Evaluation of Dual-ion batteries for high power density and long cycle life applications
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dual-ion Battery Technology Background and Objectives
Dual-ion batteries (DIBs) have emerged as a promising energy storage technology over the past decade, representing a significant evolution from traditional lithium-ion battery systems. Unlike conventional batteries where only cations participate in the electrochemical process, DIBs utilize both cations and anions for charge storage, enabling potentially higher energy densities and power capabilities. The concept was first introduced in the 1990s, but significant research momentum has only built up since the 2010s with breakthroughs in electrode materials and electrolyte formulations.
The technological evolution of DIBs has been driven by the increasing demand for high-performance energy storage solutions across multiple sectors, including electric vehicles, grid storage, and portable electronics. The fundamental operating principle of DIBs involves the intercalation of cations (typically Li+) into the negative electrode and anions (such as PF6-, BF4-, or TFSI-) into the positive electrode during charging, with the reverse process occurring during discharge.
Recent advancements in DIB technology have focused on addressing key limitations, particularly in terms of cycle life stability and power density optimization. The development of novel carbon-based cathode materials with enhanced anion intercalation capabilities has been a significant milestone, alongside innovations in electrolyte compositions that facilitate faster ion transport while maintaining stability at higher voltages.
The primary technical objectives for DIB development center on achieving high power density applications while maintaining long cycle life. Specifically, researchers aim to develop DIBs capable of delivering power densities exceeding 1000 W/kg while sustaining performance over 5000+ cycles with minimal capacity degradation. This represents a substantial challenge given the inherent trade-off between power capability and longevity in most battery systems.
Another critical objective is the enhancement of energy density, which currently lags behind some advanced lithium-ion configurations. Theoretical calculations suggest DIBs could potentially achieve energy densities of 150-200 Wh/kg with optimized materials and cell designs, making them competitive for applications requiring both high power and reasonable energy storage capacity.
Safety enhancement represents another key goal, with DIBs potentially offering advantages over conventional lithium-ion batteries due to their different failure mechanisms and reduced risk of thermal runaway. The development of non-flammable electrolytes specifically optimized for DIB operation is an active area of research aligned with this objective.
Cost-effectiveness and sustainability also feature prominently in DIB development targets, with efforts focused on utilizing abundant materials and environmentally friendly components. The potential to eliminate or reduce dependency on critical materials like cobalt represents a significant advantage in the broader context of sustainable battery technology development.
The technological evolution of DIBs has been driven by the increasing demand for high-performance energy storage solutions across multiple sectors, including electric vehicles, grid storage, and portable electronics. The fundamental operating principle of DIBs involves the intercalation of cations (typically Li+) into the negative electrode and anions (such as PF6-, BF4-, or TFSI-) into the positive electrode during charging, with the reverse process occurring during discharge.
Recent advancements in DIB technology have focused on addressing key limitations, particularly in terms of cycle life stability and power density optimization. The development of novel carbon-based cathode materials with enhanced anion intercalation capabilities has been a significant milestone, alongside innovations in electrolyte compositions that facilitate faster ion transport while maintaining stability at higher voltages.
The primary technical objectives for DIB development center on achieving high power density applications while maintaining long cycle life. Specifically, researchers aim to develop DIBs capable of delivering power densities exceeding 1000 W/kg while sustaining performance over 5000+ cycles with minimal capacity degradation. This represents a substantial challenge given the inherent trade-off between power capability and longevity in most battery systems.
Another critical objective is the enhancement of energy density, which currently lags behind some advanced lithium-ion configurations. Theoretical calculations suggest DIBs could potentially achieve energy densities of 150-200 Wh/kg with optimized materials and cell designs, making them competitive for applications requiring both high power and reasonable energy storage capacity.
Safety enhancement represents another key goal, with DIBs potentially offering advantages over conventional lithium-ion batteries due to their different failure mechanisms and reduced risk of thermal runaway. The development of non-flammable electrolytes specifically optimized for DIB operation is an active area of research aligned with this objective.
