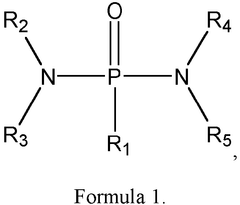
How separator and electrolyte design affect Dual-ion batteries stability and safety
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dual-ion Batteries Separator and Electrolyte Background
Dual-ion batteries (DIBs) represent an emerging energy storage technology that has gained significant attention due to their potential advantages in terms of cost, sustainability, and energy density. Unlike conventional lithium-ion batteries that rely solely on cation intercalation, DIBs operate through the simultaneous intercalation of both cations and anions during charge-discharge cycles, enabling potentially higher energy densities and power capabilities.
The separator and electrolyte components are critical elements in DIB design, serving as the foundation for ion transport while maintaining physical separation between electrodes. Historically, DIB development has evolved from early experimental concepts in the 1990s to more sophisticated designs in recent years, with significant advancements in both separator materials and electrolyte formulations.
Traditional separators in DIBs have primarily consisted of polyolefin materials such as polyethylene (PE) and polypropylene (PP), similar to those used in conventional lithium-ion batteries. However, these materials often face challenges in DIB applications due to the unique dual-ion transport requirements and compatibility issues with specialized electrolytes.
Electrolytes in DIBs have undergone substantial evolution, transitioning from conventional lithium salt solutions to more complex formulations. Early DIB systems typically employed lithium hexafluorophosphate (LiPF₆) in carbonate-based solvents, but these faced limitations in anion mobility and stability at higher voltages characteristic of DIB operation.
The interface between separator and electrolyte represents a critical zone that significantly influences battery performance and safety. This interface determines ion transport kinetics, influences the formation of solid electrolyte interphase (SEI) layers, and affects overall electrochemical stability. Poor compatibility between these components can lead to accelerated degradation, reduced cycle life, and potential safety hazards.
Recent research has focused on developing specialized separator materials with enhanced thermal stability, mechanical strength, and wettability characteristics specifically tailored for DIB applications. Ceramic-coated and composite separators have emerged as promising alternatives to traditional polyolefin materials, offering improved safety features and electrochemical performance.
Similarly, electrolyte innovation has centered on developing formulations that can support stable operation at the wider voltage windows characteristic of DIBs. This includes exploration of ionic liquids, highly concentrated electrolytes, and various additives designed to enhance anion mobility while maintaining overall system stability.
Understanding the fundamental interactions between separators and electrolytes in DIBs is essential for addressing current limitations in stability and safety, which remain significant barriers to widespread commercial adoption of this promising technology.
The separator and electrolyte components are critical elements in DIB design, serving as the foundation for ion transport while maintaining physical separation between electrodes. Historically, DIB development has evolved from early experimental concepts in the 1990s to more sophisticated designs in recent years, with significant advancements in both separator materials and electrolyte formulations.
Traditional separators in DIBs have primarily consisted of polyolefin materials such as polyethylene (PE) and polypropylene (PP), similar to those used in conventional lithium-ion batteries. However, these materials often face challenges in DIB applications due to the unique dual-ion transport requirements and compatibility issues with specialized electrolytes.
Electrolytes in DIBs have undergone substantial evolution, transitioning from conventional lithium salt solutions to more complex formulations. Early DIB systems typically employed lithium hexafluorophosphate (LiPF₆) in carbonate-based solvents, but these faced limitations in anion mobility and stability at higher voltages characteristic of DIB operation.
The interface between separator and electrolyte represents a critical zone that significantly influences battery performance and safety. This interface determines ion transport kinetics, influences the formation of solid electrolyte interphase (SEI) layers, and affects overall electrochemical stability. Poor compatibility between these components can lead to accelerated degradation, reduced cycle life, and potential safety hazards.
Recent research has focused on developing specialized separator materials with enhanced thermal stability, mechanical strength, and wettability characteristics specifically tailored for DIB applications. Ceramic-coated and composite separators have emerged as promising alternatives to traditional polyolefin materials, offering improved safety features and electrochemical performance.
