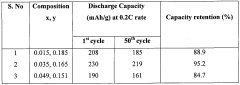
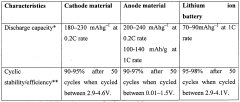
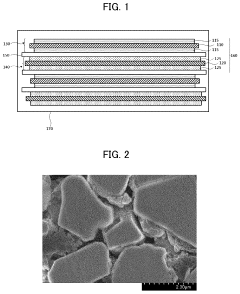
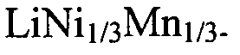Comparative analysis of Dual-ion batteries cathode materials performance and stability
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Dual-ion Batteries Evolution and Research Objectives
Dual-ion batteries (DIBs) have emerged as a promising alternative to conventional lithium-ion batteries due to their potential for higher energy density, lower cost, and improved safety characteristics. The evolution of DIBs can be traced back to the early 1990s when the concept was first introduced, but significant advancements have only been realized in the past decade with the development of novel electrode materials and electrolyte systems.
The fundamental operating principle of DIBs differs from traditional lithium-ion batteries as both anions and cations participate in the electrochemical processes. During charging, anions (typically PF6-, BF4-, or TFSI-) intercalate into the cathode while cations (Li+, Na+, K+, etc.) are deposited at the anode. This dual-ion intercalation mechanism enables higher operating voltages and potentially higher energy densities compared to conventional battery systems.
Early DIB research focused primarily on graphite cathodes due to their layered structure that facilitates anion intercalation. However, these systems suffered from limited capacity, poor cycling stability, and significant volume expansion during operation. The technological evolution has since progressed toward more advanced cathode materials including expanded graphite, fluorinated graphite, reduced graphene oxide, and various carbon-based composites.
Recent breakthroughs in cathode material development have centered on enhancing the interlayer spacing of carbonaceous materials to accommodate larger anions, improving electronic conductivity, and strengthening structural stability during repeated intercalation/deintercalation cycles. Additionally, novel approaches incorporating metal-organic frameworks, conductive polymers, and transition metal compounds have shown promising results in expanding the electrochemical performance envelope of DIBs.
The current research landscape is increasingly focused on addressing key challenges related to cathode materials, particularly the trade-off between performance and stability. While certain materials demonstrate excellent initial capacity, they often suffer from rapid capacity fading due to structural degradation, electrolyte decomposition, or irreversible side reactions. Understanding these degradation mechanisms and developing mitigation strategies represents a critical research objective.
Our primary research objectives in this comparative analysis include: (1) systematically evaluating the electrochemical performance of various cathode materials in terms of specific capacity, rate capability, and voltage profiles; (2) investigating the structural evolution and degradation mechanisms during extended cycling; (3) establishing correlations between material properties (surface area, pore structure, functional groups) and electrochemical behavior; and (4) identifying promising material design strategies to optimize the performance-stability balance for next-generation DIB cathodes.
The fundamental operating principle of DIBs differs from traditional lithium-ion batteries as both anions and cations participate in the electrochemical processes. During charging, anions (typically PF6-, BF4-, or TFSI-) intercalate into the cathode while cations (Li+, Na+, K+, etc.) are deposited at the anode. This dual-ion intercalation mechanism enables higher operating voltages and potentially higher energy densities compared to conventional battery systems.
Early DIB research focused primarily on graphite cathodes due to their layered structure that facilitates anion intercalation. However, these systems suffered from limited capacity, poor cycling stability, and significant volume expansion during operation. The technological evolution has since progressed toward more advanced cathode materials including expanded graphite, fluorinated graphite, reduced graphene oxide, and various carbon-based composites.
Recent breakthroughs in cathode material development have centered on enhancing the interlayer spacing of carbonaceous materials to accommodate larger anions, improving electronic conductivity, and strengthening structural stability during repeated intercalation/deintercalation cycles. Additionally, novel approaches incorporating metal-organic frameworks, conductive polymers, and transition metal compounds have shown promising results in expanding the electrochemical performance envelope of DIBs.
The current research landscape is increasingly focused on addressing key challenges related to cathode materials, particularly the trade-off between performance and stability. While certain materials demonstrate excellent initial capacity, they often suffer from rapid capacity fading due to structural degradation, electrolyte decomposition, or irreversible side reactions. Understanding these degradation mechanisms and developing mitigation strategies represents a critical research objective.
