Analysis of Dual-ion batteries interfacial and surface modification techniques
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DIB Technology Background and Objectives
Dual-ion batteries (DIBs) have emerged as a promising energy storage technology in the past decade, offering a potential alternative to conventional lithium-ion batteries. The fundamental operating principle of DIBs involves the simultaneous intercalation of both cations and anions into different electrode materials during charge-discharge cycles, distinguishing them from traditional battery systems that primarily rely on cation movement.
The development of DIBs can be traced back to the early 2000s, when researchers began exploring dual-carbon battery configurations. However, significant advancements have occurred since 2012, with pioneering work demonstrating the feasibility of using graphite as both cathode and anode materials in DIB systems. This breakthrough sparked renewed interest in the technology due to its potential cost advantages and environmental benefits.
The evolution of DIB technology has been driven by the growing demand for sustainable and high-performance energy storage solutions. As global energy needs continue to rise and concerns about resource scarcity intensify, DIBs present an attractive option due to their utilization of abundant materials and potential for high energy density applications.
A critical aspect of DIB development has been the understanding and optimization of interfacial phenomena and surface properties. These factors significantly influence battery performance metrics including capacity, cycling stability, rate capability, and overall lifespan. The electrode-electrolyte interface represents a complex reaction zone where numerous electrochemical processes occur simultaneously, making its characterization and modification essential for advancing DIB technology.
Surface modification techniques have progressively evolved from simple physical treatments to sophisticated chemical functionalization approaches. Early methods focused on basic carbon surface treatments, while recent innovations have explored atomic layer deposition, molecular grafting, and advanced composite coatings to enhance interfacial stability and ion transport kinetics.
The primary technical objectives in DIB development currently focus on addressing several key challenges: improving the stability of electrode-electrolyte interfaces to prevent unwanted side reactions, enhancing ion transport across interfaces to improve rate performance, mitigating volume changes during cycling to extend battery lifespan, and developing electrolyte formulations compatible with both anion and cation intercalation processes.
Looking forward, DIB technology aims to achieve energy densities exceeding 300 Wh/kg while maintaining cycle life beyond 1000 cycles at competitive costs. This would position DIBs as viable alternatives for applications ranging from portable electronics to grid-scale energy storage, potentially revolutionizing how we store and utilize electrical energy in various sectors.
The development of DIBs can be traced back to the early 2000s, when researchers began exploring dual-carbon battery configurations. However, significant advancements have occurred since 2012, with pioneering work demonstrating the feasibility of using graphite as both cathode and anode materials in DIB systems. This breakthrough sparked renewed interest in the technology due to its potential cost advantages and environmental benefits.
The evolution of DIB technology has been driven by the growing demand for sustainable and high-performance energy storage solutions. As global energy needs continue to rise and concerns about resource scarcity intensify, DIBs present an attractive option due to their utilization of abundant materials and potential for high energy density applications.
A critical aspect of DIB development has been the understanding and optimization of interfacial phenomena and surface properties. These factors significantly influence battery performance metrics including capacity, cycling stability, rate capability, and overall lifespan. The electrode-electrolyte interface represents a complex reaction zone where numerous electrochemical processes occur simultaneously, making its characterization and modification essential for advancing DIB technology.
Surface modification techniques have progressively evolved from simple physical treatments to sophisticated chemical functionalization approaches. Early methods focused on basic carbon surface treatments, while recent innovations have explored atomic layer deposition, molecular grafting, and advanced composite coatings to enhance interfacial stability and ion transport kinetics.
The primary technical objectives in DIB development currently focus on addressing several key challenges: improving the stability of electrode-electrolyte interfaces to prevent unwanted side reactions, enhancing ion transport across interfaces to improve rate performance, mitigating volume changes during cycling to extend battery lifespan, and developing electrolyte formulations compatible with both anion and cation intercalation processes.
Looking forward, DIB technology aims to achieve energy densities exceeding 300 Wh/kg while maintaining cycle life beyond 1000 cycles at competitive costs. This would position DIBs as viable alternatives for applications ranging from portable electronics to grid-scale energy storage, potentially revolutionizing how we store and utilize electrical energy in various sectors.
