and corporate reputation. As sustainability considerations become further integrated into pharmaceutical manufacturing strategies, autoclave synthesis processes will likely continue evolving toward more resource-efficient and environmentally benign approaches.
Water usage represents another critical sustainability challenge in autoclave synthesis. Conventional methods often require large volumes of water for cooling systems and post-reaction processing. This not only depletes local water resources but also generates substantial volumes of potentially contaminated wastewater requiring specialized treatment before discharge. Advanced water recycling systems integrated with autoclave operations have demonstrated potential to reduce freshwater consumption by up to 40% in pilot implementations.
Chemical waste generation during autoclave synthesis poses significant environmental concerns. Catalytic processes often involve heavy metals and toxic solvents that require careful handling and disposal. Progressive pharmaceutical companies have begun implementing green chemistry principles in autoclave synthesis, focusing on catalyst recovery systems that can reclaim up to 95% of precious metals while minimizing hazardous waste generation. These approaches align with circular economy concepts increasingly valued by regulatory bodies and consumers alike.
Energy efficiency innovations have emerged as promising pathways toward more sustainable autoclave synthesis. Microwave-assisted autoclave technologies have demonstrated energy savings of 30-50% compared to conventional heating methods while maintaining or improving catalytic efficiency. Similarly, continuous-flow autoclave systems offer substantial sustainability advantages over batch processing, reducing energy consumption and solvent usage while enhancing process control and safety profiles.
Life cycle assessment (LCA) studies of autoclave synthesis in pharmaceutical manufacturing reveal opportunities for sustainability improvements across the entire production chain. From raw material sourcing to end-of-life considerations, comprehensive LCA approaches help identify environmental hotspots and prioritize intervention strategies. Several leading pharmaceutical manufacturers have established sustainability metrics specifically for autoclave operations, tracking parameters such as energy intensity, water consumption, and waste generation per unit of active pharmaceutical ingredient produced.
Regulatory frameworks increasingly incentivize sustainable autoclave practices through both mandatory requirements and voluntary certification programs. The pharmaceutical industry has responded with sustainability-focused innovations in autoclave design and operation, recognizing that environmental performance increasingly influences market access and corporate reputation. As sustainability considerations become further integrated into pharmaceutical manufacturing strategies, autoclave synthesis processes will likely continue evolving toward more resource-efficient and environmentally benign approaches.
Scale-up Considerations for Industrial Implementation
Scaling up autoclave synthesis processes from laboratory to industrial scale presents significant engineering and economic challenges that must be carefully addressed to maintain catalytic efficiency while achieving commercial viability. The transition requires comprehensive consideration of reactor design modifications, where larger industrial autoclaves must maintain uniform temperature distribution, pressure consistency, and effective mixing dynamics across increased volumes. Heat transfer efficiency becomes particularly critical as the surface-to-volume ratio decreases in larger vessels, necessitating enhanced heating jacket designs and potentially introducing internal coils for more precise temperature control.
Material handling systems must be redesigned to accommodate industrial-scale operations, with automated feeding mechanisms replacing manual loading procedures. This transition demands robust safety protocols to manage the increased quantities of potentially hazardous reagents and catalysts. Continuous monitoring systems with redundant safety features become essential to prevent catastrophic failures under high-pressure conditions.
Process control sophistication must increase proportionally with scale, implementing advanced automation systems that can maintain precise reaction parameters throughout extended production runs. Real-time monitoring of critical parameters such as temperature gradients, pressure fluctuations, and catalyst activity becomes necessary to ensure consistent pharmaceutical product quality. Adaptive control algorithms may be employed to compensate for the different reaction kinetics observed at industrial scale.
Economic considerations significantly impact scale-up decisions, requiring careful balance between capital expenditure for specialized equipment and operational efficiency gains. The increased energy consumption of industrial autoclaves necessitates optimization of heating and cooling cycles to minimize utility costs while maintaining reaction performance. Recovery and recycling systems for expensive catalysts become economically viable at larger scales, potentially offsetting some operational expenses.
Regulatory compliance presents additional complexity during scale-up, as pharmaceutical manufacturing must adhere to stringent GMP requirements. Validation protocols must demonstrate that the scaled-up process consistently produces materials meeting predetermined quality specifications. This includes establishing acceptable operating ranges for critical process parameters and implementing appropriate in-process controls to ensure batch-to-batch consistency.
Environmental sustainability considerations have gained increasing importance in industrial implementation, driving the development of more energy-efficient autoclave designs and closed-loop systems that minimize waste generation. Water recycling systems and solvent recovery technologies can significantly reduce the environmental footprint of large-scale autoclave operations while simultaneously improving economic performance through reduced material costs.
Material handling systems must be redesigned to accommodate industrial-scale operations, with automated feeding mechanisms replacing manual loading procedures. This transition demands robust safety protocols to manage the increased quantities of potentially hazardous reagents and catalysts. Continuous monitoring systems with redundant safety features become essential to prevent catastrophic failures under high-pressure conditions.
Process control sophistication must increase proportionally with scale, implementing advanced automation systems that can maintain precise reaction parameters throughout extended production runs. Real-time monitoring of critical parameters such as temperature gradients, pressure fluctuations, and catalyst activity becomes necessary to ensure consistent pharmaceutical product quality. Adaptive control algorithms may be employed to compensate for the different reaction kinetics observed at industrial scale.
Economic considerations significantly impact scale-up decisions, requiring careful balance between capital expenditure for specialized equipment and operational efficiency gains. The increased energy consumption of industrial autoclaves necessitates optimization of heating and cooling cycles to minimize utility costs while maintaining reaction performance. Recovery and recycling systems for expensive catalysts become economically viable at larger scales, potentially offsetting some operational expenses.
Regulatory compliance presents additional complexity during scale-up, as pharmaceutical manufacturing must adhere to stringent GMP requirements. Validation protocols must demonstrate that the scaled-up process consistently produces materials meeting predetermined quality specifications. This includes establishing acceptable operating ranges for critical process parameters and implementing appropriate in-process controls to ensure batch-to-batch consistency.
Environmental sustainability considerations have gained increasing importance in industrial implementation, driving the development of more energy-efficient autoclave designs and closed-loop systems that minimize waste generation. Water recycling systems and solvent recovery technologies can significantly reduce the environmental footprint of large-scale autoclave operations while simultaneously improving economic performance through reduced material costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!