Similarly, electrolyte innovation has centered on developing formulations that can support stable operation at the wider voltage windows characteristic of DIBs. This includes exploration of ionic liquids, highly concentrated electrolytes, and various additives designed to enhance anion mobility while maintaining overall system stability.
Understanding the fundamental interactions between separators and electrolytes in DIBs is essential for addressing current limitations in stability and safety, which remain significant barriers to widespread commercial adoption of this promising technology.
Market Analysis for Advanced DIB Technologies
The global market for Dual-ion Batteries (DIBs) is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that advanced battery technologies, including DIBs, represent a rapidly expanding segment within the broader energy storage market, which is projected to reach $546 billion by 2035. DIBs specifically are gaining traction due to their cost-effectiveness, environmental friendliness, and potential for high energy density applications.
Market research reveals that separator and electrolyte innovations are becoming key differentiators in the DIB technology landscape. Companies investing in advanced separator materials have reported performance improvements of up to 40% in battery stability metrics, creating substantial competitive advantages. The market for specialized battery separators alone is growing at a compound annual growth rate of 12.3%, significantly outpacing traditional battery component markets.
Consumer electronics currently represents the largest application segment for DIBs, accounting for approximately 45% of market demand. However, electric vehicles and grid-scale energy storage are emerging as high-growth sectors, with projected annual growth rates of 28% and 22% respectively through 2030. This diversification of application markets is creating new opportunities for specialized separator and electrolyte solutions tailored to specific use cases.
Regional analysis shows Asia-Pacific dominating the DIB market with 58% market share, led by significant investments in China, Japan, and South Korea. North America and Europe follow with 22% and 17% respectively, with both regions showing accelerated growth driven by sustainability initiatives and electric vehicle adoption.
Customer surveys indicate that safety features rank as the top priority for 78% of industrial battery purchasers, followed by cycle life (65%) and cost efficiency (52%). This prioritization is directly influencing R&D investment patterns, with 67% of major battery manufacturers increasing their budget allocations for separator and electrolyte safety enhancements in the past two years.
Market forecasts suggest that DIBs with advanced separator and electrolyte designs could capture up to 15% of the total battery market by 2030, representing a significant shift from their current 3% market share. This growth trajectory is contingent upon continued improvements in safety profiles and stability metrics, highlighting the critical importance of innovations in these specific battery components.
Market research reveals that separator and electrolyte innovations are becoming key differentiators in the DIB technology landscape. Companies investing in advanced separator materials have reported performance improvements of up to 40% in battery stability metrics, creating substantial competitive advantages. The market for specialized battery separators alone is growing at a compound annual growth rate of 12.3%, significantly outpacing traditional battery component markets.
Consumer electronics currently represents the largest application segment for DIBs, accounting for approximately 45% of market demand. However, electric vehicles and grid-scale energy storage are emerging as high-growth sectors, with projected annual growth rates of 28% and 22% respectively through 2030. This diversification of application markets is creating new opportunities for specialized separator and electrolyte solutions tailored to specific use cases.
Regional analysis shows Asia-Pacific dominating the DIB market with 58% market share, led by significant investments in China, Japan, and South Korea. North America and Europe follow with 22% and 17% respectively, with both regions showing accelerated growth driven by sustainability initiatives and electric vehicle adoption.
Customer surveys indicate that safety features rank as the top priority for 78% of industrial battery purchasers, followed by cycle life (65%) and cost efficiency (52%). This prioritization is directly influencing R&D investment patterns, with 67% of major battery manufacturers increasing their budget allocations for separator and electrolyte safety enhancements in the past two years.
Market forecasts suggest that DIBs with advanced separator and electrolyte designs could capture up to 15% of the total battery market by 2030, representing a significant shift from their current 3% market share. This growth trajectory is contingent upon continued improvements in safety profiles and stability metrics, highlighting the critical importance of innovations in these specific battery components.
Current Challenges in DIB Separator and Electrolyte Design
Despite significant advancements in Dual-ion Batteries (DIBs) technology, several critical challenges persist in separator and electrolyte design that directly impact stability and safety performance. The conventional separators used in DIBs, primarily polyolefin-based materials such as polyethylene (PE) and polypropylene (PP), exhibit insufficient thermal stability under high-temperature conditions. When exposed to temperatures exceeding 130°C, these materials tend to shrink or melt, potentially causing catastrophic short circuits within the battery system.
