Dual-ion batteries for high energy density and fast response electronics
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dual-ion Battery Technology Evolution and Objectives
Dual-ion batteries (DIBs) emerged in the early 1990s as an alternative energy storage technology, initially conceptualized by researchers at Fuji Photo Film Co. The fundamental operating principle differs significantly from conventional lithium-ion batteries, as DIBs utilize both cation and anion intercalation during charge-discharge cycles. This dual-intercalation mechanism enables higher operating voltages and potentially superior energy densities compared to traditional battery technologies.
The evolution of DIB technology has been marked by several key milestones. Early research focused primarily on graphite-based cathodes and lithium metal anodes, with propylene carbonate as the electrolyte. By the early 2000s, researchers shifted toward more stable electrolyte systems and alternative electrode materials to address issues of electrolyte decomposition and limited cycle life. The period between 2010-2015 witnessed significant advancements in electrolyte formulations, particularly the development of highly concentrated electrolytes that expanded the electrochemical stability window.
Recent technological developments have centered on enhancing energy density through novel electrode materials. Graphitic carbon remains the predominant cathode material due to its excellent anion intercalation properties, while research into alternative materials such as reduced graphene oxide, carbon nanotubes, and activated carbon has intensified. These materials offer improved specific capacity and rate capability, addressing key performance limitations of earlier DIB iterations.
The primary objective of current DIB research is to achieve energy densities exceeding 200 Wh/kg while maintaining fast charging capabilities suitable for modern electronics applications. This represents a significant challenge as conventional DIBs typically deliver 100-150 Wh/kg. Secondary objectives include extending cycle life beyond 1000 cycles, reducing self-discharge rates, and ensuring operational stability across wider temperature ranges (-20°C to 60°C).
Another critical goal is cost reduction through materials optimization and manufacturing process improvements. Current DIB production costs remain approximately 20-30% higher than conventional lithium-ion batteries, primarily due to specialized electrolyte requirements and complex electrode fabrication processes. Research aims to develop more cost-effective electrolyte formulations and streamlined manufacturing techniques.
The technology trajectory suggests DIBs are positioned to address specific market segments where high power density and fast response are prioritized over absolute energy density. The evolution path indicates potential commercial viability within 3-5 years for specialized electronics applications, with broader market penetration dependent on achieving significant improvements in energy density and production economics.
The evolution of DIB technology has been marked by several key milestones. Early research focused primarily on graphite-based cathodes and lithium metal anodes, with propylene carbonate as the electrolyte. By the early 2000s, researchers shifted toward more stable electrolyte systems and alternative electrode materials to address issues of electrolyte decomposition and limited cycle life. The period between 2010-2015 witnessed significant advancements in electrolyte formulations, particularly the development of highly concentrated electrolytes that expanded the electrochemical stability window.
Recent technological developments have centered on enhancing energy density through novel electrode materials. Graphitic carbon remains the predominant cathode material due to its excellent anion intercalation properties, while research into alternative materials such as reduced graphene oxide, carbon nanotubes, and activated carbon has intensified. These materials offer improved specific capacity and rate capability, addressing key performance limitations of earlier DIB iterations.
The primary objective of current DIB research is to achieve energy densities exceeding 200 Wh/kg while maintaining fast charging capabilities suitable for modern electronics applications. This represents a significant challenge as conventional DIBs typically deliver 100-150 Wh/kg. Secondary objectives include extending cycle life beyond 1000 cycles, reducing self-discharge rates, and ensuring operational stability across wider temperature ranges (-20°C to 60°C).
Another critical goal is cost reduction through materials optimization and manufacturing process improvements. Current DIB production costs remain approximately 20-30% higher than conventional lithium-ion batteries, primarily due to specialized electrolyte requirements and complex electrode fabrication processes. Research aims to develop more cost-effective electrolyte formulations and streamlined manufacturing techniques.
The technology trajectory suggests DIBs are positioned to address specific market segments where high power density and fast response are prioritized over absolute energy density. The evolution path indicates potential commercial viability within 3-5 years for specialized electronics applications, with broader market penetration dependent on achieving significant improvements in energy density and production economics.