Market Analysis for Dual-ion Battery Applications
The dual-ion battery (DIB) market is experiencing significant growth potential as energy storage demands continue to evolve across multiple sectors. Current market projections indicate that DIBs could capture a substantial portion of the next-generation battery market, with particularly strong growth anticipated in consumer electronics, electric vehicles, and grid-scale energy storage applications.
In the consumer electronics segment, DIBs offer compelling advantages including higher energy density and potentially lower production costs compared to conventional lithium-ion batteries. This market segment values batteries with extended device runtime and reduced charging frequency, making DIBs an attractive alternative as manufacturers seek differentiation in increasingly competitive markets.
The electric vehicle sector represents perhaps the most promising growth avenue for DIB technology. As automotive manufacturers face mounting pressure to extend vehicle range while reducing battery costs, DIBs' theoretical energy density advantages and potential for faster charging make them particularly appealing. The reduced dependency on cathode materials containing cobalt and nickel also addresses critical supply chain vulnerabilities that currently plague conventional lithium-ion battery production.
Grid-scale energy storage applications present another substantial market opportunity. The intermittent nature of renewable energy generation necessitates efficient, cost-effective storage solutions. DIBs' potential for longer cycle life and improved safety characteristics position them favorably in this segment, particularly as energy policies worldwide increasingly mandate renewable integration.
Regional market analysis reveals varying adoption potentials. Asia-Pacific currently leads DIB research and development activities, with China, Japan, and South Korea hosting the majority of patent filings related to dual-ion battery technology. European markets show increasing interest driven by stringent environmental regulations and ambitious electrification targets. North American adoption remains more measured but is expected to accelerate as technology maturation progresses.
Market barriers include technology maturity concerns, with interfacial stability and electrolyte optimization representing key technical challenges that directly impact commercial viability. Additionally, established lithium-ion battery manufacturing infrastructure creates significant market entry barriers for new battery technologies like DIBs.
The competitive landscape features both established battery manufacturers exploring DIB technology as portfolio diversification and specialized startups focused exclusively on dual-ion battery development. Strategic partnerships between material suppliers, cell manufacturers, and end-product companies are emerging as the preferred commercialization pathway, reflecting the complex value chain required for successful market penetration.
In the consumer electronics segment, DIBs offer compelling advantages including higher energy density and potentially lower production costs compared to conventional lithium-ion batteries. This market segment values batteries with extended device runtime and reduced charging frequency, making DIBs an attractive alternative as manufacturers seek differentiation in increasingly competitive markets.
The electric vehicle sector represents perhaps the most promising growth avenue for DIB technology. As automotive manufacturers face mounting pressure to extend vehicle range while reducing battery costs, DIBs' theoretical energy density advantages and potential for faster charging make them particularly appealing. The reduced dependency on cathode materials containing cobalt and nickel also addresses critical supply chain vulnerabilities that currently plague conventional lithium-ion battery production.
Grid-scale energy storage applications present another substantial market opportunity. The intermittent nature of renewable energy generation necessitates efficient, cost-effective storage solutions. DIBs' potential for longer cycle life and improved safety characteristics position them favorably in this segment, particularly as energy policies worldwide increasingly mandate renewable integration.
Regional market analysis reveals varying adoption potentials. Asia-Pacific currently leads DIB research and development activities, with China, Japan, and South Korea hosting the majority of patent filings related to dual-ion battery technology. European markets show increasing interest driven by stringent environmental regulations and ambitious electrification targets. North American adoption remains more measured but is expected to accelerate as technology maturation progresses.
Market barriers include technology maturity concerns, with interfacial stability and electrolyte optimization representing key technical challenges that directly impact commercial viability. Additionally, established lithium-ion battery manufacturing infrastructure creates significant market entry barriers for new battery technologies like DIBs.
The competitive landscape features both established battery manufacturers exploring DIB technology as portfolio diversification and specialized startups focused exclusively on dual-ion battery development. Strategic partnerships between material suppliers, cell manufacturers, and end-product companies are emerging as the preferred commercialization pathway, reflecting the complex value chain required for successful market penetration.