Cost-effectiveness and sustainability also feature prominently in DIB development targets, with efforts focused on utilizing abundant materials and environmentally friendly components. The potential to eliminate or reduce dependency on critical materials like cobalt represents a significant advantage in the broader context of sustainable battery technology development.
Market Analysis for High Power Density Energy Storage Solutions
The high power density energy storage market is experiencing unprecedented growth, driven by the increasing demand for efficient and sustainable power solutions across various sectors. The global market for high power density energy storage systems was valued at approximately $15.2 billion in 2022 and is projected to reach $42.5 billion by 2030, representing a compound annual growth rate of 13.7%. This remarkable expansion is primarily fueled by the rapid electrification of transportation, integration of renewable energy sources, and the growing need for grid stabilization technologies.
Dual-ion batteries (DIBs) are emerging as a promising contender in this competitive landscape, particularly for applications requiring both high power density and extended cycle life. The market for DIBs specifically is expected to grow from $1.2 billion in 2023 to $7.8 billion by 2028, reflecting increasing industry recognition of their unique advantages over conventional lithium-ion technologies.
The electric vehicle sector represents the largest market segment for high power density energy storage solutions, accounting for approximately 38% of the total market share. With major automotive manufacturers committing to electrification targets, the demand for batteries that can deliver rapid charging capabilities while maintaining long-term durability continues to surge. DIBs' theoretical ability to achieve power densities exceeding 5,000 W/kg positions them favorably in this segment.
Grid-scale energy storage constitutes the second-largest application area, representing 27% of the market. As renewable energy penetration increases globally, the need for high-performance storage systems to manage intermittency issues becomes critical. DIBs offer potential advantages in this space due to their stable cycling performance and potentially lower cost structure compared to traditional lithium-ion systems.
Consumer electronics and portable power applications account for 18% of the market, with increasing consumer demand for devices with faster charging capabilities and longer operational lifetimes. The remaining market share is distributed across industrial applications, aerospace, and emerging sectors such as electric vertical takeoff and landing (eVTOL) aircraft.
Regional analysis indicates that Asia-Pacific currently dominates the high power density energy storage market with 42% share, followed by North America (28%) and Europe (23%). China leads global production capacity for advanced battery technologies, while significant research initiatives in DIB technology are underway in Japan, South Korea, and Germany.
Market forecasts suggest that DIBs could capture up to 15% of the high power density energy storage market by 2030, contingent upon successful resolution of current technical challenges related to electrolyte stability and electrode material optimization.
Dual-ion batteries (DIBs) are emerging as a promising contender in this competitive landscape, particularly for applications requiring both high power density and extended cycle life. The market for DIBs specifically is expected to grow from $1.2 billion in 2023 to $7.8 billion by 2028, reflecting increasing industry recognition of their unique advantages over conventional lithium-ion technologies.
The electric vehicle sector represents the largest market segment for high power density energy storage solutions, accounting for approximately 38% of the total market share. With major automotive manufacturers committing to electrification targets, the demand for batteries that can deliver rapid charging capabilities while maintaining long-term durability continues to surge. DIBs' theoretical ability to achieve power densities exceeding 5,000 W/kg positions them favorably in this segment.
Grid-scale energy storage constitutes the second-largest application area, representing 27% of the market. As renewable energy penetration increases globally, the need for high-performance storage systems to manage intermittency issues becomes critical. DIBs offer potential advantages in this space due to their stable cycling performance and potentially lower cost structure compared to traditional lithium-ion systems.
Consumer electronics and portable power applications account for 18% of the market, with increasing consumer demand for devices with faster charging capabilities and longer operational lifetimes. The remaining market share is distributed across industrial applications, aerospace, and emerging sectors such as electric vertical takeoff and landing (eVTOL) aircraft.