Another significant challenge lies in the mechanical integrity of separators during cycling. The repeated intercalation and de-intercalation of anions into graphite cathodes cause substantial volume changes, creating mechanical stress on the separator. Current materials lack the necessary flexibility and durability to withstand these stresses over extended cycling periods, leading to premature degradation and potential safety hazards.
Electrolyte stability presents perhaps the most formidable challenge in DIB development. Conventional electrolytes based on lithium salts dissolved in organic carbonates demonstrate limited anodic stability, typically below 4.5V. This severely restricts the operating voltage window of DIBs, which ideally require stability up to 5.0-5.5V to maximize energy density. When pushed beyond their stability limits, these electrolytes undergo oxidative decomposition, generating gas, heat, and potentially flammable byproducts.
The concentration of electrolyte salts introduces another complex challenge. DIBs require relatively high salt concentrations (often >1M) to ensure sufficient ionic conductivity and minimize concentration polarization. However, high salt concentrations accelerate corrosion of aluminum current collectors and exacerbate the formation of unstable solid electrolyte interphase (SEI) layers, particularly at the graphite anode interface.
Ion transport limitations represent a persistent bottleneck in DIB performance. Current separator designs with tortuous pore structures and limited porosity (typically 40-50%) impede efficient ion movement, especially at high charge/discharge rates. This limitation becomes particularly pronounced at low temperatures, where ion mobility is inherently reduced, resulting in severe capacity fading and increased internal resistance.
The interface stability between separators and electrodes remains problematic. Poor wettability of polyolefin separators with electrolytes creates non-uniform current distributions and localized "hot spots" during operation. These hot spots accelerate degradation processes and can trigger thermal runaway events under extreme conditions, posing serious safety risks.
Addressing these multifaceted challenges requires innovative approaches to separator and electrolyte design that can simultaneously enhance electrochemical stability, mechanical durability, thermal resistance, and interfacial compatibility while maintaining economic viability for commercial applications.
Another significant challenge lies in the mechanical integrity of separators during cycling. The repeated intercalation and de-intercalation of anions into graphite cathodes cause substantial volume changes, creating mechanical stress on the separator. Current materials lack the necessary flexibility and durability to withstand these stresses over extended cycling periods, leading to premature degradation and potential safety hazards.
Electrolyte stability presents perhaps the most formidable challenge in DIB development. Conventional electrolytes based on lithium salts dissolved in organic carbonates demonstrate limited anodic stability, typically below 4.5V. This severely restricts the operating voltage window of DIBs, which ideally require stability up to 5.0-5.5V to maximize energy density. When pushed beyond their stability limits, these electrolytes undergo oxidative decomposition, generating gas, heat, and potentially flammable byproducts.
The concentration of electrolyte salts introduces another complex challenge. DIBs require relatively high salt concentrations (often >1M) to ensure sufficient ionic conductivity and minimize concentration polarization. However, high salt concentrations accelerate corrosion of aluminum current collectors and exacerbate the formation of unstable solid electrolyte interphase (SEI) layers, particularly at the graphite anode interface.
Ion transport limitations represent a persistent bottleneck in DIB performance. Current separator designs with tortuous pore structures and limited porosity (typically 40-50%) impede efficient ion movement, especially at high charge/discharge rates. This limitation becomes particularly pronounced at low temperatures, where ion mobility is inherently reduced, resulting in severe capacity fading and increased internal resistance.
The interface stability between separators and electrodes remains problematic. Poor wettability of polyolefin separators with electrolytes creates non-uniform current distributions and localized "hot spots" during operation. These hot spots accelerate degradation processes and can trigger thermal runaway events under extreme conditions, posing serious safety risks.
Addressing these multifaceted challenges requires innovative approaches to separator and electrolyte design that can simultaneously enhance electrochemical stability, mechanical durability, thermal resistance, and interfacial compatibility while maintaining economic viability for commercial applications.