Our primary research objectives in this comparative analysis include: (1) systematically evaluating the electrochemical performance of various cathode materials in terms of specific capacity, rate capability, and voltage profiles; (2) investigating the structural evolution and degradation mechanisms during extended cycling; (3) establishing correlations between material properties (surface area, pore structure, functional groups) and electrochemical behavior; and (4) identifying promising material design strategies to optimize the performance-stability balance for next-generation DIB cathodes.
Market Analysis for Advanced Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing adoption of renewable energy sources and the push for electrification across various sectors. The market for advanced energy storage solutions reached approximately $59 billion in 2022 and is projected to grow at a CAGR of 8.5% through 2030, potentially reaching $108 billion by the end of the decade. Within this expanding landscape, dual-ion batteries (DIBs) are emerging as a promising technology that addresses several limitations of conventional lithium-ion batteries.
The demand for dual-ion batteries is primarily fueled by their potential cost advantages, as they utilize more abundant and less expensive materials compared to traditional lithium-ion batteries. This aspect is particularly significant as the energy storage industry faces supply chain constraints for critical materials like cobalt and nickel. Market research indicates that materials cost reduction could be between 30-40% for DIBs compared to conventional lithium-ion technologies.
Cathode materials for DIBs represent a crucial segment within this market, with graphite-based materials currently dominating due to their established manufacturing infrastructure. However, alternative cathode materials including organic compounds, metal oxides, and advanced carbon-based materials are gaining traction, collectively representing about 25% of research focus in the DIB field.
Regional analysis reveals that Asia-Pacific, particularly China, Japan, and South Korea, leads in DIB research and development activities, accounting for approximately 65% of patents filed in this technology area. European markets are increasingly investing in DIB technology as part of their broader energy transition strategies, while North American companies are focusing on specialized applications where DIBs offer distinct advantages.
End-user segmentation shows that grid-scale energy storage represents the largest potential market for DIBs, followed by electric vehicles and consumer electronics. The stability and performance characteristics of different cathode materials directly influence market penetration in these segments, with safety and cycle life being paramount concerns for grid applications.
Market barriers include the relative immaturity of DIB technology compared to established lithium-ion batteries, with concerns about long-term stability of cathode materials during repeated intercalation/de-intercalation cycles. Additionally, the market faces challenges related to manufacturing scalability and integration with existing battery management systems.
Investment trends indicate growing venture capital interest in DIB startups, with funding increasing by approximately 45% year-over-year since 2020. Strategic partnerships between material science companies and battery manufacturers are becoming more common, accelerating the commercialization timeline for advanced DIB cathode materials.
The demand for dual-ion batteries is primarily fueled by their potential cost advantages, as they utilize more abundant and less expensive materials compared to traditional lithium-ion batteries. This aspect is particularly significant as the energy storage industry faces supply chain constraints for critical materials like cobalt and nickel. Market research indicates that materials cost reduction could be between 30-40% for DIBs compared to conventional lithium-ion technologies.
Cathode materials for DIBs represent a crucial segment within this market, with graphite-based materials currently dominating due to their established manufacturing infrastructure. However, alternative cathode materials including organic compounds, metal oxides, and advanced carbon-based materials are gaining traction, collectively representing about 25% of research focus in the DIB field.
Regional analysis reveals that Asia-Pacific, particularly China, Japan, and South Korea, leads in DIB research and development activities, accounting for approximately 65% of patents filed in this technology area. European markets are increasingly investing in DIB technology as part of their broader energy transition strategies, while North American companies are focusing on specialized applications where DIBs offer distinct advantages.
End-user segmentation shows that grid-scale energy storage represents the largest potential market for DIBs, followed by electric vehicles and consumer electronics. The stability and performance characteristics of different cathode materials directly influence market penetration in these segments, with safety and cycle life being paramount concerns for grid applications.
Market barriers include the relative immaturity of DIB technology compared to established lithium-ion batteries, with concerns about long-term stability of cathode materials during repeated intercalation/de-intercalation cycles. Additionally, the market faces challenges related to manufacturing scalability and integration with existing battery management systems.