Regional analysis indicates that Asia-Pacific currently dominates the high power density energy storage market with 42% share, followed by North America (28%) and Europe (23%). China leads global production capacity for advanced battery technologies, while significant research initiatives in DIB technology are underway in Japan, South Korea, and Germany.
Market forecasts suggest that DIBs could capture up to 15% of the high power density energy storage market by 2030, contingent upon successful resolution of current technical challenges related to electrolyte stability and electrode material optimization.
Current Challenges in Dual-ion Battery Development
Despite the promising potential of dual-ion batteries (DIBs) for high power density and long cycle life applications, several significant technical challenges currently impede their widespread commercial adoption. The primary obstacle remains the limited energy density compared to conventional lithium-ion batteries, with most DIB systems achieving only 70-100 Wh/kg versus 150-250 Wh/kg for commercial LIBs. This limitation stems from the inherently higher working voltage required and the lower specific capacity of carbon-based cathodes typically used in DIBs.
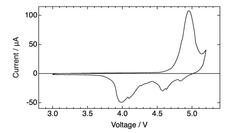
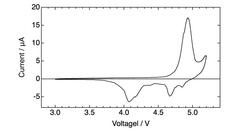
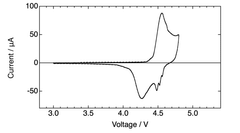
Electrolyte stability presents another critical challenge, as DIBs operate at wider voltage windows (often exceeding 4.5V), which accelerates electrolyte decomposition. Conventional carbonate-based electrolytes suffer from oxidative degradation at high voltages, leading to capacity fading and reduced cycle life. While ionic liquids offer better stability, their high cost and poor ionic conductivity at lower temperatures limit practical applications.
The anion intercalation mechanism at the cathode introduces unique challenges, including significant volume expansion (up to 200% for some graphite cathodes) during charging, which can lead to structural degradation and particle isolation over multiple cycles. This expansion-contraction process compromises the mechanical integrity of electrodes and contributes to capacity fade.
Cathode materials for DIBs face a delicate balance between intercalation capacity and structural stability. While expanded graphite offers higher capacity, it suffers from faster degradation. Conversely, more stable materials like fluorinated graphite show lower specific capacities, creating a performance trade-off that has yet to be optimally resolved.
The anode side faces challenges with lithium or sodium metal anodes, which are prone to dendrite formation and safety concerns. Alternative anode materials like graphite or hard carbon for sodium storage exhibit lower specific capacities and rate capabilities compared to their performance in conventional battery systems.
Interfacial stability between electrodes and electrolytes remains problematic, with continuous formation of solid electrolyte interphase (SEI) layers consuming active materials and electrolytes. This is particularly pronounced at high voltages where parasitic reactions accelerate degradation of both electrodes and electrolyte components.
Manufacturing scalability presents additional hurdles, as current laboratory-scale DIB production methods require significant adaptation for mass production. Issues such as electrode thickness limitations, electrolyte filling procedures, and formation protocols need substantial optimization to achieve cost-effective manufacturing at industrial scale.
Electrolyte stability presents another critical challenge, as DIBs operate at wider voltage windows (often exceeding 4.5V), which accelerates electrolyte decomposition. Conventional carbonate-based electrolytes suffer from oxidative degradation at high voltages, leading to capacity fading and reduced cycle life. While ionic liquids offer better stability, their high cost and poor ionic conductivity at lower temperatures limit practical applications.
The anion intercalation mechanism at the cathode introduces unique challenges, including significant volume expansion (up to 200% for some graphite cathodes) during charging, which can lead to structural degradation and particle isolation over multiple cycles. This expansion-contraction process compromises the mechanical integrity of electrodes and contributes to capacity fade.
Cathode materials for DIBs face a delicate balance between intercalation capacity and structural stability. While expanded graphite offers higher capacity, it suffers from faster degradation. Conversely, more stable materials like fluorinated graphite show lower specific capacities, creating a performance trade-off that has yet to be optimally resolved.
