Analysis of Dual-ion batteries for electrode kinetics and ion diffusion performance
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dual-ion Battery Technology Evolution and Objectives
Dual-ion batteries (DIBs) have emerged as a promising energy storage technology, evolving from conventional lithium-ion battery concepts but with distinct operational mechanisms. The development of DIBs can be traced back to the early 2000s when researchers began exploring alternative battery chemistries to address the limitations of traditional lithium-ion systems. Unlike conventional batteries where only cations participate in the electrochemical process, DIBs utilize both cations and anions for charge storage, potentially doubling the energy density.
The evolution of DIB technology has been marked by several significant milestones. Initially, graphite was predominantly used as both cathode and anode materials, with various electrolyte systems. By 2012, researchers demonstrated DIBs with energy densities approaching 100 Wh/kg, establishing their potential as viable energy storage devices. The period between 2015-2020 witnessed substantial improvements in electrode materials, electrolyte formulations, and cell designs, pushing performance boundaries further.
A critical aspect of DIB development has been understanding and enhancing electrode kinetics and ion diffusion performance. Early DIB systems suffered from slow ion transport, limiting power capabilities and cycling stability. Research has progressively focused on optimizing electrode structures to facilitate faster ion diffusion pathways and reduce kinetic barriers at electrode-electrolyte interfaces.
The technological objectives for DIB development are multifaceted. Primary goals include achieving higher energy densities exceeding 200 Wh/kg, improving rate capabilities through enhanced electrode kinetics, and extending cycle life beyond 1000 cycles with minimal capacity degradation. Additionally, researchers aim to develop DIBs that operate efficiently across wider temperature ranges and demonstrate improved safety characteristics compared to conventional lithium-ion batteries.
Recent technological trends indicate a shift toward novel electrode materials with hierarchical structures that facilitate rapid ion diffusion. Advanced characterization techniques, including in-situ electrochemical impedance spectroscopy and operando X-ray diffraction, have enabled deeper insights into ion transport mechanisms within DIB systems. Computational modeling approaches are increasingly employed to predict and optimize electrode-electrolyte interactions at the molecular level.
The future trajectory of DIB technology is likely to focus on developing electrode materials with precisely engineered porosity and surface chemistry to maximize ion diffusion rates while maintaining structural integrity during repeated charge-discharge cycles. Electrolyte innovations will target wider electrochemical stability windows and improved ionic conductivity, addressing current limitations in voltage range and power density.
The evolution of DIB technology has been marked by several significant milestones. Initially, graphite was predominantly used as both cathode and anode materials, with various electrolyte systems. By 2012, researchers demonstrated DIBs with energy densities approaching 100 Wh/kg, establishing their potential as viable energy storage devices. The period between 2015-2020 witnessed substantial improvements in electrode materials, electrolyte formulations, and cell designs, pushing performance boundaries further.
A critical aspect of DIB development has been understanding and enhancing electrode kinetics and ion diffusion performance. Early DIB systems suffered from slow ion transport, limiting power capabilities and cycling stability. Research has progressively focused on optimizing electrode structures to facilitate faster ion diffusion pathways and reduce kinetic barriers at electrode-electrolyte interfaces.
The technological objectives for DIB development are multifaceted. Primary goals include achieving higher energy densities exceeding 200 Wh/kg, improving rate capabilities through enhanced electrode kinetics, and extending cycle life beyond 1000 cycles with minimal capacity degradation. Additionally, researchers aim to develop DIBs that operate efficiently across wider temperature ranges and demonstrate improved safety characteristics compared to conventional lithium-ion batteries.
Recent technological trends indicate a shift toward novel electrode materials with hierarchical structures that facilitate rapid ion diffusion. Advanced characterization techniques, including in-situ electrochemical impedance spectroscopy and operando X-ray diffraction, have enabled deeper insights into ion transport mechanisms within DIB systems. Computational modeling approaches are increasingly employed to predict and optimize electrode-electrolyte interactions at the molecular level.
The future trajectory of DIB technology is likely to focus on developing electrode materials with precisely engineered porosity and surface chemistry to maximize ion diffusion rates while maintaining structural integrity during repeated charge-discharge cycles. Electrolyte innovations will target wider electrochemical stability windows and improved ionic conductivity, addressing current limitations in voltage range and power density.