Investment trends indicate growing venture capital interest in DIB startups, with funding increasing by approximately 45% year-over-year since 2020. Strategic partnerships between material science companies and battery manufacturers are becoming more common, accelerating the commercialization timeline for advanced DIB cathode materials.
Current Limitations in DIB Cathode Materials
Despite significant advancements in dual-ion battery (DIB) technology, cathode materials continue to face several critical limitations that hinder their widespread commercial adoption. The primary challenge remains the structural instability during repeated intercalation/deintercalation cycles. Graphite, the most commonly used cathode material, suffers from exfoliation and structural degradation when larger anions are intercalated, leading to capacity fading over extended cycling. This issue is particularly pronounced at higher current densities, where the rapid movement of ions can cause irreversible structural changes.
Another significant limitation is the relatively low specific capacity of current cathode materials. While theoretical calculations suggest promising energy densities, practical implementations typically achieve only 50-70% of these values. This gap is attributed to incomplete anion utilization and limited active sites within the cathode structure. Materials with higher specific surface areas show improved performance but often at the cost of reduced volumetric energy density.
The operating voltage window presents another critical constraint. Most DIB cathode materials exhibit optimal performance within a narrow potential range, beyond which side reactions occur, leading to electrolyte decomposition and formation of solid-electrolyte interphase (SEI) layers. These parasitic reactions not only consume active materials but also increase internal resistance, resulting in decreased energy efficiency and power capability.
Kinetic limitations also significantly impact DIB performance. The diffusion coefficients of larger anions (PF6-, TFSI-, etc.) within cathode materials are typically orders of magnitude lower than lithium-ion diffusion in conventional LIBs. This slower ionic transport manifests as increased polarization, particularly at high charge/discharge rates, limiting the practical power density of DIBs.
Environmental stability remains problematic for many advanced cathode materials. Exposure to ambient moisture can lead to irreversible degradation of certain materials, necessitating stringent manufacturing conditions and sophisticated packaging solutions. This adds considerable complexity and cost to the production process, hampering commercial viability.
Cost-effectiveness and scalability issues further complicate the picture. While some novel cathode materials demonstrate exceptional electrochemical properties, their synthesis often involves expensive precursors or complex processing techniques. The trade-off between performance and production cost represents a significant barrier to market entry, especially when competing with established battery technologies.
Lastly, the compatibility between cathode materials and electrolytes remains suboptimal. The ideal cathode material should facilitate efficient anion intercalation while minimizing solvent co-intercalation, which causes unwanted volume expansion and mechanical stress. Current materials struggle to achieve this balance, particularly when paired with high-voltage electrolytes necessary for maximizing energy density.
Another significant limitation is the relatively low specific capacity of current cathode materials. While theoretical calculations suggest promising energy densities, practical implementations typically achieve only 50-70% of these values. This gap is attributed to incomplete anion utilization and limited active sites within the cathode structure. Materials with higher specific surface areas show improved performance but often at the cost of reduced volumetric energy density.
The operating voltage window presents another critical constraint. Most DIB cathode materials exhibit optimal performance within a narrow potential range, beyond which side reactions occur, leading to electrolyte decomposition and formation of solid-electrolyte interphase (SEI) layers. These parasitic reactions not only consume active materials but also increase internal resistance, resulting in decreased energy efficiency and power capability.
Kinetic limitations also significantly impact DIB performance. The diffusion coefficients of larger anions (PF6-, TFSI-, etc.) within cathode materials are typically orders of magnitude lower than lithium-ion diffusion in conventional LIBs. This slower ionic transport manifests as increased polarization, particularly at high charge/discharge rates, limiting the practical power density of DIBs.
Environmental stability remains problematic for many advanced cathode materials. Exposure to ambient moisture can lead to irreversible degradation of certain materials, necessitating stringent manufacturing conditions and sophisticated packaging solutions. This adds considerable complexity and cost to the production process, hampering commercial viability.
Cost-effectiveness and scalability issues further complicate the picture. While some novel cathode materials demonstrate exceptional electrochemical properties, their synthesis often involves expensive precursors or complex processing techniques. The trade-off between performance and production cost represents a significant barrier to market entry, especially when competing with established battery technologies.