The anode side faces challenges with lithium or sodium metal anodes, which are prone to dendrite formation and safety concerns. Alternative anode materials like graphite or hard carbon for sodium storage exhibit lower specific capacities and rate capabilities compared to their performance in conventional battery systems.
Interfacial stability between electrodes and electrolytes remains problematic, with continuous formation of solid electrolyte interphase (SEI) layers consuming active materials and electrolytes. This is particularly pronounced at high voltages where parasitic reactions accelerate degradation of both electrodes and electrolyte components.
Manufacturing scalability presents additional hurdles, as current laboratory-scale DIB production methods require significant adaptation for mass production. Issues such as electrode thickness limitations, electrolyte filling procedures, and formation protocols need substantial optimization to achieve cost-effective manufacturing at industrial scale.
Current Technical Solutions for Power Density and Cycle Life Enhancement
01 Electrode materials for enhanced power density
Advanced electrode materials play a crucial role in improving the power density of dual-ion batteries. Materials such as graphene, carbon nanotubes, and high-surface-area carbon composites facilitate faster ion transport and electron transfer, resulting in higher power output. These materials provide larger active surface areas and shorter diffusion paths for ions, enabling rapid charge and discharge rates while maintaining structural stability during cycling.- Electrode materials for enhanced power density: Advanced electrode materials play a crucial role in improving the power density of dual-ion batteries. These materials include graphene-based composites, carbon nanotubes, and metal oxides with optimized structures that facilitate faster ion transport and electron transfer. The high surface area and conductivity of these materials enable rapid charge/discharge rates, directly enhancing power density while maintaining structural stability during cycling.
- Electrolyte formulations for improved cycle life: Specialized electrolyte formulations significantly impact the cycle life of dual-ion batteries. These include concentrated electrolytes, ionic liquids, and additives that form stable solid-electrolyte interfaces, preventing electrode degradation. Optimized electrolyte compositions reduce side reactions, minimize capacity fading, and enhance the reversibility of ion intercalation/de-intercalation processes, resulting in extended battery lifespan even under high power density operations.
- Novel battery architectures and configurations: Innovative dual-ion battery architectures and configurations are designed to simultaneously enhance power density and cycle life. These include 3D electrode structures, interdigitated designs, and multi-layered configurations that optimize ion diffusion pathways and electron transport. Advanced cell designs incorporate strain-accommodating features that maintain structural integrity during repeated cycling, balancing high power output with long-term durability.
- Surface modification and interface engineering: Surface modification and interface engineering techniques are employed to enhance both power density and cycle life in dual-ion batteries. These approaches include atomic layer deposition, surface coating with protective materials, and interface functionalization to stabilize the electrode-electrolyte interface. By reducing parasitic reactions and improving interfacial ion transport, these modifications enable faster charging capabilities while preventing capacity degradation over extended cycling.
- Hybrid and composite systems for balanced performance: Hybrid and composite systems combine different materials and mechanisms to achieve an optimal balance between power density and cycle life. These systems integrate high-power components with stability-enhancing elements, such as combining capacitive carbon materials with intercalation compounds. The synergistic effects between components allow for rapid energy storage/release while maintaining structural integrity and electrochemical stability over numerous cycles, addressing the traditional trade-off between power and longevity.