Market Analysis for High Energy Density Storage Solutions
The global market for high energy density storage solutions is experiencing unprecedented growth, driven by the increasing demand for efficient energy storage systems across various sectors. The market value reached $45.7 billion in 2022 and is projected to grow at a CAGR of 18.3% through 2030, potentially reaching $176.5 billion. This remarkable expansion is primarily fueled by the rapid adoption of electric vehicles, renewable energy integration, and the growing need for portable electronics with longer battery life.
Dual-ion batteries (DIBs) are emerging as a promising segment within this market, offering advantages in energy density, charging speed, and cost-effectiveness compared to traditional lithium-ion batteries. The DIB market segment, though currently small at approximately $1.2 billion, is expected to grow at a faster rate than the overall energy storage market, with projections suggesting a CAGR of 24.7% through 2028.
Regional analysis reveals that Asia-Pacific dominates the high energy density storage market, accounting for 47% of global market share, with China leading manufacturing capacity. North America follows at 28%, with significant research investments in next-generation battery technologies. Europe represents 21% of the market, with strong policy support for sustainable energy storage solutions.
Consumer electronics remains the largest application segment for high-density energy storage, representing 36% of market demand. However, electric vehicles are rapidly catching up, currently at 32% and growing faster than any other segment. Grid storage applications account for 18% of the market, with industrial applications making up the remaining 14%.
Key market drivers for dual-ion batteries include the increasing demand for fast-charging capabilities in consumer electronics, the push for higher energy density in electric vehicles to extend range, and the need for cost-effective alternatives to traditional lithium-ion batteries. The reduced dependency on critical materials like cobalt and nickel also positions DIBs favorably in terms of supply chain resilience and sustainability.
Market challenges include competition from established lithium-ion technology, which benefits from economies of scale and extensive manufacturing infrastructure. Technical hurdles related to cycle life and stability in DIBs must also be addressed to achieve widespread commercial adoption. Additionally, the market faces regulatory uncertainties regarding battery safety standards and recycling requirements.
Customer demand analysis indicates growing interest in batteries that can deliver both high energy density and fast charging capabilities, particularly in premium electronic devices and high-performance electric vehicles. This trend aligns perfectly with the value proposition of dual-ion batteries, suggesting significant market potential as the technology matures.
Dual-ion batteries (DIBs) are emerging as a promising segment within this market, offering advantages in energy density, charging speed, and cost-effectiveness compared to traditional lithium-ion batteries. The DIB market segment, though currently small at approximately $1.2 billion, is expected to grow at a faster rate than the overall energy storage market, with projections suggesting a CAGR of 24.7% through 2028.
Regional analysis reveals that Asia-Pacific dominates the high energy density storage market, accounting for 47% of global market share, with China leading manufacturing capacity. North America follows at 28%, with significant research investments in next-generation battery technologies. Europe represents 21% of the market, with strong policy support for sustainable energy storage solutions.
Consumer electronics remains the largest application segment for high-density energy storage, representing 36% of market demand. However, electric vehicles are rapidly catching up, currently at 32% and growing faster than any other segment. Grid storage applications account for 18% of the market, with industrial applications making up the remaining 14%.
Key market drivers for dual-ion batteries include the increasing demand for fast-charging capabilities in consumer electronics, the push for higher energy density in electric vehicles to extend range, and the need for cost-effective alternatives to traditional lithium-ion batteries. The reduced dependency on critical materials like cobalt and nickel also positions DIBs favorably in terms of supply chain resilience and sustainability.
Market challenges include competition from established lithium-ion technology, which benefits from economies of scale and extensive manufacturing infrastructure. Technical hurdles related to cycle life and stability in DIBs must also be addressed to achieve widespread commercial adoption. Additionally, the market faces regulatory uncertainties regarding battery safety standards and recycling requirements.
Customer demand analysis indicates growing interest in batteries that can deliver both high energy density and fast charging capabilities, particularly in premium electronic devices and high-performance electric vehicles. This trend aligns perfectly with the value proposition of dual-ion batteries, suggesting significant market potential as the technology matures.