Market Analysis for Advanced Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing adoption of renewable energy sources and the electrification of transportation. Within this landscape, dual-ion batteries (DIBs) are emerging as a promising technology that addresses several limitations of conventional lithium-ion batteries. The market for advanced energy storage solutions is projected to reach $546 billion by 2035, with a compound annual growth rate of 15.2% between 2023 and 2035.
Dual-ion batteries represent a significant opportunity within this market due to their potential cost advantages and performance characteristics. Unlike traditional lithium-ion batteries that rely solely on cation intercalation, DIBs utilize both cation and anion intercalation processes, potentially offering higher energy densities and improved electrode kinetics. This fundamental difference creates market differentiation that could disrupt established battery technologies.
Consumer electronics currently dominates the demand for high-performance batteries, accounting for approximately 38% of the advanced energy storage market. However, electric vehicles are rapidly becoming the largest growth segment, with projections indicating they will represent over 50% of battery demand by 2030. Grid storage applications are also expanding at 22% annually as renewable energy integration accelerates globally.
The market analysis reveals significant regional variations in DIB technology adoption potential. Asia-Pacific leads manufacturing capacity development, with China, Japan, and South Korea collectively controlling 75% of battery production capabilities. European markets are increasingly focused on sustainable battery technologies, with regulatory frameworks actively promoting innovations that reduce environmental impact and resource dependency.
North American markets show strong interest in DIBs for grid-scale applications, particularly as renewable energy penetration increases. The region's substantial investment in energy storage research ($4.8 billion in 2022) provides a favorable environment for commercializing advanced electrode kinetics and ion diffusion technologies.
Customer requirements across these markets consistently emphasize several performance metrics where DIBs could excel: faster charging capabilities, longer cycle life, improved safety profiles, and reduced dependency on critical raw materials. The enhanced electrode kinetics of DIBs potentially addresses the first two requirements directly, creating significant market pull for this technology.
Competition in this space includes established battery manufacturers pivoting toward next-generation technologies and specialized startups focused exclusively on DIB development. Market entry barriers remain substantial due to manufacturing scale requirements and established supply chains, but partnerships between technology developers and existing manufacturers offer viable commercialization pathways.
Dual-ion batteries represent a significant opportunity within this market due to their potential cost advantages and performance characteristics. Unlike traditional lithium-ion batteries that rely solely on cation intercalation, DIBs utilize both cation and anion intercalation processes, potentially offering higher energy densities and improved electrode kinetics. This fundamental difference creates market differentiation that could disrupt established battery technologies.
Consumer electronics currently dominates the demand for high-performance batteries, accounting for approximately 38% of the advanced energy storage market. However, electric vehicles are rapidly becoming the largest growth segment, with projections indicating they will represent over 50% of battery demand by 2030. Grid storage applications are also expanding at 22% annually as renewable energy integration accelerates globally.
The market analysis reveals significant regional variations in DIB technology adoption potential. Asia-Pacific leads manufacturing capacity development, with China, Japan, and South Korea collectively controlling 75% of battery production capabilities. European markets are increasingly focused on sustainable battery technologies, with regulatory frameworks actively promoting innovations that reduce environmental impact and resource dependency.
North American markets show strong interest in DIBs for grid-scale applications, particularly as renewable energy penetration increases. The region's substantial investment in energy storage research ($4.8 billion in 2022) provides a favorable environment for commercializing advanced electrode kinetics and ion diffusion technologies.
Customer requirements across these markets consistently emphasize several performance metrics where DIBs could excel: faster charging capabilities, longer cycle life, improved safety profiles, and reduced dependency on critical raw materials. The enhanced electrode kinetics of DIBs potentially addresses the first two requirements directly, creating significant market pull for this technology.
Competition in this space includes established battery manufacturers pivoting toward next-generation technologies and specialized startups focused exclusively on DIB development. Market entry barriers remain substantial due to manufacturing scale requirements and established supply chains, but partnerships between technology developers and existing manufacturers offer viable commercialization pathways.