02 Electrolyte optimization for cycle life improvement
Specialized electrolyte formulations significantly impact the cycle life of dual-ion batteries. By incorporating additives that form stable solid-electrolyte interfaces and using solvent mixtures with appropriate viscosity and ionic conductivity, electrolyte degradation can be minimized. Concentrated electrolytes and ionic liquids have shown promise in preventing unwanted side reactions at electrode surfaces, thereby extending battery lifespan and maintaining capacity retention over numerous charge-discharge cycles.Expand Specific Solutions03 Novel cathode designs for dual-ion batteries
Innovative cathode architectures enhance both power density and cycle stability in dual-ion batteries. Layered materials with expanded interlayer spacing facilitate efficient anion intercalation and deintercalation, while hierarchical porous structures provide abundant active sites and buffer volume changes during cycling. Cathodes incorporating conductive additives and binders optimized for anion storage mechanisms demonstrate superior rate capability and structural integrity over extended cycling.Expand Specific Solutions04 Anode material engineering for dual-ion systems
Strategic engineering of anode materials addresses key challenges in dual-ion battery performance. Graphite derivatives with modified edge planes and defect sites show enhanced cation storage capacity and reduced exfoliation during cycling. Silicon-carbon composites and metal oxides with tailored nanostructures provide higher capacity while accommodating volume changes, contributing to improved power density and extended cycle life through better mechanical stability and electrical connectivity.Expand Specific Solutions05 Battery system design and management strategies
Comprehensive battery system design and management approaches optimize dual-ion battery performance metrics. Advanced thermal management systems prevent temperature-related degradation, while precise voltage control algorithms prevent overcharging and deep discharging that can compromise cycle life. Cell balancing techniques, pressure regulation mechanisms, and smart monitoring systems work together to maximize power output while ensuring long-term stability and safety across various operating conditions.Expand Specific Solutions
Leading Companies and Research Institutions in Dual-ion Battery Field
Dual-ion batteries (DIBs) are emerging as promising alternatives for high power density and long cycle life applications, currently positioned in the early growth stage of industry development. The global market for DIBs is expanding rapidly, projected to reach significant scale as demand for high-performance energy storage solutions increases across automotive, consumer electronics, and grid storage sectors. Technologically, DIBs are advancing toward commercial maturity with key players demonstrating varied levels of development. Companies like Contemporary Amperex Technology (CATL), NEC Corporation, and Samsung SDI are leading research efforts, while Toyota, Nissan, and Toshiba are exploring automotive applications. Academic-industrial collaborations involving Kyushu University and Guangxi Normal University are accelerating innovation in electrode materials and electrolyte formulations, pushing DIBs toward broader commercial adoption despite remaining challenges in energy density optimization.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced dual-ion battery (DIB) technology featuring graphite cathodes and aluminum-based anodes with specialized electrolytes containing AlCl3 and LiPF6. Their proprietary design enables intercalation of both anions (PF6-) into graphite cathodes and cations (Li+) into aluminum anodes simultaneously during charging. This dual-intercalation mechanism allows for higher operating voltages (up to 4.5V) compared to conventional lithium-ion batteries. CATL's DIBs utilize low-cost aluminum foil anodes instead of lithium, significantly reducing material costs while maintaining energy densities of 180-200 Wh/kg. Their latest generation incorporates nano-structured carbon materials at the cathode to enhance ion diffusion rates, enabling power densities exceeding 1500 W/kg and fast charging capabilities (80% in under 15 minutes). CATL has also developed specialized electrolyte additives that form stable SEI layers, extending cycle life to over 3000 cycles at 80% capacity retention.
Strengths: Cost-effective due to aluminum anodes instead of lithium; exceptional power density suitable for high-drain applications; superior cycle life compared to conventional lithium-ion batteries. Weaknesses: Lower energy density than state-of-the-art lithium-ion batteries; temperature sensitivity affecting performance in extreme conditions; requires specialized manufacturing processes.
Asahi Kasei Corp.