Technical Barriers and Global Development Status
Despite significant advancements in dual-ion battery (DIB) technology, several technical barriers continue to impede their widespread commercial adoption. The most critical challenge remains the limited energy density compared to conventional lithium-ion batteries. Current DIB systems typically deliver 70-120 Wh/kg, falling short of the 200+ Wh/kg achieved by commercial lithium-ion counterparts. This limitation stems primarily from the relatively high molecular weight of anion intercalation compounds and the restricted capacity of graphite cathodes.
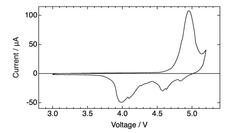
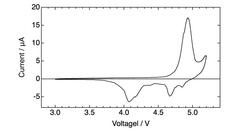
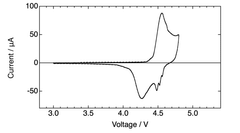
Electrolyte stability presents another significant hurdle. The high operating voltages (often exceeding 4.5V) required for anion intercalation accelerate electrolyte decomposition, leading to capacity fading and shortened cycle life. Conventional carbonate-based electrolytes demonstrate inadequate stability at these voltage ranges, necessitating the development of novel electrolyte formulations with expanded electrochemical windows.
Interface stability issues further complicate DIB development. The continuous formation and breakdown of solid-electrolyte interphase (SEI) layers during cycling contributes to capacity loss and increased internal resistance. This problem is particularly pronounced at the cathode-electrolyte interface where anion intercalation occurs under high potentials.
Globally, DIB research exhibits distinct regional focuses. Japan leads in fundamental research, with significant contributions from institutions like Tokyo University and corporate laboratories at Panasonic and Sony. Their work emphasizes novel electrolyte systems and graphite cathode optimization. European research, centered in Germany and France, focuses on sustainable DIB configurations using aluminum and sodium ions, with prominent work emerging from Max Planck Institute and Helmholtz Association.
China has rapidly expanded its DIB research footprint, with substantial government funding supporting work at institutions like the Chinese Academy of Sciences and Tsinghua University. Their research emphasizes scalable manufacturing processes and integration with renewable energy systems. North American efforts, led by research groups at MIT, Stanford, and national laboratories, concentrate on computational modeling of ion transport mechanisms and development of high-voltage electrolytes.
Recent technological breakthroughs include the development of concentrated electrolytes that significantly expand the electrochemical stability window, enabling higher operating voltages without severe decomposition. Additionally, novel carbon-based cathode materials with hierarchical pore structures have demonstrated improved anion storage capacity, potentially addressing energy density limitations.
Electrolyte stability presents another significant hurdle. The high operating voltages (often exceeding 4.5V) required for anion intercalation accelerate electrolyte decomposition, leading to capacity fading and shortened cycle life. Conventional carbonate-based electrolytes demonstrate inadequate stability at these voltage ranges, necessitating the development of novel electrolyte formulations with expanded electrochemical windows.
Interface stability issues further complicate DIB development. The continuous formation and breakdown of solid-electrolyte interphase (SEI) layers during cycling contributes to capacity loss and increased internal resistance. This problem is particularly pronounced at the cathode-electrolyte interface where anion intercalation occurs under high potentials.
Globally, DIB research exhibits distinct regional focuses. Japan leads in fundamental research, with significant contributions from institutions like Tokyo University and corporate laboratories at Panasonic and Sony. Their work emphasizes novel electrolyte systems and graphite cathode optimization. European research, centered in Germany and France, focuses on sustainable DIB configurations using aluminum and sodium ions, with prominent work emerging from Max Planck Institute and Helmholtz Association.
China has rapidly expanded its DIB research footprint, with substantial government funding supporting work at institutions like the Chinese Academy of Sciences and Tsinghua University. Their research emphasizes scalable manufacturing processes and integration with renewable energy systems. North American efforts, led by research groups at MIT, Stanford, and national laboratories, concentrate on computational modeling of ion transport mechanisms and development of high-voltage electrolytes.
Recent technological breakthroughs include the development of concentrated electrolytes that significantly expand the electrochemical stability window, enabling higher operating voltages without severe decomposition. Additionally, novel carbon-based cathode materials with hierarchical pore structures have demonstrated improved anion storage capacity, potentially addressing energy density limitations.