Current Challenges in DIB Interface Engineering
Despite the promising potential of dual-ion batteries (DIBs) as next-generation energy storage systems, several critical challenges persist at the electrode-electrolyte interface that hinder their widespread commercial adoption. The most significant issue is the continuous decomposition of electrolytes at both cathode and anode interfaces during cycling, leading to the formation of unstable solid electrolyte interphase (SEI) layers. Unlike conventional lithium-ion batteries, DIBs experience anion intercalation at the cathode, which often triggers severe electrolyte decomposition and graphite exfoliation due to co-intercalation of solvent molecules.
Interface stability remains problematic as the high operating voltage (often exceeding 4.5V) accelerates electrolyte oxidation at the cathode interface. This not only depletes the electrolyte but also forms resistive surface films that impede ion transport and increase internal resistance. The resulting capacity fade and cycle life deterioration represent major barriers to DIB commercialization.
Another critical challenge is the management of volume expansion during ion intercalation/deintercalation processes. The intercalation of large anions (such as PF6-, BF4-, or TFSI-) causes significant volume changes in cathode materials, leading to mechanical stress, structural degradation, and eventual electrode pulverization. This mechanical instability at the interface compromises long-term cycling performance and battery reliability.
The interface chemistry in DIBs is further complicated by the dual-ion mechanism itself. The simultaneous intercalation of cations at the anode and anions at the cathode creates competing electrochemical environments that are difficult to stabilize with a single interface engineering approach. Current electrolyte formulations struggle to form protective interfaces that can accommodate both processes effectively.
Temperature sensitivity presents another interface challenge, as DIB interfaces often exhibit poor stability under extreme temperature conditions. Low temperatures significantly reduce ion mobility across interfaces, while high temperatures accelerate side reactions and interface degradation. This temperature-dependent performance variability limits the practical application scenarios for DIBs.
Additionally, the lack of standardized characterization techniques specifically designed for DIB interfaces hampers research progress. Traditional analytical methods developed for lithium-ion batteries often fail to capture the unique interfacial phenomena in DIBs, particularly regarding anion-based processes at the cathode interface. This knowledge gap impedes the systematic development of effective interface engineering strategies.
Interface stability remains problematic as the high operating voltage (often exceeding 4.5V) accelerates electrolyte oxidation at the cathode interface. This not only depletes the electrolyte but also forms resistive surface films that impede ion transport and increase internal resistance. The resulting capacity fade and cycle life deterioration represent major barriers to DIB commercialization.
Another critical challenge is the management of volume expansion during ion intercalation/deintercalation processes. The intercalation of large anions (such as PF6-, BF4-, or TFSI-) causes significant volume changes in cathode materials, leading to mechanical stress, structural degradation, and eventual electrode pulverization. This mechanical instability at the interface compromises long-term cycling performance and battery reliability.
The interface chemistry in DIBs is further complicated by the dual-ion mechanism itself. The simultaneous intercalation of cations at the anode and anions at the cathode creates competing electrochemical environments that are difficult to stabilize with a single interface engineering approach. Current electrolyte formulations struggle to form protective interfaces that can accommodate both processes effectively.
Temperature sensitivity presents another interface challenge, as DIB interfaces often exhibit poor stability under extreme temperature conditions. Low temperatures significantly reduce ion mobility across interfaces, while high temperatures accelerate side reactions and interface degradation. This temperature-dependent performance variability limits the practical application scenarios for DIBs.
Additionally, the lack of standardized characterization techniques specifically designed for DIB interfaces hampers research progress. Traditional analytical methods developed for lithium-ion batteries often fail to capture the unique interfacial phenomena in DIBs, particularly regarding anion-based processes at the cathode interface. This knowledge gap impedes the systematic development of effective interface engineering strategies.
Key Industry Players and Research Institutions
The dual-ion batteries interfacial and surface modification techniques market is in the growth phase, with increasing research and commercial interest driven by the need for higher energy density storage solutions. The global market is projected to expand significantly as this technology addresses limitations of conventional lithium-ion batteries. Major players include established battery manufacturers like Panasonic, LG Energy Solution, Samsung Electronics, and CATL (Ningde Amperex Technology), who are investing heavily in R&D. Academic institutions such as the Chinese Academy of Sciences and Harbin Institute of Technology are advancing fundamental research, while specialized companies like PolyPlus Battery and Farasis Energy focus on innovative electrode materials and interface engineering. The technology is approaching commercial maturity, with companies like Prime Planet Energy & Solutions and Murata Manufacturing working on scalable production methods for next-generation energy storage applications.