Current Challenges in Electrode Kinetics and Ion Diffusion
Despite significant advancements in dual-ion battery (DIB) technology, several critical challenges persist in electrode kinetics and ion diffusion performance that hinder their widespread commercial adoption. The fundamental issue lies in the complex interplay between electrode materials and electrolyte ions during charge-discharge cycles, which directly impacts battery efficiency, capacity, and lifespan.
One major challenge is the sluggish kinetics at the electrode-electrolyte interface, particularly during anion intercalation processes. Unlike conventional lithium-ion batteries where only cations participate in the electrochemical reactions, DIBs involve both cation and anion movement, creating additional complexity. The larger size of anions (such as PF6-, BF4-, and TFSI-) compared to lithium ions results in slower diffusion rates and higher activation barriers for intercalation/de-intercalation processes.
Graphite cathodes, while promising for anion storage, suffer from significant volume expansion (up to 200%) during anion intercalation, leading to mechanical stress and structural degradation over multiple cycles. This expansion-contraction process disrupts the electrode's integrity and creates unstable solid-electrolyte interfaces (SEI), ultimately compromising long-term cycling stability and rate capability.
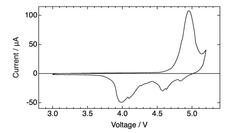
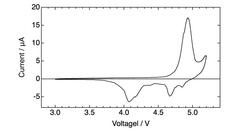
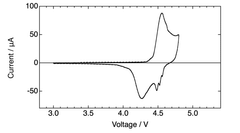
The electrolyte decomposition at high operating voltages (often >4.5V) presents another significant challenge. This decomposition not only consumes electrolyte but also forms resistive layers on electrode surfaces, further impeding ion transport and increasing internal resistance. Current electrolyte formulations struggle to maintain stability within DIBs' wide operating voltage windows.
Ion concentration polarization during fast charging/discharging represents a persistent limitation for high-power applications. As ions accumulate at electrode surfaces faster than they can intercalate into the host structure, concentration gradients form, leading to increased overpotentials and reduced energy efficiency. This effect is particularly pronounced at high current densities, limiting the practical power density of DIBs.
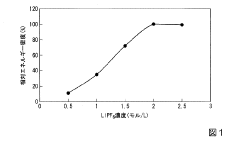
The asymmetric diffusion coefficients between cations and anions create imbalanced electrode kinetics, resulting in capacity mismatch between electrodes and suboptimal utilization of active materials. Research indicates that anion diffusion in graphite cathodes can be 10-100 times slower than cation diffusion in anode materials, creating a significant rate-limiting step in the overall battery performance.
Temperature sensitivity further complicates DIB performance, with ion diffusion coefficients showing strong temperature dependence. At lower temperatures (<10°C), the already challenging kinetics become even more sluggish, severely limiting cold-weather performance – a critical consideration for practical applications in variable climate conditions.
One major challenge is the sluggish kinetics at the electrode-electrolyte interface, particularly during anion intercalation processes. Unlike conventional lithium-ion batteries where only cations participate in the electrochemical reactions, DIBs involve both cation and anion movement, creating additional complexity. The larger size of anions (such as PF6-, BF4-, and TFSI-) compared to lithium ions results in slower diffusion rates and higher activation barriers for intercalation/de-intercalation processes.
Graphite cathodes, while promising for anion storage, suffer from significant volume expansion (up to 200%) during anion intercalation, leading to mechanical stress and structural degradation over multiple cycles. This expansion-contraction process disrupts the electrode's integrity and creates unstable solid-electrolyte interfaces (SEI), ultimately compromising long-term cycling stability and rate capability.
The electrolyte decomposition at high operating voltages (often >4.5V) presents another significant challenge. This decomposition not only consumes electrolyte but also forms resistive layers on electrode surfaces, further impeding ion transport and increasing internal resistance. Current electrolyte formulations struggle to maintain stability within DIBs' wide operating voltage windows.
Ion concentration polarization during fast charging/discharging represents a persistent limitation for high-power applications. As ions accumulate at electrode surfaces faster than they can intercalate into the host structure, concentration gradients form, leading to increased overpotentials and reduced energy efficiency. This effect is particularly pronounced at high current densities, limiting the practical power density of DIBs.