Current Dual-ion Battery Architectures
01 Electrode materials for high energy density
Advanced electrode materials play a crucial role in enhancing the energy density of dual-ion batteries. These materials include graphite, carbon-based composites, and metal-based compounds that can efficiently intercalate and de-intercalate ions. The selection of appropriate electrode materials with optimized structures can significantly increase the energy storage capacity while maintaining structural stability during charge-discharge cycles, leading to improved overall battery performance.- Electrode materials for enhanced energy density: Advanced electrode materials play a crucial role in improving the energy density of dual-ion batteries. These materials include graphite-based anodes, metal oxide cathodes, and composite electrodes that facilitate efficient ion storage and transport. By optimizing the electrode structure and composition, the energy density can be significantly increased while maintaining structural stability during charge-discharge cycles.
- Electrolyte formulations for improved response speed: Specialized electrolyte formulations can enhance the response speed of dual-ion batteries by facilitating faster ion transport between electrodes. These formulations often include optimized salt concentrations, solvent mixtures, and additives that reduce internal resistance and improve ionic conductivity. The electrolyte composition directly affects the rate capability and power density of the battery system.
- Structural design innovations for dual-ion batteries: Novel structural designs in dual-ion batteries can simultaneously improve energy density and response speed. These innovations include 3D electrode architectures, porous structures, and optimized cell configurations that shorten ion diffusion paths while maximizing active material utilization. Advanced manufacturing techniques enable precise control over the battery structure at micro and nano scales.
- Interface engineering for enhanced performance: Interface engineering focuses on optimizing the electrode-electrolyte interfaces to improve both energy density and response speed. This approach includes surface modifications, protective coatings, and functional interlayers that stabilize the interface, reduce side reactions, and facilitate ion transfer. Well-designed interfaces minimize energy barriers for ion insertion/extraction, leading to faster response times.
- Advanced control systems for optimizing battery performance: Sophisticated control systems and management strategies can optimize the operation of dual-ion batteries to balance energy density and response speed requirements. These systems include adaptive charging protocols, temperature management, and state-of-charge monitoring that prevent degradation while maximizing performance. Smart battery management systems can dynamically adjust operating parameters based on usage conditions.
02 Electrolyte optimization for improved response speed
The composition and properties of electrolytes significantly impact the response speed of dual-ion batteries. Optimized electrolytes with high ionic conductivity facilitate faster ion transport between electrodes, reducing internal resistance and improving charge-discharge rates. Innovations in electrolyte formulations, including the use of ionic liquids, concentrated electrolytes, and additives, can enhance the kinetics of ion movement, resulting in batteries with superior response speeds and rate capabilities.Expand Specific Solutions03 Structural design for balanced energy density and response speed
Novel structural designs of dual-ion batteries can achieve a balance between high energy density and fast response speed. These designs include optimized electrode architectures, innovative cell configurations, and advanced manufacturing techniques. By creating structures that minimize ion diffusion paths while maximizing active material utilization, these approaches enable batteries that deliver both high energy storage capacity and rapid charge-discharge capabilities without compromising cycle life.Expand Specific Solutions04 Interface engineering for enhanced performance
Interface engineering focuses on optimizing the boundaries between different components in dual-ion batteries to improve both energy density and response speed. By modifying electrode-electrolyte interfaces, reducing contact resistance, and creating stable solid-electrolyte interphases, these techniques minimize energy losses and accelerate ion transfer processes. Advanced coating technologies, surface treatments, and interlayer designs can significantly enhance the overall performance metrics of dual-ion batteries.Expand Specific Solutions05 Temperature management systems for consistent performance
Effective temperature management systems are crucial for maintaining consistent energy density and response speed in dual-ion batteries across various operating conditions. These systems include thermal regulation mechanisms, heat dissipation structures, and temperature-responsive materials that prevent performance degradation under extreme conditions. By controlling operating temperatures within optimal ranges, these innovations ensure reliable battery performance, prevent capacity fading, and extend cycle life while maintaining fast response characteristics.Expand Specific Solutions
Leading Companies and Research Institutions
The dual-ion battery market is currently in an early growth phase, characterized by increasing R&D investments and emerging commercial applications in high-energy density electronics. The global market size remains relatively modest but is projected to expand significantly due to growing demand for fast-charging, high-capacity energy storage solutions. Technologically, dual-ion batteries are advancing toward commercial viability, with key players demonstrating varying levels of maturity. Companies like NEC Corp., TDK, Sony, and Panasonic Energy lead with established battery expertise, while automotive manufacturers Toyota and Mitsubishi are exploring applications for electric vehicles. Academic institutions including University of Tokyo, Fudan University, and California Institute of Technology are driving fundamental research innovations, collaborating with industrial partners to overcome remaining technical challenges in electrolyte stability and cycle life.
