How electrolyte formulation impacts Dual-ion batteries long term performance
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Formulation in DIBs: Background and Objectives
Dual-ion batteries (DIBs) have emerged as a promising energy storage technology in recent years, offering potential advantages in terms of cost, sustainability, and energy density compared to conventional lithium-ion batteries. The evolution of DIBs can be traced back to the early 2000s, when researchers began exploring alternative battery chemistries to address the limitations of traditional lithium-ion systems. The fundamental operating principle of DIBs involves the simultaneous intercalation of both cations and anions into different electrode materials during charge-discharge cycles, distinguishing them from conventional batteries that primarily utilize cation intercalation.
The electrolyte formulation represents a critical component in DIB technology, serving as the medium for ion transport between electrodes while significantly influencing the battery's performance characteristics. Historical developments in electrolyte research have progressed from simple salt-solvent combinations to sophisticated multi-component systems designed to enhance specific performance metrics. This evolution reflects the growing understanding of the complex interactions between electrolyte components and electrode materials in the DIB environment.
Current technical trends in DIB electrolyte formulation focus on addressing several key challenges, including enhancing ionic conductivity, expanding the electrochemical stability window, mitigating electrode degradation, and improving cycling efficiency. The field is witnessing a shift toward electrolyte systems that can facilitate faster ion transport while maintaining stability during extended cycling periods, which is essential for long-term performance.
The primary technical objective of this investigation is to establish a comprehensive understanding of how electrolyte formulation parameters—including salt concentration, solvent composition, additive incorporation, and impurity levels—impact the long-term performance stability of DIBs. Specifically, we aim to identify the correlation between electrolyte chemical properties and battery degradation mechanisms that manifest over extended cycling periods.
Secondary objectives include quantifying the influence of electrolyte formulation on the solid electrolyte interphase (SEI) formation dynamics, determining optimal electrolyte compositions for different electrode material combinations, and developing predictive models that can accelerate the design of next-generation DIB electrolytes. These objectives align with the broader industry goal of developing DIB systems with enhanced cycle life, improved capacity retention, and reliable performance under various operating conditions.
The significance of this research extends beyond academic interest, as electrolyte optimization represents a potentially cost-effective approach to improving DIB performance without necessitating fundamental changes to electrode materials or battery architecture. Understanding these relationships will provide valuable insights for the strategic development of DIB technology for various applications, from grid-scale energy storage to electric vehicles.
The electrolyte formulation represents a critical component in DIB technology, serving as the medium for ion transport between electrodes while significantly influencing the battery's performance characteristics. Historical developments in electrolyte research have progressed from simple salt-solvent combinations to sophisticated multi-component systems designed to enhance specific performance metrics. This evolution reflects the growing understanding of the complex interactions between electrolyte components and electrode materials in the DIB environment.
Current technical trends in DIB electrolyte formulation focus on addressing several key challenges, including enhancing ionic conductivity, expanding the electrochemical stability window, mitigating electrode degradation, and improving cycling efficiency. The field is witnessing a shift toward electrolyte systems that can facilitate faster ion transport while maintaining stability during extended cycling periods, which is essential for long-term performance.
The primary technical objective of this investigation is to establish a comprehensive understanding of how electrolyte formulation parameters—including salt concentration, solvent composition, additive incorporation, and impurity levels—impact the long-term performance stability of DIBs. Specifically, we aim to identify the correlation between electrolyte chemical properties and battery degradation mechanisms that manifest over extended cycling periods.
Secondary objectives include quantifying the influence of electrolyte formulation on the solid electrolyte interphase (SEI) formation dynamics, determining optimal electrolyte compositions for different electrode material combinations, and developing predictive models that can accelerate the design of next-generation DIB electrolytes. These objectives align with the broader industry goal of developing DIB systems with enhanced cycle life, improved capacity retention, and reliable performance under various operating conditions.
The significance of this research extends beyond academic interest, as electrolyte optimization represents a potentially cost-effective approach to improving DIB performance without necessitating fundamental changes to electrode materials or battery architecture. Understanding these relationships will provide valuable insights for the strategic development of DIB technology for various applications, from grid-scale energy storage to electric vehicles.