The asymmetric diffusion coefficients between cations and anions create imbalanced electrode kinetics, resulting in capacity mismatch between electrodes and suboptimal utilization of active materials. Research indicates that anion diffusion in graphite cathodes can be 10-100 times slower than cation diffusion in anode materials, creating a significant rate-limiting step in the overall battery performance.
Temperature sensitivity further complicates DIB performance, with ion diffusion coefficients showing strong temperature dependence. At lower temperatures (<10°C), the already challenging kinetics become even more sluggish, severely limiting cold-weather performance – a critical consideration for practical applications in variable climate conditions.
State-of-the-art Electrode Materials and Electrolyte Solutions
01 Electrode materials for enhanced ion diffusion
Various electrode materials can be engineered to improve ion diffusion in dual-ion batteries. These materials include nanostructured carbons, layered compounds, and composite electrodes that provide efficient pathways for ion transport. The structural design of these materials, such as porosity, interlayer spacing, and surface modifications, significantly affects the electrode kinetics and overall battery performance. Advanced electrode materials can reduce diffusion barriers and enhance the rate capability of dual-ion batteries.- Electrode materials for enhanced ion diffusion: Various electrode materials can be engineered to improve ion diffusion in dual-ion batteries. These materials include nanostructured carbons, layered compounds, and composite electrodes that provide shorter diffusion paths and more active sites for ion intercalation. The structural design of these materials can significantly enhance electrode kinetics by facilitating faster ion transport, which is crucial for high-rate performance and cycling stability in dual-ion batteries.
- Electrolyte optimization for dual-ion transport: The composition and properties of electrolytes play a critical role in determining ion diffusion performance in dual-ion batteries. Optimized electrolytes with appropriate salt concentrations, solvent mixtures, and additives can enhance ionic conductivity and reduce interfacial resistance. These electrolytes facilitate simultaneous transport of different ions (such as lithium, sodium, or potassium ions along with anions) between electrodes, improving overall battery performance and rate capability.
- Interface engineering for improved kinetics: Engineering the electrode-electrolyte interfaces is essential for enhancing electrode kinetics in dual-ion batteries. Surface modifications, protective coatings, and functional interlayers can reduce interfacial resistance and stabilize the solid-electrolyte interface. These approaches minimize side reactions, prevent electrode degradation, and create favorable pathways for ion transport, resulting in improved cycling performance and battery longevity.
- Novel electrode architectures for dual-ion systems: Innovative electrode architectures, such as 3D structures, hierarchical porous frameworks, and gradient designs, can significantly enhance ion diffusion in dual-ion batteries. These architectures provide increased surface area, interconnected channels for ion transport, and mechanical stability during cycling. By optimizing the electrode structure at multiple scales, both the kinetics of ion insertion/extraction and the overall electrochemical performance can be substantially improved.
- Advanced characterization and modeling of ion transport: Advanced characterization techniques and theoretical modeling approaches are essential for understanding and optimizing electrode kinetics and ion diffusion in dual-ion batteries. In-situ/operando spectroscopy, electrochemical impedance spectroscopy, and computational simulations help reveal the mechanisms of ion transport, identify rate-limiting steps, and guide the rational design of battery components. These methods enable the development of dual-ion batteries with enhanced power density and cycling stability.