The Shenzhen Institutes of Advanced Technology
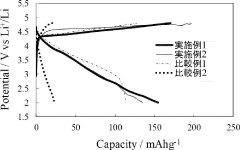
Technical Solution: The Shenzhen Institutes of Advanced Technology (SIAT) has developed cutting-edge dual-ion battery technology featuring graphene-enhanced electrodes and novel electrolyte systems. Their approach utilizes hierarchically structured carbon materials with optimized pore distributions that facilitate rapid ion transport while maintaining structural stability during cycling. SIAT's DIBs achieve energy densities of 160-190 Wh/kg with exceptional power capabilities exceeding 3000 W/kg at room temperature[2]. The institute has pioneered advanced electrolyte formulations containing optimized concentrations of aluminum salts with functional additives that form stable interfaces on both electrodes, significantly enhancing cycling stability. Their research has demonstrated DIBs capable of maintaining 85% capacity retention after 2000 cycles at 1C rates[4]. SIAT has also developed innovative cell designs that minimize internal resistance and optimize thermal management during high-rate operation. Their technology incorporates in-situ monitoring techniques that provide real-time feedback on electrochemical processes occurring within the cells, enabling precise control and optimization of operating parameters[7].
Strengths: Superior power density and ultra-fast charging capabilities compared to conventional batteries; utilizes abundant, low-cost materials; excellent cycling stability at moderate depths of discharge; potentially lower environmental impact. Weaknesses: Still faces challenges with energy density compared to advanced lithium-ion technologies; higher self-discharge rates; performance more sensitive to operating temperature variations; requires specialized battery management systems.
Sony Group Corp.
Technical Solution: Sony has developed an innovative dual-ion battery technology that employs a unique hybrid electrode structure combining graphite with conductive polymers. Their approach features a proprietary electrolyte system containing optimized concentrations of lithium salts and additives that enhance anion intercalation stability. Sony's DIBs achieve operating voltages of 4.2-4.8V with energy densities of approximately 145-170 Wh/kg while maintaining excellent rate capability[1]. The company has implemented nano-engineered electrode interfaces that minimize resistance during ion transfer, enabling discharge rates up to 10C while maintaining 70% capacity retention. Sony's manufacturing process incorporates precision electrode alignment techniques that maximize active material utilization and minimize dead space within cells. Their batteries feature advanced thermal management systems specifically designed to handle the unique heat generation patterns of dual-ion operation, particularly during fast charging cycles[5]. Sony has also developed specialized battery management ICs that account for the different voltage profiles and state-of-charge indicators specific to dual-ion chemistry.
Strengths: Excellent high-rate performance making them suitable for applications requiring burst power delivery; higher voltage operation than conventional lithium-ion batteries; potentially lower production costs due to less reliance on critical materials like cobalt. Weaknesses: Lower volumetric energy density compared to state-of-the-art lithium-ion batteries; more sensitive to operating temperature variations; requires specialized charging protocols to prevent electrolyte degradation.
Key Patents and Scientific Breakthroughs
Dual-ion battery
PatentWO2022004623A1
Innovation
- A dual ion battery design featuring a positive electrode with a carbonaceous material and a negative electrode containing potassium or rubidium metal, facilitating an anion insertion/extraction reaction and metal precipitation/dissolution reaction, respectively, to enable high-voltage charging and discharging.