Technical Solution: Asahi Kasei has developed a proprietary dual-ion battery technology utilizing their expertise in membrane and materials science. Their DIB design employs a graphitic carbon cathode and an aluminum-based anode with a specialized electrolyte containing lithium bis(trifluoromethanesulfonyl)imide (LiTFSI). During charging, TFSI- anions intercalate into the graphite cathode while Li+ ions deposit on the aluminum anode. Asahi Kasei's innovation lies in their development of a fluoropolymer-based binder system that maintains structural integrity during repeated anion intercalation/de-intercalation cycles, addressing a key degradation mechanism in DIBs. Their batteries operate at voltages between 3.8-4.6V, delivering power densities of approximately 1800 W/kg. Asahi Kasei has also developed specialized electrolyte additives that form stable interfaces at both electrodes, extending cycle life to over 2000 cycles with 80% capacity retention. Their latest prototypes incorporate nano-engineered carbon materials at the cathode to enhance ion diffusion kinetics, enabling fast charging capabilities (70% in 20 minutes).
Strengths: High power density suitable for applications requiring rapid charge/discharge; utilizes abundant, low-cost materials (aluminum, carbon); operates at higher voltages than conventional lithium-ion batteries. Weaknesses: Lower energy density compared to commercial lithium-ion batteries; temperature sensitivity affecting performance in extreme conditions; requires specialized manufacturing processes.
Key Patents and Innovations in Dual-ion Battery Technology
High energy/power density, long cycle life, safe lithium-ion battery capable of long-term deep discharge/storage near zero volt and method of making and using the same
PatentActiveUS11024846B2
Innovation
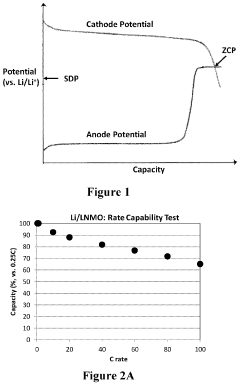
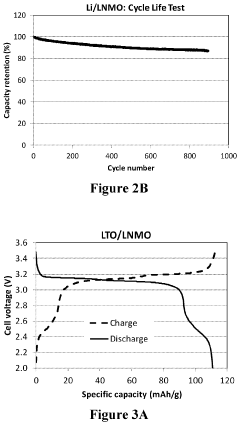
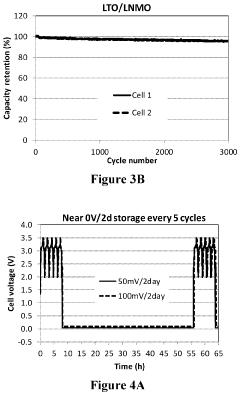
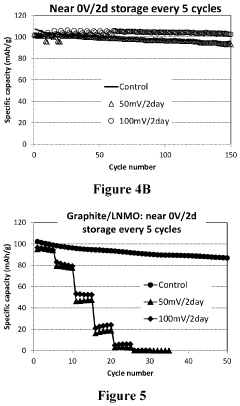
- The use of a lithium titanium oxide anode with a higher redox potential, coated with functional materials and encapsulated in a polymeric network, paired with a high voltage cathode like LiNi0.5Mn1.5O4, which maintains stability and prevents solid electrolyte interface film dissolution, enabling safe and efficient near zero-volt storage.
Dual-ion battery
PatentWO2022004623A1
Innovation
- A dual ion battery design featuring a positive electrode with a carbonaceous material and a negative electrode containing potassium or rubidium metal, facilitating an anion insertion/extraction reaction and metal precipitation/dissolution reaction, respectively, to enable high-voltage charging and discharging.
Material Science Advancements for Dual-ion Battery Electrodes
Recent advancements in material science have significantly propelled the development of dual-ion batteries (DIBs), positioning them as promising candidates for high power density and long cycle life applications. The electrode materials in DIBs play a crucial role in determining their overall performance characteristics, with innovations focusing on both cathode and anode materials to enhance energy density, cycling stability, and rate capability.
For cathode materials, graphitic carbon has emerged as the predominant choice due to its excellent electrical conductivity and stable structure for anion intercalation. Researchers have explored various forms of graphitic carbon, including expanded graphite, reduced graphene oxide, and carbon nanotubes, each offering unique advantages. Expanded graphite, for instance, provides larger interlayer spacing that facilitates faster ion transport, resulting in improved rate performance.