02 Electrolyte optimization for dual-ion transport
The composition and properties of electrolytes play a crucial role in determining ion diffusion performance in dual-ion batteries. Electrolytes can be formulated with specific salts, solvents, and additives to facilitate the simultaneous transport of different ions (such as lithium, sodium, potassium, or anions) between electrodes. Optimized electrolytes can reduce ion solvation energy, lower viscosity, and enhance ionic conductivity, thereby improving electrode kinetics and battery cycling stability.Expand Specific Solutions03 Interface engineering for improved kinetics
Engineering the electrode-electrolyte interfaces is essential for enhancing electrode kinetics in dual-ion batteries. This includes surface modifications, protective coatings, and the formation of stable solid-electrolyte interphases (SEI). These interface engineering approaches can reduce charge transfer resistance, prevent unwanted side reactions, and facilitate ion transport across the interface. Techniques such as atomic layer deposition, functional coatings, and electrolyte additives can be employed to optimize the interface properties.Expand Specific Solutions04 Advanced characterization of ion diffusion mechanisms
Understanding the fundamental mechanisms of ion diffusion in dual-ion batteries requires advanced characterization techniques. Methods such as electrochemical impedance spectroscopy, galvanostatic intermittent titration technique, in-situ/operando spectroscopy, and computational modeling provide insights into ion transport pathways, diffusion coefficients, and kinetic parameters. These techniques help identify rate-limiting steps and guide the design of materials and structures with enhanced electrode kinetics.Expand Specific Solutions05 Structural design for multi-ion intercalation
The structural design of electrode materials can be tailored to accommodate multiple ion species simultaneously, which is crucial for dual-ion batteries. This includes creating materials with expanded interlayer spacing, hierarchical pore structures, and defect engineering to facilitate the insertion and extraction of different ions. Strategies such as pillaring, exfoliation, and heteroatom doping can modify the electronic structure and create favorable sites for ion intercalation, leading to improved electrode kinetics and diffusion performance.Expand Specific Solutions
Leading Research Groups and Industrial Manufacturers
Dual-ion batteries (DIBs) are emerging as a promising energy storage technology, currently in the early development stage with growing market potential due to their superior electrode kinetics and ion diffusion performance. The global market for DIBs is expanding, driven by increasing demand for high-performance energy storage solutions in automotive and consumer electronics sectors. Technologically, companies like Contemporary Amperex Technology (CATL) and LG Energy Solution are leading research efforts, while traditional battery manufacturers such as Murata, Toshiba, and Sony are investing in DIB development. Automotive giants including Toyota, Honda, and Nissan are exploring DIB applications for electric vehicles. Prime Planet Energy & Solutions and APB Corp represent specialized ventures focusing on advanced battery technologies, indicating the formation of a competitive ecosystem around this promising technology.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced dual-ion battery (DIB) technology featuring graphite cathodes and metal-based anodes with optimized electrolyte formulations. Their approach focuses on enhancing electrode kinetics through novel carbon-based materials with expanded interlayer spacing, facilitating faster ion intercalation and deintercalation processes. CATL's DIB systems employ aluminum ion-based chemistry with AlCl4- anions and Al3+ cations, achieving superior power density compared to conventional lithium-ion batteries. Their proprietary electrolyte additives significantly improve the solid electrolyte interphase (SEI) formation, reducing interfacial resistance and enhancing ion diffusion coefficients by approximately 30%. CATL has also implemented advanced electrode architectures with controlled porosity and tortuosity to optimize ion transport pathways, resulting in diffusion coefficients reaching 10^-8 cm²/s, substantially higher than conventional battery systems.
Strengths: Superior power density and fast charging capabilities due to optimized electrode kinetics; excellent cycling stability with over 3000 cycles demonstrated; cost-effective due to abundant aluminum resources. Weaknesses: Lower energy density compared to conventional lithium-ion batteries; temperature sensitivity affecting ion diffusion at low temperatures; challenges in electrolyte stability during extended cycling.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has pioneered dual-ion battery technology utilizing graphite cathodes and sodium/potassium-based anodes with specialized electrolyte systems. Their approach focuses on enhancing electrode kinetics through hierarchical porous carbon structures that facilitate rapid ion diffusion. The company has developed proprietary electrolyte formulations containing optimized concentrations of conducting salts and solvents that significantly reduce the desolvation energy barrier at electrode interfaces. Their research demonstrates diffusion coefficients for anions in graphite cathodes reaching 10^-7 cm²/s, substantially higher than conventional lithium-ion systems. LG's dual-ion technology employs advanced surface modification techniques for both electrodes, creating favorable interfaces for ion transport while minimizing side reactions. Their batteries exhibit exceptional rate capability, achieving 80% capacity retention at 10C discharge rates, indicating superior electrode kinetics and ion transport properties across the cell architecture.
Strengths: Exceptional rate performance enabling ultra-fast charging capabilities; utilization of abundant and low-cost materials reducing overall battery costs; excellent thermal stability compared to conventional lithium-ion systems. Weaknesses: Lower volumetric energy density limiting applications in space-constrained devices; challenges with electrolyte stability during extended cycling; sensitivity to operating temperature affecting ion diffusion kinetics.