Market Analysis for Advanced Dual-ion Battery Technologies
The global market for dual-ion batteries (DIBs) is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that the advanced battery sector, which includes DIBs, is projected to reach $240 billion by 2027, with DIBs potentially capturing 5-8% of this market. This growth trajectory is supported by the rising adoption of electric vehicles, renewable energy integration, and portable electronics requiring high-performance energy storage.
Dual-ion batteries offer several market advantages that position them favorably against traditional lithium-ion technologies. Their potential for lower production costs (approximately 30% less than conventional lithium-ion batteries) stems from the use of more abundant materials like aluminum and graphite. Additionally, DIBs demonstrate superior safety profiles with reduced thermal runaway risks, addressing a critical market concern in energy storage applications.
Regional market analysis reveals that Asia-Pacific currently dominates DIB research and development, with China, Japan, and South Korea leading commercialization efforts. European markets show increasing interest, particularly in Germany and France, where research institutions have established partnerships with industrial manufacturers to accelerate DIB technology transfer. North American markets remain more focused on traditional lithium-ion technologies but are gradually increasing investments in alternative battery chemistries including DIBs.
Market segmentation indicates that stationary energy storage represents the most promising initial application for DIBs, with grid-scale storage solutions potentially accounting for 45% of early DIB market adoption. The automotive sector presents significant long-term potential, though current performance limitations related to electrolyte formulation challenges must be addressed before widespread implementation.
Consumer demand patterns demonstrate growing preference for batteries with longer cycle life and improved sustainability metrics. DIBs with optimized electrolyte formulations that enhance long-term performance could capture premium market segments willing to pay 15-20% more for extended battery lifespans. This represents a significant market opportunity for manufacturers who can overcome current electrolyte stability challenges.
Investment trends show increasing venture capital interest in advanced DIB technologies, with funding rounds for electrolyte innovation startups growing by 65% between 2020 and 2023. Strategic partnerships between electrolyte manufacturers and battery producers have increased by 40% in the same period, indicating strong market confidence in DIB technology's commercial potential once long-term performance issues are resolved.
Dual-ion batteries offer several market advantages that position them favorably against traditional lithium-ion technologies. Their potential for lower production costs (approximately 30% less than conventional lithium-ion batteries) stems from the use of more abundant materials like aluminum and graphite. Additionally, DIBs demonstrate superior safety profiles with reduced thermal runaway risks, addressing a critical market concern in energy storage applications.
Regional market analysis reveals that Asia-Pacific currently dominates DIB research and development, with China, Japan, and South Korea leading commercialization efforts. European markets show increasing interest, particularly in Germany and France, where research institutions have established partnerships with industrial manufacturers to accelerate DIB technology transfer. North American markets remain more focused on traditional lithium-ion technologies but are gradually increasing investments in alternative battery chemistries including DIBs.
Market segmentation indicates that stationary energy storage represents the most promising initial application for DIBs, with grid-scale storage solutions potentially accounting for 45% of early DIB market adoption. The automotive sector presents significant long-term potential, though current performance limitations related to electrolyte formulation challenges must be addressed before widespread implementation.
Consumer demand patterns demonstrate growing preference for batteries with longer cycle life and improved sustainability metrics. DIBs with optimized electrolyte formulations that enhance long-term performance could capture premium market segments willing to pay 15-20% more for extended battery lifespans. This represents a significant market opportunity for manufacturers who can overcome current electrolyte stability challenges.
Investment trends show increasing venture capital interest in advanced DIB technologies, with funding rounds for electrolyte innovation startups growing by 65% between 2020 and 2023. Strategic partnerships between electrolyte manufacturers and battery producers have increased by 40% in the same period, indicating strong market confidence in DIB technology's commercial potential once long-term performance issues are resolved.