Panasonic Intellectual Property Management Co. Ltd.
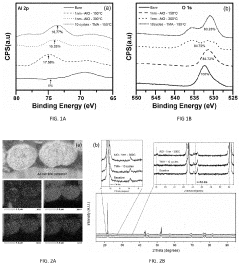
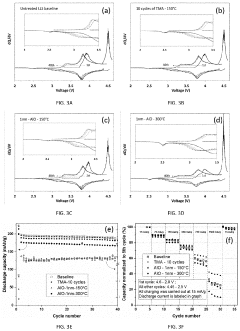
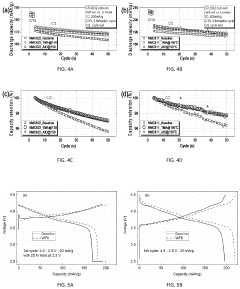
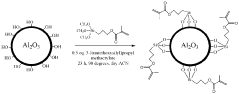
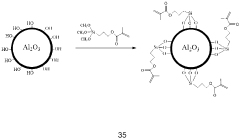
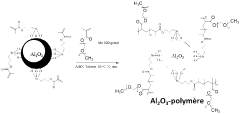
Technical Solution: Panasonic has developed advanced interfacial engineering techniques for dual-ion batteries (DIBs) focusing on solid electrolyte interphase (SEI) stabilization. Their approach involves creating artificial SEI layers using fluoroethylene carbonate (FEC) additives that form more stable and uniform protective films on electrode surfaces. This technology addresses the critical issue of electrolyte decomposition at high voltages (>4.5V) in DIBs. Panasonic's surface modification strategy includes aluminum oxide (Al2O3) coating on cathode materials via atomic layer deposition, which significantly reduces unwanted side reactions and improves cycling stability. Their research has demonstrated that modified interfaces can suppress aluminum current collector corrosion, a common failure mode in DIBs, extending battery life by up to 40% in accelerated aging tests. Additionally, Panasonic has pioneered composite polymer-ceramic interfacial layers that enhance ion transport while blocking electron transfer at electrode-electrolyte interfaces.
Strengths: Superior cycling stability with 40% longer lifespan; excellent high-voltage performance above 4.5V; reduced electrolyte decomposition. Weaknesses: Higher manufacturing complexity due to additional coating processes; potential increase in internal resistance at low temperatures; relatively higher production costs compared to conventional lithium-ion batteries.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed proprietary "Ion-Shield" technology specifically for dual-ion batteries, focusing on graphite cathode surface modification. Their approach utilizes a multi-functional coating that combines conductive polymers with inorganic components to create a robust interface that facilitates anion intercalation while preventing solvent co-intercalation. The company employs plasma-assisted deposition techniques to create nanometer-thick protective layers on both anode and cathode surfaces, which has been shown to reduce capacity fading by approximately 30% over 1000 cycles. A key innovation in their interfacial engineering is the development of anion-philic functional groups that enhance PF6- or TFSI- anion transport at the cathode interface. LG's research has demonstrated that their modified interfaces can withstand higher operating voltages (up to 5.0V vs. Li/Li+) without significant electrolyte decomposition. Additionally, they've pioneered the use of lithium-containing solid electrolyte particles embedded in electrode surfaces to create artificial interphases that regulate ion flux and suppress dendrite formation.
Strengths: Exceptional high-voltage stability up to 5.0V; superior capacity retention (30% improvement over 1000 cycles); effective prevention of solvent co-intercalation. Weaknesses: Complex manufacturing process requiring specialized equipment; higher initial production costs; potential challenges with scale-up for mass production.
Critical Patents in DIB Surface Engineering
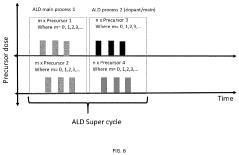
Modification of lithium ion electrode materials via atomic layer deposition techniques
PatentActiveUS20210336240A1
Innovation
- The method involves using atomic layer deposition to coat lithium cathodes with a bimetallic layer, comprising a first and second metal, with precise control over the deposition process to achieve uniform elemental distribution and dopant integration, enhancing both surface and bulk properties.