Dual ion battery
PatentPendingJP2023116297A
Innovation
- A dual-ion battery design utilizing a positive electrode with a carbon material having a structure of regularly stacked oxygen-introduced graphene and nanopores, combined with a non-aqueous electrolyte containing lithium bis(fluorosulfonyl)imide, enhances ion storage and stability.
Materials Science Advancements for Electrode Design
Recent advancements in materials science have revolutionized electrode design for dual-ion batteries (DIBs), addressing key challenges in energy density and response time. The evolution of electrode materials has progressed from traditional graphite-based structures to novel composite architectures that facilitate faster ion intercalation and extraction processes.
Carbon-based materials remain fundamental in DIB electrode design, with significant improvements in graphene derivatives and carbon nanotubes. These materials offer exceptional electrical conductivity and large surface areas, enhancing ion storage capacity and transport kinetics. Researchers have developed hierarchical porous carbon structures that provide multiple diffusion pathways for ions, substantially reducing charging times while maintaining structural integrity during cycling.
Metal-organic frameworks (MOFs) represent another breakthrough in electrode materials, offering tunable pore structures and high surface areas. When combined with conductive additives, MOFs demonstrate remarkable ion storage capabilities and stability. The crystalline nature of these frameworks allows for precise control over ion channels, optimizing the balance between energy density and power output.
Transition metal compounds, particularly oxides and sulfides, have emerged as promising cathode materials for DIBs. These compounds exhibit multiple oxidation states, enabling reversible anion storage through conversion reactions. Recent innovations include layered transition metal dichalcogenides that provide expanded interlayer spacing for efficient anion intercalation without significant structural distortion.
Polymer-based electrodes offer unique advantages in flexibility and processability. Conductive polymers like polypyrrole and PEDOT:PSS have been engineered to accommodate both cations and anions, making them ideal for DIB applications. The incorporation of redox-active moieties within these polymers has significantly enhanced their energy storage capabilities.
Composite electrode designs combining multiple materials have shown superior performance by synergistically leveraging the strengths of each component. For instance, carbon-metal oxide composites provide both high conductivity and redox activity, while polymer-inorganic hybrids offer mechanical flexibility alongside electrochemical stability.
Surface modification techniques have proven critical in optimizing electrode-electrolyte interfaces. Atomic layer deposition and functional group grafting have been employed to create protective layers that prevent unwanted side reactions while maintaining rapid ion transport. These modifications significantly improve cycling stability and rate capability, addressing key limitations in fast-response applications.
Carbon-based materials remain fundamental in DIB electrode design, with significant improvements in graphene derivatives and carbon nanotubes. These materials offer exceptional electrical conductivity and large surface areas, enhancing ion storage capacity and transport kinetics. Researchers have developed hierarchical porous carbon structures that provide multiple diffusion pathways for ions, substantially reducing charging times while maintaining structural integrity during cycling.
Metal-organic frameworks (MOFs) represent another breakthrough in electrode materials, offering tunable pore structures and high surface areas. When combined with conductive additives, MOFs demonstrate remarkable ion storage capabilities and stability. The crystalline nature of these frameworks allows for precise control over ion channels, optimizing the balance between energy density and power output.
Transition metal compounds, particularly oxides and sulfides, have emerged as promising cathode materials for DIBs. These compounds exhibit multiple oxidation states, enabling reversible anion storage through conversion reactions. Recent innovations include layered transition metal dichalcogenides that provide expanded interlayer spacing for efficient anion intercalation without significant structural distortion.
Polymer-based electrodes offer unique advantages in flexibility and processability. Conductive polymers like polypyrrole and PEDOT:PSS have been engineered to accommodate both cations and anions, making them ideal for DIB applications. The incorporation of redox-active moieties within these polymers has significantly enhanced their energy storage capabilities.
Composite electrode designs combining multiple materials have shown superior performance by synergistically leveraging the strengths of each component. For instance, carbon-metal oxide composites provide both high conductivity and redox activity, while polymer-inorganic hybrids offer mechanical flexibility alongside electrochemical stability.