Current Electrolyte Challenges in Dual-ion Battery Systems
Dual-ion batteries (DIBs) represent a promising energy storage technology, yet their long-term performance is significantly constrained by current electrolyte limitations. Unlike conventional lithium-ion batteries, DIBs involve the intercalation of both cations and anions during charge-discharge cycles, placing unique demands on electrolyte formulations.
The primary challenge facing DIB electrolytes is their limited electrochemical stability window. Most conventional electrolytes decompose at high voltages (>4.5V), whereas DIBs typically operate at voltage ranges of 3.0-5.0V. This decomposition leads to capacity fading and reduced cycle life, severely limiting the practical application of these battery systems in long-term scenarios.
Electrolyte solvent degradation presents another significant hurdle. Carbonate-based solvents, commonly used in DIBs, undergo oxidative decomposition at the cathode interface during high-voltage operation. This results in the formation of unstable solid electrolyte interphase (SEI) layers that continue to consume electrolyte over time, leading to increased internal resistance and diminished capacity retention.
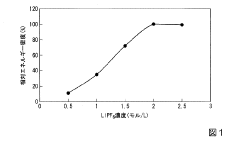
Salt concentration and composition critically impact DIB performance. Traditional lithium salts like LiPF6 exhibit limited anion mobility and stability issues at high voltages. Furthermore, the concentration gradient that develops during cycling causes polarization effects that accelerate capacity fade. Finding the optimal salt concentration that balances ionic conductivity with electrochemical stability remains challenging.
Temperature sensitivity of electrolytes significantly affects DIB longevity. At elevated temperatures, electrolyte decomposition accelerates dramatically, while at low temperatures, increased viscosity and reduced ionic conductivity lead to severe performance limitations. This narrow operational temperature window restricts the practical deployment of DIBs in various environmental conditions.
The co-intercalation phenomenon, where solvent molecules insert alongside ions into electrode materials, causes structural degradation of electrodes over extended cycling. Current electrolyte formulations have not adequately addressed this issue, resulting in progressive capacity loss and mechanical failure of electrode materials.
Electrolyte-electrode compatibility issues further complicate DIB development. The diverse range of electrode materials used in DIBs (graphite, metal oxides, organic compounds) each interact differently with electrolytes. These interactions often lead to parasitic reactions that consume active materials and electrolyte components, progressively degrading battery performance.
Water contamination represents another critical challenge, as even trace amounts of moisture can trigger hydrolysis reactions with electrolyte salts, producing harmful byproducts like HF that attack electrode materials and current collectors, accelerating corrosion and capacity fade during long-term operation.
The primary challenge facing DIB electrolytes is their limited electrochemical stability window. Most conventional electrolytes decompose at high voltages (>4.5V), whereas DIBs typically operate at voltage ranges of 3.0-5.0V. This decomposition leads to capacity fading and reduced cycle life, severely limiting the practical application of these battery systems in long-term scenarios.
Electrolyte solvent degradation presents another significant hurdle. Carbonate-based solvents, commonly used in DIBs, undergo oxidative decomposition at the cathode interface during high-voltage operation. This results in the formation of unstable solid electrolyte interphase (SEI) layers that continue to consume electrolyte over time, leading to increased internal resistance and diminished capacity retention.
Salt concentration and composition critically impact DIB performance. Traditional lithium salts like LiPF6 exhibit limited anion mobility and stability issues at high voltages. Furthermore, the concentration gradient that develops during cycling causes polarization effects that accelerate capacity fade. Finding the optimal salt concentration that balances ionic conductivity with electrochemical stability remains challenging.
Temperature sensitivity of electrolytes significantly affects DIB longevity. At elevated temperatures, electrolyte decomposition accelerates dramatically, while at low temperatures, increased viscosity and reduced ionic conductivity lead to severe performance limitations. This narrow operational temperature window restricts the practical deployment of DIBs in various environmental conditions.
The co-intercalation phenomenon, where solvent molecules insert alongside ions into electrode materials, causes structural degradation of electrodes over extended cycling. Current electrolyte formulations have not adequately addressed this issue, resulting in progressive capacity loss and mechanical failure of electrode materials.