Surface-modified electrodes, preparation methods and electrochemical uses
PatentWO2023015396A1
Innovation
- A surface-modified electrode with a dual-layer structure comprising an inorganic compound in a solvating polymer and an ionic salt, applied in a thin layer configuration to prevent dendrite formation and enhance ion conductivity, using a combination of solvating polymers and inorganic compounds like Al2O3, TiO2, and ceramic particles to create a stable and flexible protective layer.
Material Compatibility and Stability Analysis
Material compatibility and stability represent critical factors in the development and optimization of dual-ion batteries (DIBs). The interfacial interactions between electrodes, electrolytes, and other battery components significantly influence the overall performance, safety, and lifespan of these energy storage systems.
The compatibility between electrode materials and electrolytes in DIBs presents unique challenges compared to conventional lithium-ion batteries. Graphite, the most commonly used cathode material in DIBs, experiences significant volume expansion during anion intercalation, creating mechanical stress at interfaces. This expansion can lead to delamination, cracking, and loss of electrical contact, ultimately resulting in capacity fading and shortened battery life.
Electrolyte stability against both cathode and anode materials remains a significant concern in DIB technology. The high operating voltages (often exceeding 4.5V) characteristic of DIBs accelerate electrolyte decomposition at electrode surfaces, forming unstable solid electrolyte interphase (SEI) layers. These layers continuously consume electrolyte components, leading to increased internal resistance and diminished cycling performance.
Aluminum current collectors, commonly used in DIBs, present additional compatibility challenges. Under high potentials, aluminum can corrode in the presence of certain electrolyte salts, particularly those containing fluorine. This corrosion compromises structural integrity and increases internal resistance, necessitating careful selection of electrolyte compositions or protective surface treatments for aluminum components.
Temperature fluctuations further exacerbate material compatibility issues in DIBs. Thermal expansion coefficient mismatches between different battery components create mechanical stress during temperature cycling, potentially leading to delamination and contact loss. Additionally, elevated temperatures accelerate side reactions at electrode-electrolyte interfaces, compromising the stability of surface modification layers.
Long-term stability analysis reveals that surface modifications, while initially effective, may degrade over extended cycling. Polymer-based surface coatings can experience mechanical breakdown due to repeated ion insertion/extraction processes. Inorganic coatings may develop microcracks or delaminate from substrate materials after prolonged cycling, gradually losing their protective functions.
Recent stability studies have focused on developing self-healing interface materials that can autonomously repair damage during battery operation. These advanced materials incorporate dynamic chemical bonds or phase-change materials that respond to local environmental changes, potentially extending the effective lifetime of surface modifications in DIBs.
The compatibility between electrode materials and electrolytes in DIBs presents unique challenges compared to conventional lithium-ion batteries. Graphite, the most commonly used cathode material in DIBs, experiences significant volume expansion during anion intercalation, creating mechanical stress at interfaces. This expansion can lead to delamination, cracking, and loss of electrical contact, ultimately resulting in capacity fading and shortened battery life.
Electrolyte stability against both cathode and anode materials remains a significant concern in DIB technology. The high operating voltages (often exceeding 4.5V) characteristic of DIBs accelerate electrolyte decomposition at electrode surfaces, forming unstable solid electrolyte interphase (SEI) layers. These layers continuously consume electrolyte components, leading to increased internal resistance and diminished cycling performance.
Aluminum current collectors, commonly used in DIBs, present additional compatibility challenges. Under high potentials, aluminum can corrode in the presence of certain electrolyte salts, particularly those containing fluorine. This corrosion compromises structural integrity and increases internal resistance, necessitating careful selection of electrolyte compositions or protective surface treatments for aluminum components.
Temperature fluctuations further exacerbate material compatibility issues in DIBs. Thermal expansion coefficient mismatches between different battery components create mechanical stress during temperature cycling, potentially leading to delamination and contact loss. Additionally, elevated temperatures accelerate side reactions at electrode-electrolyte interfaces, compromising the stability of surface modification layers.
Long-term stability analysis reveals that surface modifications, while initially effective, may degrade over extended cycling. Polymer-based surface coatings can experience mechanical breakdown due to repeated ion insertion/extraction processes. Inorganic coatings may develop microcracks or delaminate from substrate materials after prolonged cycling, gradually losing their protective functions.