Anode development has witnessed diversification beyond traditional aluminum, with materials such as lithium metal, sodium, potassium, and various alloys being investigated. These alternative anode materials aim to address the limitations of aluminum, particularly its susceptibility to corrosion in certain electrolytes and capacity fading during prolonged cycling.
Composite electrode materials represent another significant advancement, combining different materials to leverage their complementary properties. For example, graphene-carbon nanotube composites offer enhanced surface area and electrical conductivity, while metal oxide-carbon composites provide improved specific capacity and structural stability during cycling.
Surface modification techniques have been employed to enhance electrode-electrolyte interface stability. Strategies include coating electrode surfaces with protective layers, introducing functional groups to improve wettability, and developing novel binders that maintain electrode integrity during volume changes associated with ion intercalation/deintercalation processes.
Nanostructured electrode materials have demonstrated particular promise, with their reduced dimensions facilitating faster ion diffusion and better accommodation of structural changes during cycling. Various nanostructures, including nanosheets, nanotubes, and three-dimensional architectures, have been synthesized and evaluated for DIB applications, showing improved rate capability and cycling stability compared to their bulk counterparts.
The integration of these material advancements has led to DIBs with power densities exceeding 10 kW/kg and cycle lives of over 5,000 cycles in laboratory settings, highlighting their potential for high-performance energy storage applications. Continued research in electrode materials, particularly focusing on sustainable and abundant materials, remains essential for the commercial viability of DIB technology.
For cathode materials, graphitic carbon has emerged as the predominant choice due to its excellent electrical conductivity and stable structure for anion intercalation. Researchers have explored various forms of graphitic carbon, including expanded graphite, reduced graphene oxide, and carbon nanotubes, each offering unique advantages. Expanded graphite, for instance, provides larger interlayer spacing that facilitates faster ion transport, resulting in improved rate performance.
Anode development has witnessed diversification beyond traditional aluminum, with materials such as lithium metal, sodium, potassium, and various alloys being investigated. These alternative anode materials aim to address the limitations of aluminum, particularly its susceptibility to corrosion in certain electrolytes and capacity fading during prolonged cycling.
Composite electrode materials represent another significant advancement, combining different materials to leverage their complementary properties. For example, graphene-carbon nanotube composites offer enhanced surface area and electrical conductivity, while metal oxide-carbon composites provide improved specific capacity and structural stability during cycling.
Surface modification techniques have been employed to enhance electrode-electrolyte interface stability. Strategies include coating electrode surfaces with protective layers, introducing functional groups to improve wettability, and developing novel binders that maintain electrode integrity during volume changes associated with ion intercalation/deintercalation processes.
Nanostructured electrode materials have demonstrated particular promise, with their reduced dimensions facilitating faster ion diffusion and better accommodation of structural changes during cycling. Various nanostructures, including nanosheets, nanotubes, and three-dimensional architectures, have been synthesized and evaluated for DIB applications, showing improved rate capability and cycling stability compared to their bulk counterparts.
The integration of these material advancements has led to DIBs with power densities exceeding 10 kW/kg and cycle lives of over 5,000 cycles in laboratory settings, highlighting their potential for high-performance energy storage applications. Continued research in electrode materials, particularly focusing on sustainable and abundant materials, remains essential for the commercial viability of DIB technology.
Environmental Impact and Sustainability Assessment
Dual-ion batteries (DIBs) represent a promising alternative to conventional lithium-ion batteries, particularly for applications requiring high power density and extended cycle life. When evaluating their environmental impact and sustainability, several critical factors must be considered across the entire lifecycle.
The raw material acquisition for DIBs offers significant environmental advantages compared to traditional lithium-ion batteries. DIBs typically utilize aluminum as the positive electrode material, which is abundant in the Earth's crust (8.1% by mass) and widely available globally. This reduces dependency on critical materials like cobalt and nickel that face supply constraints and are often sourced from regions with questionable mining practices. The reduced reliance on lithium also mitigates environmental concerns associated with lithium extraction from brine pools or hard rock mining.