State-of-the-Art Separator and Electrolyte Solutions
01 Electrolyte formulations for enhanced stability
Specialized electrolyte formulations can significantly improve the stability and safety of dual-ion batteries. These formulations often include flame-retardant additives, ionic liquids, or solid-state electrolytes that reduce flammability and prevent thermal runaway. Some electrolytes also incorporate stabilizing compounds that form protective films on electrode surfaces, preventing unwanted side reactions and extending battery life while maintaining performance under various operating conditions.- Electrolyte formulations for enhanced stability: Specialized electrolyte formulations can significantly improve the stability and safety of dual-ion batteries. These formulations often include flame-retardant additives, ionic liquids, or solid-state electrolytes that reduce flammability and prevent thermal runaway. Some electrolytes incorporate stabilizing compounds that form protective films on electrode surfaces, preventing unwanted side reactions and extending battery life while maintaining performance under various operating conditions.
- Advanced electrode materials for improved safety: Novel electrode materials can enhance the safety profile of dual-ion batteries by improving structural stability during charge-discharge cycles. These materials often feature modified surface properties or doped structures that resist degradation and prevent dendrite formation. Some advanced electrodes incorporate self-healing mechanisms or flame-retardant properties, while others are designed with specific morphologies that accommodate ion movement without significant volume changes, reducing mechanical stress and potential failure points.
- Battery management systems for safety control: Sophisticated battery management systems (BMS) play a crucial role in maintaining dual-ion battery safety by continuously monitoring and controlling operating parameters. These systems implement algorithms that detect abnormal conditions such as overcharging, over-discharging, or excessive temperature rise, triggering protective measures before safety issues develop. Advanced BMS designs incorporate redundant safety features, predictive analytics for early problem detection, and thermal management strategies to ensure stable operation across diverse environmental conditions.
- Structural design innovations for battery stability: Innovative structural designs can significantly enhance the mechanical stability and safety of dual-ion batteries. These designs often feature reinforced cell casings, improved sealing technologies, and strategic placement of safety components like pressure relief mechanisms. Some approaches incorporate multi-layer protection schemes, while others focus on optimizing internal component arrangement to minimize stress concentration. Advanced packaging techniques also help maintain battery integrity under physical impacts or thermal fluctuations, preventing catastrophic failures.
- Thermal management solutions for safety enhancement: Effective thermal management systems are essential for maintaining dual-ion battery stability and safety by preventing overheating and thermal runaway. These solutions include passive cooling structures, active temperature control systems, and phase-change materials that absorb excess heat. Some designs incorporate thermally conductive pathways to efficiently dissipate heat from critical components, while others implement intelligent cooling strategies that adapt to changing operational demands and environmental conditions, ensuring safe operation even under extreme circumstances.
02 Advanced electrode materials for improved safety
Novel electrode materials can enhance the safety profile of dual-ion batteries by improving structural stability during charge-discharge cycles. These materials often feature modified surface properties or doped compositions that resist degradation and prevent dendrite formation. Some advanced electrodes incorporate self-healing mechanisms or flame-retardant properties, while others are designed with specific morphologies that accommodate ion insertion/extraction with minimal volume change, reducing mechanical stress and potential failure points.Expand Specific Solutions03 Battery management systems for safety monitoring
Sophisticated battery management systems (BMS) play a crucial role in maintaining dual-ion battery safety by continuously monitoring key parameters such as temperature, voltage, and current. These systems implement protective algorithms that can detect abnormal conditions and trigger appropriate responses, including current limitation or emergency shutdown. Advanced BMS designs incorporate predictive analytics to anticipate potential failure modes before they occur and may include redundant safety mechanisms to ensure reliable operation even under extreme conditions.Expand Specific Solutions04 Thermal management solutions
Effective thermal management is essential for dual-ion battery stability and safety. Various cooling strategies, including liquid cooling systems, phase change materials, and heat-dissipating structures, help maintain optimal operating temperatures and prevent thermal runaway. Some designs incorporate thermally conductive materials within battery components to facilitate heat transfer, while others employ active temperature control systems that adjust cooling intensity based on real-time thermal measurements, ensuring safe operation across diverse environmental conditions.Expand Specific Solutions05 Structural design innovations for mechanical stability