Electrolyte-electrode compatibility issues further complicate DIB development. The diverse range of electrode materials used in DIBs (graphite, metal oxides, organic compounds) each interact differently with electrolytes. These interactions often lead to parasitic reactions that consume active materials and electrolyte components, progressively degrading battery performance.
Water contamination represents another critical challenge, as even trace amounts of moisture can trigger hydrolysis reactions with electrolyte salts, producing harmful byproducts like HF that attack electrode materials and current collectors, accelerating corrosion and capacity fade during long-term operation.
Current Electrolyte Formulation Strategies for Enhanced Performance
01 Electrode materials for enhanced long-term performance
Advanced electrode materials play a crucial role in improving the long-term performance of dual-ion batteries. These materials include specially designed cathodes and anodes that can withstand repeated intercalation and deintercalation of ions without significant structural degradation. Innovations in electrode composition, such as carbon-based materials with optimized pore structures or metal-organic frameworks, help maintain capacity retention over extended cycling. These materials minimize volume changes during charge-discharge cycles, reducing mechanical stress and extending battery lifespan.- Electrode materials for improved cycling stability: Advanced electrode materials play a crucial role in enhancing the long-term performance of dual-ion batteries. Materials such as graphite, carbon-based composites, and metal oxides with optimized structures can significantly improve cycling stability by maintaining structural integrity during repeated charge-discharge cycles. These materials minimize volume changes and prevent electrode degradation, leading to extended battery lifespan and consistent performance over thousands of cycles.
- Electrolyte formulations for enhanced ion transport: Specialized electrolyte formulations are essential for maintaining dual-ion battery performance over extended periods. These formulations typically include optimized salt concentrations, solvent mixtures, and additives that improve ion mobility while reducing side reactions at electrode interfaces. Advanced electrolytes can suppress dendrite formation, minimize electrolyte decomposition, and enhance the stability of the solid-electrolyte interphase (SEI), resulting in improved capacity retention and extended cycle life.
- Battery management systems for performance optimization: Sophisticated battery management systems (BMS) are critical for optimizing the long-term performance of dual-ion batteries. These systems monitor and control key parameters such as temperature, voltage, and current to prevent conditions that accelerate degradation. Advanced BMS implementations include adaptive charging protocols, state-of-health estimation algorithms, and thermal management strategies that collectively extend battery lifespan by maintaining optimal operating conditions throughout the battery's service life.
- Novel cell designs and architectures: Innovative cell designs and architectures significantly impact the long-term performance of dual-ion batteries. These designs focus on optimizing ion diffusion pathways, reducing internal resistance, and improving mechanical stability. Advanced configurations may include structured electrodes, optimized separator designs, and novel cell geometries that collectively enhance capacity retention, power capability, and overall lifespan by addressing fundamental limitations in conventional battery designs.
- Protective coatings and interface engineering: Protective coatings and interface engineering techniques are employed to enhance the long-term stability of dual-ion batteries. These approaches involve applying nanoscale protective layers on electrode surfaces or modifying the electrode-electrolyte interfaces to suppress parasitic reactions. Such engineering strategies effectively mitigate degradation mechanisms like electrode dissolution, electrolyte decomposition, and gas evolution, resulting in improved cycling performance and extended calendar life under various operating conditions.