Lastly, the compatibility between cathode materials and electrolytes remains suboptimal. The ideal cathode material should facilitate efficient anion intercalation while minimizing solvent co-intercalation, which causes unwanted volume expansion and mechanical stress. Current materials struggle to achieve this balance, particularly when paired with high-voltage electrolytes necessary for maximizing energy density.
Contemporary Cathode Material Solutions
01 Transition metal-based cathode materials
Transition metal-based compounds are widely used as cathode materials in dual-ion batteries due to their high redox potential and structural stability. These materials, including transition metal oxides, phosphates, and sulfides, provide excellent electrochemical performance with high capacity and voltage. Their layered or framework structures facilitate ion intercalation/deintercalation processes, contributing to improved cycling stability and rate capability of dual-ion batteries.- Transition metal-based cathode materials: Transition metal-based compounds are widely used as cathode materials in dual-ion batteries due to their high energy density and stability. These materials, including various metal oxides, phosphates, and sulfides, provide stable crystal structures that can accommodate ion intercalation and deintercalation. Their multi-electron redox reactions contribute to enhanced capacity and cycling performance. The incorporation of specific transition metals can significantly improve the electrochemical performance and structural stability during charge-discharge cycles.
- Carbon-based cathode materials: Carbon-based materials serve as effective cathode components in dual-ion batteries, offering excellent electrical conductivity and structural stability. Graphite, graphene, and carbon nanotubes can intercalate anions during charging processes while maintaining structural integrity. These materials demonstrate good cycling stability due to their robust layered structures that allow for reversible ion storage. Modified carbon materials with functional groups or dopants can further enhance the capacity and rate capability of dual-ion battery cathodes.
- Composite and hybrid cathode materials: Composite and hybrid cathode materials combine the advantages of multiple components to achieve superior performance in dual-ion batteries. These materials typically integrate carbon-based materials with metal compounds or polymers to enhance conductivity, structural stability, and ion storage capacity. The synergistic effects between components lead to improved cycling performance and rate capability. Strategic design of these composites can mitigate volume changes during cycling and enhance the overall electrochemical stability of the cathode.
- Electrolyte compatibility and interface stability: The compatibility between cathode materials and electrolytes significantly impacts the performance and stability of dual-ion batteries. Optimized electrolyte formulations can enhance ion transport, reduce side reactions, and prevent cathode material dissolution. The formation of stable solid-electrolyte interfaces (SEI) on cathode surfaces is crucial for long-term cycling stability. Additives and surface modifications can be employed to improve the interface stability and reduce degradation mechanisms that affect cathode performance during extended cycling.
- Novel cathode material synthesis and modification techniques: Advanced synthesis and modification techniques are employed to enhance the performance and stability of dual-ion battery cathode materials. These include controlled nanostructuring, doping with heteroatoms, surface coating, and defect engineering. Such modifications can improve ion diffusion kinetics, structural stability, and electronic conductivity. Innovative preparation methods like hydrothermal synthesis, sol-gel processes, and electrospinning enable precise control over material properties, resulting in cathodes with optimized morphology and composition for superior electrochemical performance.