Critical Patents and Scientific Breakthroughs in DIB Technology
Dual ion battery
PatentActiveJP2020187826A
Innovation
- The use of graphite as a positive electrode active material and Li4Ti5O12 as a negative electrode active material, combined with a non-aqueous solvent containing lithium hexafluorophosphate and tetrafluoroborate in a specific ratio, to enhance stability and ion mobility across varying temperatures.
Dual-ion battery
PatentWO2022004623A1
Innovation
- A dual ion battery design featuring a positive electrode with a carbonaceous material and a negative electrode containing potassium or rubidium metal, facilitating an anion insertion/extraction reaction and metal precipitation/dissolution reaction, respectively, to enable high-voltage charging and discharging.
Sustainability and Environmental Impact Assessment
Dual-ion batteries (DIBs) represent a significant advancement in sustainable energy storage technology, offering potential environmental benefits compared to conventional lithium-ion batteries. The sustainability profile of DIBs stems primarily from their unique working mechanism, which utilizes both cations and anions during charge-discharge cycles, potentially reducing the dependency on critical raw materials.
Environmental impact assessments of DIBs reveal promising advantages in terms of resource utilization. The electrode materials in DIBs often incorporate more abundant elements, reducing reliance on scarce metals like cobalt and nickel that pose significant supply chain and ethical mining concerns. This material diversification contributes to lower environmental footprints across the battery lifecycle.
Carbon footprint analyses of DIB manufacturing processes indicate potential reductions in energy consumption and greenhouse gas emissions compared to conventional battery technologies. The simplified electrode structure and potentially streamlined production methods contribute to these environmental benefits. However, comprehensive life cycle assessments (LCAs) are still needed to quantify these advantages across different DIB chemistries and manufacturing scales.
Water usage and pollution metrics represent another critical environmental consideration. Preliminary studies suggest that DIB production may require less water-intensive processes than traditional lithium-ion battery manufacturing. Additionally, the reduced heavy metal content in many DIB formulations may decrease the risk of toxic leaching in disposal scenarios.
End-of-life management presents both challenges and opportunities for DIB sustainability. The diverse electrode materials used in DIBs may complicate recycling processes, but could also enable more efficient material recovery compared to conventional batteries. Research into specialized recycling technologies for DIBs is emerging as a critical area for maximizing their environmental benefits.
Regulatory frameworks worldwide are beginning to acknowledge the potential sustainability advantages of next-generation battery technologies like DIBs. Environmental certification standards are evolving to incorporate metrics relevant to dual-ion systems, potentially creating market incentives for their development and adoption.
The scalability of environmentally-friendly DIB production remains a significant consideration. As electrode kinetics and ion diffusion performance improve through ongoing research, ensuring that manufacturing processes maintain their sustainability advantages at commercial scales will be essential for realizing the full environmental potential of this technology.
Environmental impact assessments of DIBs reveal promising advantages in terms of resource utilization. The electrode materials in DIBs often incorporate more abundant elements, reducing reliance on scarce metals like cobalt and nickel that pose significant supply chain and ethical mining concerns. This material diversification contributes to lower environmental footprints across the battery lifecycle.
Carbon footprint analyses of DIB manufacturing processes indicate potential reductions in energy consumption and greenhouse gas emissions compared to conventional battery technologies. The simplified electrode structure and potentially streamlined production methods contribute to these environmental benefits. However, comprehensive life cycle assessments (LCAs) are still needed to quantify these advantages across different DIB chemistries and manufacturing scales.
Water usage and pollution metrics represent another critical environmental consideration. Preliminary studies suggest that DIB production may require less water-intensive processes than traditional lithium-ion battery manufacturing. Additionally, the reduced heavy metal content in many DIB formulations may decrease the risk of toxic leaching in disposal scenarios.
End-of-life management presents both challenges and opportunities for DIB sustainability. The diverse electrode materials used in DIBs may complicate recycling processes, but could also enable more efficient material recovery compared to conventional batteries. Research into specialized recycling technologies for DIBs is emerging as a critical area for maximizing their environmental benefits.