02 Electrolyte formulations for stability and cycle life
Specialized electrolyte formulations significantly impact the long-term performance of dual-ion batteries. These formulations include optimized salt concentrations, solvent mixtures, and additives that form stable solid-electrolyte interfaces, preventing continuous electrolyte decomposition. Advanced electrolytes minimize side reactions at electrode surfaces, reduce gas generation during cycling, and maintain ionic conductivity over extended periods. Some formulations incorporate flame-retardant components or ionic liquids to enhance safety while preserving long-term electrochemical performance.Expand Specific Solutions03 Battery management systems for performance optimization
Sophisticated battery management systems (BMS) are essential for optimizing the long-term performance of dual-ion batteries. These systems employ advanced algorithms to monitor and control charging/discharging parameters, preventing operation in detrimental voltage ranges. The BMS can implement adaptive charging protocols that adjust based on battery age and usage patterns, minimizing stress factors that accelerate degradation. Real-time monitoring of temperature, voltage, and current helps maintain optimal operating conditions, while predictive analytics can forecast performance decline and recommend preventive maintenance.Expand Specific Solutions04 Structural design innovations for durability
Innovative structural designs enhance the long-term durability of dual-ion batteries. These include novel cell architectures that accommodate volume changes during cycling, specialized separators that prevent dendrite formation, and reinforced current collectors that maintain electrical contact over extended use. Some designs incorporate pressure-regulation mechanisms to manage gas evolution, while others feature self-healing components that can repair minor damage during operation. Advanced packaging techniques minimize exposure to environmental factors like moisture and oxygen that accelerate degradation.Expand Specific Solutions05 Thermal management solutions for extended lifespan
Effective thermal management systems are critical for extending the lifespan of dual-ion batteries. These solutions include passive and active cooling mechanisms that prevent temperature spikes during fast charging or high-power discharge events. Some systems incorporate phase-change materials that absorb excess heat, while others use liquid cooling circuits for more demanding applications. Advanced thermal management also includes insulation strategies to maintain optimal operating temperatures in extreme environments. By preventing thermal runaway and minimizing temperature-related degradation mechanisms, these systems significantly improve long-term battery performance.Expand Specific Solutions
Leading Manufacturers and Research Institutions in DIB Technology
The dual-ion battery (DIB) market is currently in an early growth phase, characterized by increasing research activity but limited commercial deployment. The global market size remains relatively small compared to conventional lithium-ion batteries, though projections indicate significant expansion potential as the technology matures. Key players like Samsung SDI, Contemporary Amperex Technology (CATL), and SK Innovation are investing heavily in electrolyte formulation research to overcome DIB performance limitations. Technical maturity varies significantly across companies, with established battery manufacturers such as LG Chem, Panasonic, and Tesla focusing on electrolyte stability improvements, while specialized firms like Sion Power and Guangzhou Tinci Materials Technology concentrate on novel electrolyte compositions. Research institutions including Changchun Institute of Applied Chemistry and Qingdao Institute of Bioenergy are advancing fundamental understanding of electrolyte-electrode interactions for long-term DIB performance.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed advanced electrolyte formulations for dual-ion batteries (DIBs) focusing on high-voltage stability and long-term cycling performance. Their proprietary electrolyte system incorporates fluorinated carbonates and lithium salts with specific additives to mitigate aluminum current collector corrosion at high voltages. The company's research has demonstrated that controlling the solid electrolyte interphase (SEI) formation through tailored electrolyte composition significantly improves capacity retention over extended cycling. Samsung's formulations typically include 1M LiPF6 in EC/DMC with 2-5% FEC and phosphorus-based additives, which has shown to reduce electrolyte decomposition at the graphite anode during anion intercalation processes. Their recent developments include electrolytes with enhanced thermal stability up to 60°C, addressing a critical limitation in conventional DIB systems.
Strengths: Superior high-voltage stability (>4.5V) enabling higher energy density; excellent capacity retention (>80% after 1000 cycles); reduced self-discharge rates. Weaknesses: Higher production costs due to specialty additives; potential safety concerns at elevated temperatures despite improvements; limited performance in extreme low-temperature environments.
Contemporary Amperex Technology Co., Ltd.