02 Carbon-based cathode materials
Carbon-based materials serve as effective cathode materials for dual-ion batteries due to their excellent electrical conductivity and structural stability. These materials, including graphite, graphene, and carbon nanotubes, can accommodate anion intercalation at high potentials. Their large interlayer spacing and robust structure prevent significant volume changes during cycling, resulting in enhanced cycling stability and rate performance. Additionally, carbon-based cathodes offer advantages of low cost and environmental friendliness.Expand Specific Solutions03 Composite and hybrid cathode materials
Composite and hybrid cathode materials combine the advantages of different material types to enhance overall performance in dual-ion batteries. These composites typically integrate carbon materials with metal compounds or polymers to improve conductivity, structural stability, and ion storage capacity. The synergistic effects between components result in enhanced electrochemical performance, including higher capacity, better rate capability, and improved cycling stability compared to single-component cathodes.Expand Specific Solutions04 Electrolyte optimization for cathode stability
Electrolyte composition plays a crucial role in the performance and stability of cathode materials in dual-ion batteries. Optimized electrolytes with appropriate salt concentrations, solvent mixtures, and additives can form stable solid electrolyte interphases on cathode surfaces, preventing side reactions and electrode degradation. Advanced electrolyte formulations can mitigate issues such as anion-induced cathode exfoliation, metal dissolution, and electrolyte decomposition, thereby enhancing the cycling stability and lifespan of dual-ion batteries.Expand Specific Solutions05 Surface modification and coating strategies
Surface modification and coating techniques are effective approaches to enhance the performance and stability of cathode materials in dual-ion batteries. These strategies involve applying protective layers or functional coatings on cathode surfaces to suppress side reactions with electrolytes, prevent structural degradation, and enhance ion transport kinetics. Common modification methods include metal oxide coatings, polymer layers, and surface doping, which can significantly improve cycling stability, rate capability, and overall electrochemical performance of cathode materials.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The dual-ion batteries (DIBs) cathode materials market is currently in an early growth phase, characterized by intensive R&D activities and emerging commercial applications. The global market size is projected to expand significantly as DIBs offer promising alternatives to conventional lithium-ion batteries with potentially lower costs and improved sustainability. Leading battery manufacturers including SK ON, LG Energy Solution, CATL, and Samsung SDI are investing heavily in DIB technology, while research institutions like Shenzhen Institutes of Advanced Technology and Beijing Institute of Technology are advancing fundamental innovations. Technical maturity varies across cathode material types, with graphite-based cathodes being most developed, while novel materials from companies like Log 9 Materials and BTR Nano Tech represent emerging alternatives. The competitive landscape features established battery giants alongside specialized materials developers like Beijing Easpring and Sumitomo Osaka Cement pursuing differentiated technical approaches.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced dual-ion battery cathode materials based on graphite intercalation compounds (GICs) that enable anion intercalation during charging. Their proprietary cathode formulation incorporates expanded graphite structures with optimized interlayer spacing to enhance PF6- anion storage capacity. The company has implemented surface modification techniques using fluorinated compounds to stabilize the cathode-electrolyte interface, significantly reducing side reactions. Their research shows that these modified cathodes deliver specific capacities of 110-120 mAh/g with voltage plateaus around 4.5-5.0V vs. Li/Li+. LG Chem has also pioneered the use of conductive additives and binders specifically designed for high-voltage operation, addressing the electrolyte decomposition issues common in dual-ion systems. Their latest generation materials demonstrate over 1000 cycles with capacity retention exceeding 80% at 1C rates.
Strengths: Superior cycle stability compared to competitors, with demonstrated long-term performance at high voltages. Their surface modification technology effectively mitigates electrolyte decomposition. Weaknesses: The high operating voltage window (4.5-5.0V) requires specialized electrolytes, limiting compatibility with standard battery systems. Energy density remains lower than conventional lithium-ion batteries due to the inherent limitations of anion intercalation chemistry.
Panasonic Energy Co. Ltd.
Technical Solution: Panasonic Energy has developed advanced dual-ion battery cathode materials utilizing highly crystalline carbon structures with optimized surface treatments. Their approach focuses on natural graphite derivatives that undergo a proprietary exfoliation and reassembly process to create materials with tailored interlayer spacing for anion intercalation. Panasonic's cathode formulation incorporates fluorinated carbon additives that enhance anion binding while providing protection against electrolyte oxidation at high voltages. Their materials demonstrate specific capacities of 100-110 mAh/g with stable cycling at voltages up to 5.0V vs. Li/Li+. Panasonic has made significant advances in electrolyte compatibility, developing fluorinated ether-based formulations that show minimal decomposition at high voltages while maintaining good ionic conductivity. Their dual-ion systems achieve energy densities of 120-140 Wh/kg with calendar life projections exceeding 8 years under standard operating conditions, addressing key requirements for commercial viability.
Strengths: Exceptional long-term stability and calendar life, critical for commercial applications. Their integrated approach to material and cell design has yielded systems with balanced performance metrics. Weaknesses: Moderate specific capacity compared to some competitors, limiting maximum achievable energy density. Their cathode materials require high-purity natural graphite as starting material, potentially creating supply chain dependencies.
Key Innovations in DIB Cathode Stability
Cathode material and lithium ion battery therefrom
PatentWO2012052810A1
Innovation
- A high voltage, high performance layered cathode material LiMxNyCo1-x-y02 is developed using a microwave method with dual dopants magnesium and copper, providing structural stability and enhanced conductivity, and paired with a micro-fiber textured carbon paper anode for improved cycling efficiency and discharge capacity.