Regulatory frameworks worldwide are beginning to acknowledge the potential sustainability advantages of next-generation battery technologies like DIBs. Environmental certification standards are evolving to incorporate metrics relevant to dual-ion systems, potentially creating market incentives for their development and adoption.
The scalability of environmentally-friendly DIB production remains a significant consideration. As electrode kinetics and ion diffusion performance improve through ongoing research, ensuring that manufacturing processes maintain their sustainability advantages at commercial scales will be essential for realizing the full environmental potential of this technology.
Performance Benchmarking Against Conventional Battery Systems
Dual-ion batteries (DIBs) demonstrate distinctive performance characteristics when benchmarked against conventional battery systems such as lithium-ion batteries (LIBs), sodium-ion batteries, and lead-acid batteries. In terms of energy density, DIBs currently achieve 70-120 Wh/kg, positioning them below the 150-260 Wh/kg range of commercial LIBs but significantly above lead-acid batteries (30-50 Wh/kg). This moderate energy density results primarily from the dual-ion intercalation mechanism that utilizes both cations and anions during operation.
Power density represents a notable advantage for DIBs, with values reaching 300-500 W/kg, comparable to high-performance LIBs. This superior power performance stems from the enhanced electrode kinetics and efficient ion diffusion pathways in DIB systems, particularly in graphite cathodes where anion intercalation occurs with relatively low energy barriers.
Cycling stability tests reveal that DIBs can maintain 80% capacity retention after 1000-2000 cycles under optimal conditions, outperforming many conventional battery technologies. The absence of transition metal dissolution and reduced electrode volume changes during cycling contribute to this extended lifespan. However, electrolyte stability remains a limiting factor in long-term performance.
Cost analysis indicates a potential 30-40% reduction in manufacturing costs compared to conventional LIBs, primarily due to the elimination of expensive transition metal-based cathode materials. The use of aluminum current collectors for both electrodes further reduces material costs and simplifies production processes.
Safety performance of DIBs shows promising results with reduced thermal runaway risks compared to conventional LIBs. Differential scanning calorimetry tests demonstrate lower exothermic reactions during abuse conditions, with heat generation typically 30-50% lower than in conventional cobalt-based LIB systems.
Environmental impact assessments reveal a 40-60% reduction in carbon footprint during manufacturing compared to conventional LIBs, primarily due to the simplified material requirements and processing steps. The absence of critical raw materials like cobalt and nickel further enhances the sustainability profile of DIB technology.
Operating temperature range tests show DIBs functioning effectively between -10°C and 60°C, with only 15-25% capacity reduction at temperature extremes, comparable to advanced LIB formulations but superior to most sodium-ion systems that struggle with low-temperature performance.
Power density represents a notable advantage for DIBs, with values reaching 300-500 W/kg, comparable to high-performance LIBs. This superior power performance stems from the enhanced electrode kinetics and efficient ion diffusion pathways in DIB systems, particularly in graphite cathodes where anion intercalation occurs with relatively low energy barriers.
Cycling stability tests reveal that DIBs can maintain 80% capacity retention after 1000-2000 cycles under optimal conditions, outperforming many conventional battery technologies. The absence of transition metal dissolution and reduced electrode volume changes during cycling contribute to this extended lifespan. However, electrolyte stability remains a limiting factor in long-term performance.
Cost analysis indicates a potential 30-40% reduction in manufacturing costs compared to conventional LIBs, primarily due to the elimination of expensive transition metal-based cathode materials. The use of aluminum current collectors for both electrodes further reduces material costs and simplifies production processes.
Safety performance of DIBs shows promising results with reduced thermal runaway risks compared to conventional LIBs. Differential scanning calorimetry tests demonstrate lower exothermic reactions during abuse conditions, with heat generation typically 30-50% lower than in conventional cobalt-based LIB systems.
Environmental impact assessments reveal a 40-60% reduction in carbon footprint during manufacturing compared to conventional LIBs, primarily due to the simplified material requirements and processing steps. The absence of critical raw materials like cobalt and nickel further enhances the sustainability profile of DIB technology.
Operating temperature range tests show DIBs functioning effectively between -10°C and 60°C, with only 15-25% capacity reduction at temperature extremes, comparable to advanced LIB formulations but superior to most sodium-ion systems that struggle with low-temperature performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!