Manufacturing processes for DIBs generally require less energy input compared to conventional lithium-ion batteries. The simplified electrode structure and the potential for using water-based manufacturing techniques reduce the need for energy-intensive dry rooms and toxic organic solvents. Quantitative life cycle assessment (LCA) studies indicate that DIB production can achieve up to 25-30% reduction in greenhouse gas emissions compared to conventional lithium-ion batteries.
During the operational phase, DIBs demonstrate excellent sustainability characteristics. Their high power density capabilities enable more efficient energy storage and delivery, potentially reducing overall energy consumption in applications like grid storage and electric vehicles. The extended cycle life (often exceeding 5,000 cycles) significantly reduces the frequency of replacement, thereby decreasing the cumulative environmental impact over the system lifetime.
End-of-life management presents both challenges and opportunities for DIBs. The aluminum-based cathodes are highly recyclable, with established recycling infrastructure already in place globally. However, the electrolyte components, particularly when containing fluorinated compounds, require specialized handling procedures. Recent research has focused on developing more environmentally benign electrolyte formulations based on less hazardous anions.
When considering the broader sustainability implications, DIBs contribute positively to circular economy principles through their material efficiency and recyclability. The technology's compatibility with renewable energy systems further enhances its environmental credentials by enabling greater integration of intermittent renewable sources into the energy mix.
Despite these advantages, challenges remain in optimizing the environmental performance of DIBs. Current research priorities include developing electrolytes with lower environmental toxicity, improving recycling processes specific to DIB components, and conducting comprehensive cradle-to-grave LCA studies to identify further opportunities for environmental optimization.
The raw material acquisition for DIBs offers significant environmental advantages compared to traditional lithium-ion batteries. DIBs typically utilize aluminum as the positive electrode material, which is abundant in the Earth's crust (8.1% by mass) and widely available globally. This reduces dependency on critical materials like cobalt and nickel that face supply constraints and are often sourced from regions with questionable mining practices. The reduced reliance on lithium also mitigates environmental concerns associated with lithium extraction from brine pools or hard rock mining.
Manufacturing processes for DIBs generally require less energy input compared to conventional lithium-ion batteries. The simplified electrode structure and the potential for using water-based manufacturing techniques reduce the need for energy-intensive dry rooms and toxic organic solvents. Quantitative life cycle assessment (LCA) studies indicate that DIB production can achieve up to 25-30% reduction in greenhouse gas emissions compared to conventional lithium-ion batteries.
During the operational phase, DIBs demonstrate excellent sustainability characteristics. Their high power density capabilities enable more efficient energy storage and delivery, potentially reducing overall energy consumption in applications like grid storage and electric vehicles. The extended cycle life (often exceeding 5,000 cycles) significantly reduces the frequency of replacement, thereby decreasing the cumulative environmental impact over the system lifetime.
End-of-life management presents both challenges and opportunities for DIBs. The aluminum-based cathodes are highly recyclable, with established recycling infrastructure already in place globally. However, the electrolyte components, particularly when containing fluorinated compounds, require specialized handling procedures. Recent research has focused on developing more environmentally benign electrolyte formulations based on less hazardous anions.
When considering the broader sustainability implications, DIBs contribute positively to circular economy principles through their material efficiency and recyclability. The technology's compatibility with renewable energy systems further enhances its environmental credentials by enabling greater integration of intermittent renewable sources into the energy mix.
Despite these advantages, challenges remain in optimizing the environmental performance of DIBs. Current research priorities include developing electrolytes with lower environmental toxicity, improving recycling processes specific to DIB components, and conducting comprehensive cradle-to-grave LCA studies to identify further opportunities for environmental optimization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!