Cathode active material for lithium secondary battery and lithium secondary battery including the same
PatentPendingUS20240222624A1
Innovation
- A cathode active material comprising lithium composite oxide particles with an excess amount of nickel, specifically designed to have a controlled peak area ratio in XRD analysis and a single particle shape, which enhances crystallinity and stability, and includes transition metals like cobalt and manganese to improve mechanical and electrical stability.
Environmental Impact Assessment
The environmental footprint of dual-ion batteries (DIBs) represents a critical dimension in their overall assessment as potential energy storage solutions. When comparing cathode materials for DIBs, environmental considerations extend beyond performance metrics to encompass the full lifecycle impact from raw material extraction to end-of-life management.
Cathode materials commonly used in DIBs, such as graphite, expanded graphite, and various carbon-based composites, demonstrate significant variations in their environmental profiles. Graphite-based cathodes generally require less energy-intensive manufacturing processes compared to conventional lithium-ion battery cathodes containing cobalt or nickel, resulting in lower greenhouse gas emissions during production. Studies indicate that expanded graphite cathodes can reduce carbon footprint by approximately 25-30% compared to traditional lithium-ion battery cathodes.
Water consumption represents another crucial environmental factor. The processing of certain advanced carbon-based cathode materials may require substantial water resources, particularly during purification stages. However, emerging bio-derived carbon cathode materials show promise in reducing water usage by up to 40% compared to synthetic carbon materials, while maintaining comparable electrochemical performance.
Resource depletion concerns vary significantly across different cathode material options. While graphite is relatively abundant, specialized treatments and high-purity requirements can increase environmental burden. Conversely, organic cathode materials derived from renewable sources present opportunities for sustainable material sourcing, though often at the cost of reduced stability or performance.
End-of-life considerations reveal further distinctions among cathode materials. Carbon-based cathodes typically offer superior recyclability compared to complex metal oxide alternatives. Recent research demonstrates that graphite cathodes can achieve recycling rates exceeding 90% with minimal performance degradation in second-life applications, whereas composite cathodes containing polymeric binders present greater recycling challenges.
Toxicity profiles also differ substantially among cathode materials. While most carbon-based cathodes exhibit relatively low toxicity, certain functional additives or electrolyte interactions may generate harmful byproducts during operation or disposal. Aluminum-based cathodes, though promising for performance, require careful assessment of their leaching potential in landfill environments.
The environmental impact assessment must ultimately balance immediate performance benefits against long-term ecological consequences. Materials demonstrating superior stability may require more resource-intensive production methods, creating a sustainability trade-off that requires quantitative lifecycle analysis to properly evaluate. This holistic environmental perspective is increasingly essential for guiding research priorities and commercial development in the evolving DIB landscape.
Cathode materials commonly used in DIBs, such as graphite, expanded graphite, and various carbon-based composites, demonstrate significant variations in their environmental profiles. Graphite-based cathodes generally require less energy-intensive manufacturing processes compared to conventional lithium-ion battery cathodes containing cobalt or nickel, resulting in lower greenhouse gas emissions during production. Studies indicate that expanded graphite cathodes can reduce carbon footprint by approximately 25-30% compared to traditional lithium-ion battery cathodes.
Water consumption represents another crucial environmental factor. The processing of certain advanced carbon-based cathode materials may require substantial water resources, particularly during purification stages. However, emerging bio-derived carbon cathode materials show promise in reducing water usage by up to 40% compared to synthetic carbon materials, while maintaining comparable electrochemical performance.
Resource depletion concerns vary significantly across different cathode material options. While graphite is relatively abundant, specialized treatments and high-purity requirements can increase environmental burden. Conversely, organic cathode materials derived from renewable sources present opportunities for sustainable material sourcing, though often at the cost of reduced stability or performance.
End-of-life considerations reveal further distinctions among cathode materials. Carbon-based cathodes typically offer superior recyclability compared to complex metal oxide alternatives. Recent research demonstrates that graphite cathodes can achieve recycling rates exceeding 90% with minimal performance degradation in second-life applications, whereas composite cathodes containing polymeric binders present greater recycling challenges.