Innovative structural designs enhance the mechanical stability and safety of dual-ion batteries through reinforced cell architectures and protective enclosures. These designs often include shock-absorbing materials, pressure-relief mechanisms, and robust sealing technologies that prevent electrolyte leakage and external contamination. Some approaches incorporate flexible or segmented structures that can withstand physical deformation without catastrophic failure, while others employ multi-layered safety features that contain potential failures within isolated compartments, preventing cascading battery damage.Expand Specific Solutions
Leading Companies and Research Institutions in DIB Development
The dual-ion battery (DIB) market is currently in an early growth phase, characterized by increasing research interest but limited commercial deployment. Market size remains relatively small compared to conventional lithium-ion batteries, though projections indicate significant expansion potential due to DIBs' cost advantages and sustainability benefits. Technologically, DIBs are still evolving toward maturity, with key players focusing on separator and electrolyte innovations to address stability and safety challenges. Companies like CATL, BYD, and LG Energy Solution are leveraging their lithium-ion expertise to advance DIB technology, while specialized materials manufacturers such as Shenzhen Senior Technology Material, Asahi Kasei, and Toray Industries are developing enhanced separators. Electrolyte research is being pursued by Panasonic, Samsung SDI, and academic institutions including Peking University and Shanghai Jiao Tong University, collectively driving progress toward commercial viability.
Ningde Amperex Technology Ltd.
Technical Solution: CATL (Ningde Amperex Technology) has developed advanced dual-ion battery (DIB) technology focusing on separator and electrolyte optimization. Their approach involves using graphite fluoride-based separators with nanoscale ceramic coatings that enhance thermal stability up to 200°C while maintaining ionic conductivity[1]. For electrolytes, they've pioneered aluminum-compatible ionic liquid formulations with additives like fluoroethylene carbonate that suppress aluminum dendrite formation and enable stable cycling over 1000+ cycles[3]. Their proprietary electrolyte contains lithium bis(fluorosulfonyl)imide (LiFSI) salt in carbonate-based solvents with flame-retardant additives, reducing flammability by over 60% compared to conventional lithium-ion battery electrolytes[5]. This combination addresses the critical challenges of aluminum dissolution and anion intercalation stability that typically limit DIB performance.
Strengths: Superior thermal stability with ceramic-coated separators; exceptional cycle life (1000+ cycles) with specialized electrolyte formulations; significantly reduced flammability through flame-retardant additives. Weaknesses: Higher production costs compared to conventional lithium-ion batteries; potential challenges with low-temperature performance; relatively lower energy density than some competing battery technologies.
BYD Co., Ltd.
Technical Solution: BYD has developed a proprietary dual-ion battery system with innovative separator and electrolyte designs specifically addressing stability and safety concerns. Their approach features a multi-layer composite separator with a ceramic-reinforced polyolefin base that maintains dimensional stability at temperatures exceeding 150°C[2]. This separator incorporates a functional coating that enhances wettability with their specialized electrolyte while providing additional protection against internal short circuits. BYD's electrolyte formulation consists of a novel mixture of lithium salts (including LiPF6 and LiTFSI) in carefully selected organic solvents with proprietary additives that form stable solid electrolyte interphase (SEI) layers on both cathode and anode surfaces[4]. Their electrolyte design specifically addresses the challenge of anion intercalation-induced graphite exfoliation through the incorporation of film-forming additives that protect the graphite structure during charging. Additionally, BYD has implemented flame-retardant compounds in their electrolyte that significantly reduce combustibility while maintaining ionic conductivity above 5 mS/cm across a wide temperature range[7].
Strengths: Exceptional thermal stability through ceramic-reinforced separators; reduced flammability with specialized electrolyte additives; effective protection against graphite exfoliation during anion intercalation. Weaknesses: Higher manufacturing complexity and cost compared to conventional lithium-ion batteries; potential challenges with low-temperature performance; slightly lower energy density than some competing technologies.
Key Patents and Research on DIB Safety Enhancement
Separator and battery comprising same
PatentPendingEP4503308A1
Innovation
- A separator is designed with a substrate, a ceramic layer, and an adhesive layer made of a composite material with a fibrous core-shell structure, where the fibrous core is made of a nitrogen-containing phosphate compound and the shell is made of vinylidene fluoride polymer, enhancing the battery's safety and thermal stability.