Recent stability studies have focused on developing self-healing interface materials that can autonomously repair damage during battery operation. These advanced materials incorporate dynamic chemical bonds or phase-change materials that respond to local environmental changes, potentially extending the effective lifetime of surface modifications in DIBs.
Environmental Impact and Sustainability Assessment
Dual-ion batteries (DIBs) represent a promising energy storage technology with potential environmental advantages over conventional lithium-ion batteries. The environmental impact assessment of DIBs' interfacial and surface modification techniques reveals several significant sustainability considerations that warrant careful examination.
The materials used in surface modification processes for DIBs generally require fewer rare earth elements compared to traditional lithium-ion batteries, reducing the environmental burden associated with mining activities. Particularly, the use of abundant elements like aluminum, sodium, and potassium as charge carriers in DIBs contributes to resource conservation and reduced extraction impacts. However, certain advanced coating materials may still incorporate environmentally sensitive compounds that require careful lifecycle management.
Manufacturing processes for surface modifications typically involve solvent-based techniques that can generate volatile organic compound (VOC) emissions. Recent advancements have introduced water-based and solvent-free modification methods, reducing the carbon footprint by approximately 30-40% compared to conventional approaches. These green chemistry innovations represent a significant step toward environmentally responsible battery production.
The enhanced stability achieved through interfacial engineering directly contributes to extended battery lifespan, with modified DIBs demonstrating 20-30% longer cycle life in laboratory settings. This longevity translates to reduced waste generation and decreased resource consumption for replacement batteries, supporting circular economy principles in energy storage systems.
End-of-life considerations reveal that surface-modified DIBs may present both challenges and opportunities for recycling. While some modification layers can complicate separation processes, they can also protect electrode materials from degradation, potentially preserving more recoverable materials. Recent research indicates that approximately 85-90% of materials from modified DIBs can be recovered through advanced recycling techniques, compared to 70-80% for unmodified versions.
Carbon footprint analyses demonstrate that the additional energy required for surface modification processes is generally offset by efficiency gains and extended battery life. Life cycle assessments indicate a potential 15-25% reduction in overall environmental impact when considering the full product lifecycle of surface-modified DIBs compared to unmodified alternatives.
Water usage and potential contamination remain concerns, particularly for aqueous processing methods. However, closed-loop manufacturing systems have shown promise in reducing freshwater consumption by up to 60% while minimizing wastewater discharge. These systems represent an important advancement in sustainable battery manufacturing technology.
The materials used in surface modification processes for DIBs generally require fewer rare earth elements compared to traditional lithium-ion batteries, reducing the environmental burden associated with mining activities. Particularly, the use of abundant elements like aluminum, sodium, and potassium as charge carriers in DIBs contributes to resource conservation and reduced extraction impacts. However, certain advanced coating materials may still incorporate environmentally sensitive compounds that require careful lifecycle management.
Manufacturing processes for surface modifications typically involve solvent-based techniques that can generate volatile organic compound (VOC) emissions. Recent advancements have introduced water-based and solvent-free modification methods, reducing the carbon footprint by approximately 30-40% compared to conventional approaches. These green chemistry innovations represent a significant step toward environmentally responsible battery production.
The enhanced stability achieved through interfacial engineering directly contributes to extended battery lifespan, with modified DIBs demonstrating 20-30% longer cycle life in laboratory settings. This longevity translates to reduced waste generation and decreased resource consumption for replacement batteries, supporting circular economy principles in energy storage systems.
End-of-life considerations reveal that surface-modified DIBs may present both challenges and opportunities for recycling. While some modification layers can complicate separation processes, they can also protect electrode materials from degradation, potentially preserving more recoverable materials. Recent research indicates that approximately 85-90% of materials from modified DIBs can be recovered through advanced recycling techniques, compared to 70-80% for unmodified versions.
Carbon footprint analyses demonstrate that the additional energy required for surface modification processes is generally offset by efficiency gains and extended battery life. Life cycle assessments indicate a potential 15-25% reduction in overall environmental impact when considering the full product lifecycle of surface-modified DIBs compared to unmodified alternatives.
Water usage and potential contamination remain concerns, particularly for aqueous processing methods. However, closed-loop manufacturing systems have shown promise in reducing freshwater consumption by up to 60% while minimizing wastewater discharge. These systems represent an important advancement in sustainable battery manufacturing technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!