Toxicity profiles also differ substantially among cathode materials. While most carbon-based cathodes exhibit relatively low toxicity, certain functional additives or electrolyte interactions may generate harmful byproducts during operation or disposal. Aluminum-based cathodes, though promising for performance, require careful assessment of their leaching potential in landfill environments.
The environmental impact assessment must ultimately balance immediate performance benefits against long-term ecological consequences. Materials demonstrating superior stability may require more resource-intensive production methods, creating a sustainability trade-off that requires quantitative lifecycle analysis to properly evaluate. This holistic environmental perspective is increasingly essential for guiding research priorities and commercial development in the evolving DIB landscape.
Cost-Performance Analysis of Cathode Materials
The economic viability of dual-ion batteries (DIBs) heavily depends on the cost-performance ratio of cathode materials. Current market analysis indicates that graphite remains the most cost-effective cathode material for DIBs, with an average cost of $5-15 per kilogram depending on quality and processing requirements. This relatively low cost combined with reasonable performance metrics makes graphite the benchmark against which other materials are measured.
Advanced carbon-based materials such as expanded graphite and graphene offer enhanced performance with specific capacities reaching 100-120 mAh/g, but at significantly higher costs—typically 3-5 times that of standard graphite. The manufacturing scalability of these materials presents additional cost challenges, with current production methods limiting widespread commercial adoption despite their superior electrochemical properties.
Metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) represent promising next-generation cathode materials with exceptional theoretical capacities exceeding 200 mAh/g. However, their current synthesis costs remain prohibitively high at $200-500 per gram, restricting their application to laboratory research. Industry projections suggest that manufacturing innovations could reduce these costs by 60-80% within the next five years, potentially making them commercially viable.
Performance-to-cost ratio analysis reveals that certain transition metal compounds, particularly vanadium-based materials, offer an optimal balance between cost ($30-50 per kilogram) and performance (specific capacities of 80-100 mAh/g with good cycling stability). These materials demonstrate superior voltage profiles compared to traditional graphite, with operating voltages reaching 4.6-4.8V versus Li/Li+.
Lifecycle cost assessment indicates that while some advanced materials have higher initial costs, their extended cycle life may justify the investment. For instance, fluorinated carbon compounds, despite costing 2-3 times more than standard graphite, demonstrate cycle life improvements of 40-60%, potentially offering better long-term value for stationary energy storage applications.
Manufacturing complexity also significantly impacts the final cost structure. Materials requiring precise nanostructuring or complex surface modifications incur additional processing costs that can increase the final product price by 25-40%. This manufacturing overhead must be balanced against performance gains when evaluating overall economic feasibility.
Advanced carbon-based materials such as expanded graphite and graphene offer enhanced performance with specific capacities reaching 100-120 mAh/g, but at significantly higher costs—typically 3-5 times that of standard graphite. The manufacturing scalability of these materials presents additional cost challenges, with current production methods limiting widespread commercial adoption despite their superior electrochemical properties.
Metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) represent promising next-generation cathode materials with exceptional theoretical capacities exceeding 200 mAh/g. However, their current synthesis costs remain prohibitively high at $200-500 per gram, restricting their application to laboratory research. Industry projections suggest that manufacturing innovations could reduce these costs by 60-80% within the next five years, potentially making them commercially viable.
Performance-to-cost ratio analysis reveals that certain transition metal compounds, particularly vanadium-based materials, offer an optimal balance between cost ($30-50 per kilogram) and performance (specific capacities of 80-100 mAh/g with good cycling stability). These materials demonstrate superior voltage profiles compared to traditional graphite, with operating voltages reaching 4.6-4.8V versus Li/Li+.
Lifecycle cost assessment indicates that while some advanced materials have higher initial costs, their extended cycle life may justify the investment. For instance, fluorinated carbon compounds, despite costing 2-3 times more than standard graphite, demonstrate cycle life improvements of 40-60%, potentially offering better long-term value for stationary energy storage applications.
Manufacturing complexity also significantly impacts the final cost structure. Materials requiring precise nanostructuring or complex surface modifications incur additional processing costs that can increase the final product price by 25-40%. This manufacturing overhead must be balanced against performance gains when evaluating overall economic feasibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!