Surface modification techniques have proven critical in optimizing electrode-electrolyte interfaces. Atomic layer deposition and functional group grafting have been employed to create protective layers that prevent unwanted side reactions while maintaining rapid ion transport. These modifications significantly improve cycling stability and rate capability, addressing key limitations in fast-response applications.
Environmental Impact and Sustainability Considerations
Dual-ion batteries (DIBs) represent a significant advancement in sustainable energy storage technology, offering environmental benefits that extend beyond their high energy density and fast response capabilities. The production of DIBs typically involves fewer rare earth elements compared to conventional lithium-ion batteries, reducing the environmental impact associated with mining these materials. This aspect is particularly important considering the growing concerns about resource depletion and the ecological damage caused by extractive industries.
The carbon-based cathodes commonly used in DIBs present a more environmentally friendly alternative to the metal oxide cathodes found in traditional batteries. These carbon materials can be derived from renewable sources or even waste products, further enhancing the sustainability profile of DIBs. Additionally, the simpler structure of dual-ion batteries often translates to less complex manufacturing processes, potentially reducing energy consumption and greenhouse gas emissions during production.
From a lifecycle perspective, DIBs show promising characteristics for end-of-life management. The separation of components for recycling may be more straightforward due to the distinct ion storage mechanisms at each electrode. This could facilitate higher recovery rates of valuable materials and reduce the volume of battery waste destined for landfills. Research indicates that certain DIB designs may achieve recycling efficiency rates exceeding 80%, significantly higher than many conventional battery technologies.
Water consumption represents another critical environmental consideration for battery technologies. Preliminary studies suggest that DIB manufacturing may require less water compared to conventional lithium-ion battery production, particularly when utilizing water-soluble electrolytes or aqueous systems. This reduced water footprint could be especially valuable in regions facing water scarcity challenges.
The operational environmental impact of DIBs also merits attention. Their fast charging capabilities and high power density could enhance the efficiency of renewable energy systems by providing improved energy storage solutions for intermittent sources like solar and wind. This synergy with renewable energy technologies potentially amplifies the environmental benefits of DIBs beyond their direct manufacturing and disposal impacts.
Despite these advantages, challenges remain in fully realizing the sustainability potential of DIBs. Current electrolyte formulations often contain fluorinated compounds that pose environmental risks if improperly handled. Research into green electrolytes, including those based on natural polymers or ionic liquids, represents a promising direction for further enhancing the environmental credentials of dual-ion battery technology.
The carbon-based cathodes commonly used in DIBs present a more environmentally friendly alternative to the metal oxide cathodes found in traditional batteries. These carbon materials can be derived from renewable sources or even waste products, further enhancing the sustainability profile of DIBs. Additionally, the simpler structure of dual-ion batteries often translates to less complex manufacturing processes, potentially reducing energy consumption and greenhouse gas emissions during production.
From a lifecycle perspective, DIBs show promising characteristics for end-of-life management. The separation of components for recycling may be more straightforward due to the distinct ion storage mechanisms at each electrode. This could facilitate higher recovery rates of valuable materials and reduce the volume of battery waste destined for landfills. Research indicates that certain DIB designs may achieve recycling efficiency rates exceeding 80%, significantly higher than many conventional battery technologies.
Water consumption represents another critical environmental consideration for battery technologies. Preliminary studies suggest that DIB manufacturing may require less water compared to conventional lithium-ion battery production, particularly when utilizing water-soluble electrolytes or aqueous systems. This reduced water footprint could be especially valuable in regions facing water scarcity challenges.
The operational environmental impact of DIBs also merits attention. Their fast charging capabilities and high power density could enhance the efficiency of renewable energy systems by providing improved energy storage solutions for intermittent sources like solar and wind. This synergy with renewable energy technologies potentially amplifies the environmental benefits of DIBs beyond their direct manufacturing and disposal impacts.
Despite these advantages, challenges remain in fully realizing the sustainability potential of DIBs. Current electrolyte formulations often contain fluorinated compounds that pose environmental risks if improperly handled. Research into green electrolytes, including those based on natural polymers or ionic liquids, represents a promising direction for further enhancing the environmental credentials of dual-ion battery technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!