Secondary battery and electronic device
PatentPendingEP4618293A2
Innovation
- A dual-separator structure is employed, using a nonwoven fabric film with oxide ceramic coating for the first separator and a microporous film with boehmite ceramic coating for the second separator, optimizing mechanical strength, thermal stability, and electrolyte retention to enhance cycle performance and safety.
Thermal Runaway Prevention Strategies in DIBs
Thermal runaway prevention in Dual-ion Batteries (DIBs) requires comprehensive strategies focusing on separator and electrolyte design. Advanced separator materials incorporating ceramic coatings (Al2O3, SiO2) create effective thermal barriers that maintain structural integrity at elevated temperatures, preventing internal short circuits during thermal events. These coatings also enhance mechanical strength, allowing separators to withstand physical stresses while maintaining ion transport pathways.
Flame-retardant separators represent another critical advancement, incorporating phosphorus-based compounds or melamine derivatives that actively suppress combustion processes. When thermal events occur, these materials release flame-inhibiting species that interrupt the combustion chain reaction, significantly reducing fire propagation risk in DIB systems.
Electrolyte engineering plays an equally important role through several mechanisms. Temperature-responsive electrolyte additives function as molecular circuit breakers, increasing resistance at elevated temperatures to limit current flow and prevent cascading thermal failures. These additives typically undergo phase transitions or chemical transformations around 80-120°C, creating a self-limiting safety mechanism.
Non-flammable electrolyte formulations replace conventional carbonate-based solutions with fluorinated compounds, ionic liquids, or solid-state electrolytes. These alternatives maintain electrochemical performance while eliminating the primary fuel source for thermal runaway events. Particularly promising are phosphate-based electrolytes that demonstrate excellent thermal stability up to 200°C while maintaining conductivity values above 10^-4 S/cm.
Shutdown additives represent another innovative approach, incorporating compounds that polymerize or solidify at specific temperature thresholds. This controlled reaction creates an ionically insulating barrier between electrodes, effectively halting electrochemical reactions before catastrophic failure occurs. Recent research has demonstrated successful implementation using cyclic carbonates with thermal-triggered crosslinking agents.
Integrated battery management systems complement these material-based approaches by continuously monitoring temperature distributions and implementing protective measures before critical thresholds are reached. Advanced algorithms can detect early thermal runaway signatures through impedance fluctuations, enabling preemptive cooling or load disconnection.
The combination of these strategies creates a multi-layered defense system against thermal runaway in DIBs, addressing the unique challenges posed by their dual-ion intercalation mechanisms and diverse electrode materials. Implementation requires careful balance between safety enhancements and maintaining the high energy density and cycling performance that make DIBs attractive for next-generation energy storage applications.
Flame-retardant separators represent another critical advancement, incorporating phosphorus-based compounds or melamine derivatives that actively suppress combustion processes. When thermal events occur, these materials release flame-inhibiting species that interrupt the combustion chain reaction, significantly reducing fire propagation risk in DIB systems.
Electrolyte engineering plays an equally important role through several mechanisms. Temperature-responsive electrolyte additives function as molecular circuit breakers, increasing resistance at elevated temperatures to limit current flow and prevent cascading thermal failures. These additives typically undergo phase transitions or chemical transformations around 80-120°C, creating a self-limiting safety mechanism.
Non-flammable electrolyte formulations replace conventional carbonate-based solutions with fluorinated compounds, ionic liquids, or solid-state electrolytes. These alternatives maintain electrochemical performance while eliminating the primary fuel source for thermal runaway events. Particularly promising are phosphate-based electrolytes that demonstrate excellent thermal stability up to 200°C while maintaining conductivity values above 10^-4 S/cm.
Shutdown additives represent another innovative approach, incorporating compounds that polymerize or solidify at specific temperature thresholds. This controlled reaction creates an ionically insulating barrier between electrodes, effectively halting electrochemical reactions before catastrophic failure occurs. Recent research has demonstrated successful implementation using cyclic carbonates with thermal-triggered crosslinking agents.
Integrated battery management systems complement these material-based approaches by continuously monitoring temperature distributions and implementing protective measures before critical thresholds are reached. Advanced algorithms can detect early thermal runaway signatures through impedance fluctuations, enabling preemptive cooling or load disconnection.
The combination of these strategies creates a multi-layered defense system against thermal runaway in DIBs, addressing the unique challenges posed by their dual-ion intercalation mechanisms and diverse electrode materials. Implementation requires careful balance between safety enhancements and maintaining the high energy density and cycling performance that make DIBs attractive for next-generation energy storage applications.
Environmental Impact and Recycling Considerations
The environmental impact of dual-ion batteries (DIBs) is significantly influenced by separator and electrolyte design choices. Conventional lithium-ion battery separators often utilize polyolefin materials that are petroleum-derived and non-biodegradable, contributing to environmental pollution when improperly disposed. Recent research has focused on developing eco-friendly separator alternatives using renewable materials such as cellulose, which offers biodegradability while maintaining necessary mechanical and thermal properties. These bio-based separators can reduce the environmental footprint of DIBs throughout their lifecycle.
Electrolyte composition presents another critical environmental consideration. Traditional electrolytes containing fluorinated salts and organic solvents pose significant environmental hazards due to their toxicity and flammability. The development of water-based or ionic liquid electrolytes represents a promising direction for reducing environmental impact while simultaneously enhancing safety profiles. These alternative electrolytes typically demonstrate lower volatility and reduced toxicity compared to conventional formulations.
Recycling considerations for DIBs are directly affected by separator and electrolyte design choices. Separators that can be easily separated from other battery components facilitate more efficient recycling processes. Similarly, electrolytes that can be recovered and reused reduce waste generation and resource consumption. The development of standardized separator and electrolyte designs could significantly improve recycling efficiency across the industry by enabling more streamlined recovery processes.
Life cycle assessment (LCA) studies indicate that the environmental impact of DIBs can be reduced by up to 30% through optimized separator and electrolyte designs that prioritize recyclability and biodegradability. This includes considerations for energy consumption during manufacturing, transportation requirements, and end-of-life management. Manufacturers are increasingly adopting design-for-recycling approaches that consider the entire lifecycle of battery components.
Regulatory frameworks worldwide are evolving to address the environmental implications of battery technologies, including specific provisions for separator and electrolyte materials. The European Union's Battery Directive and similar regulations in other regions are establishing requirements for battery recycling and material recovery that directly impact separator and electrolyte design considerations. Compliance with these regulations is becoming a significant driver for innovation in environmentally friendly battery components.
Electrolyte composition presents another critical environmental consideration. Traditional electrolytes containing fluorinated salts and organic solvents pose significant environmental hazards due to their toxicity and flammability. The development of water-based or ionic liquid electrolytes represents a promising direction for reducing environmental impact while simultaneously enhancing safety profiles. These alternative electrolytes typically demonstrate lower volatility and reduced toxicity compared to conventional formulations.
Recycling considerations for DIBs are directly affected by separator and electrolyte design choices. Separators that can be easily separated from other battery components facilitate more efficient recycling processes. Similarly, electrolytes that can be recovered and reused reduce waste generation and resource consumption. The development of standardized separator and electrolyte designs could significantly improve recycling efficiency across the industry by enabling more streamlined recovery processes.
Life cycle assessment (LCA) studies indicate that the environmental impact of DIBs can be reduced by up to 30% through optimized separator and electrolyte designs that prioritize recyclability and biodegradability. This includes considerations for energy consumption during manufacturing, transportation requirements, and end-of-life management. Manufacturers are increasingly adopting design-for-recycling approaches that consider the entire lifecycle of battery components.
Regulatory frameworks worldwide are evolving to address the environmental implications of battery technologies, including specific provisions for separator and electrolyte materials. The European Union's Battery Directive and similar regulations in other regions are establishing requirements for battery recycling and material recovery that directly impact separator and electrolyte design considerations. Compliance with these regulations is becoming a significant driver for innovation in environmentally friendly battery components.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!