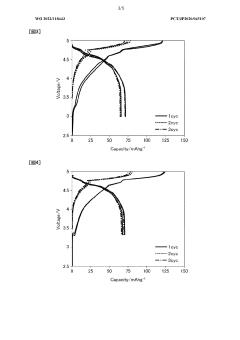
Technical Solution: CATL has pioneered a dual-salt electrolyte system specifically engineered for dual-ion batteries that addresses the critical challenges of anion intercalation and electrode stability. Their formulation combines conventional lithium salts (LiPF6) with specialized anion donors like lithium bis(fluorosulfonyl)imide (LiFSI) at optimized concentrations to enhance anion mobility and intercalation kinetics. CATL's research has demonstrated that controlling the solvation structure of the electrolyte through precise solvent ratios (typically using EC/DEC/FEC mixtures) significantly improves the reversibility of anion intercalation into graphite cathodes. Their proprietary additives package includes film-forming compounds that protect aluminum current collectors from corrosion at high voltages (>4.5V), a common failure mode in DIBs. CATL has also developed concentration-gradient electrolytes that create favorable SEI compositions at both electrodes simultaneously, addressing the competing requirements of anion and cation intercalation processes. Their electrolyte formulations have achieved remarkable cycling stability with >85% capacity retention after 2000 cycles at 1C rate.
Strengths: Exceptional cycling stability with minimal capacity fade; superior rate capability enabling fast charging applications; excellent compatibility with graphite cathodes preventing exfoliation. Weaknesses: Higher cost compared to conventional lithium-ion battery electrolytes; temperature sensitivity limiting performance below 0°C; potential safety concerns at elevated temperatures requiring additional thermal management.
Critical Patents and Research on Electrolyte-DIB Interface Mechanisms
Dual ion battery
PatentActiveJP2020187826A
Innovation
- The use of graphite as a positive electrode active material and Li4Ti5O12 as a negative electrode active material, combined with a non-aqueous solvent containing lithium hexafluorophosphate and tetrafluoroborate in a specific ratio, to enhance stability and ion mobility across varying temperatures.
Electrolyte and dual ion battery
PatentWO2022118443A1
Innovation
- A dual ion battery design incorporating a positive electrode with a lithium salt, a heterocyclic non-aromatic cation, and a bis(trifluoromethanesulfonyl)imide anion, along with a negative electrode active material, and an electrolyte containing specific compounds like sulfite, organoborane, and cyclic sulfonic acid esters, enables reversible electrochemical reactions.
Sustainability Impact of Electrolyte Materials in Battery Production
The environmental footprint of electrolyte materials in dual-ion batteries represents a critical sustainability consideration that extends beyond performance metrics. Conventional electrolytes often contain fluorinated salts and carbonate-based solvents derived from petrochemical sources, raising significant environmental concerns throughout their lifecycle. The mining and processing of lithium hexafluorophosphate (LiPF6) and other fluorinated compounds generate substantial greenhouse gas emissions and require extensive water usage, contributing to resource depletion in vulnerable ecosystems.
Production processes for these electrolytes typically involve energy-intensive synthesis methods and hazardous chemical reactions, resulting in considerable carbon emissions. For every kilogram of conventional electrolyte produced, approximately 5-7 kg of CO2 equivalent is released into the atmosphere, significantly impacting the overall carbon footprint of battery manufacturing operations.
The toxicity profile of traditional electrolyte components presents additional environmental challenges. Fluorinated compounds can persist in the environment for extended periods, potentially contaminating water sources and soil when improperly disposed. Studies indicate that leaching of these materials from discarded batteries can lead to bioaccumulation in aquatic organisms and disrupt local ecosystems, with potential long-term consequences for biodiversity.
Recent sustainability assessments have highlighted the end-of-life management challenges associated with electrolyte materials. The difficulty in separating and recovering electrolytes from spent batteries results in significant material loss and prevents the establishment of closed-loop recycling systems. This inefficiency undermines circular economy principles and contributes to the growing electronic waste problem globally.
Alternative green electrolyte formulations show promising sustainability improvements. Bio-derived solvents and water-based electrolytes can reduce environmental impact by up to 40% compared to conventional options, while maintaining acceptable electrochemical performance. Ionic liquids, despite their higher production costs, offer extended cycle life that may offset their initial environmental footprint through longer battery service life.
The sustainability profile of electrolyte materials directly influences regulatory compliance and market acceptance. As environmental regulations become increasingly stringent worldwide, manufacturers face growing pressure to adopt greener formulations. Companies pioneering sustainable electrolyte technologies gain competitive advantages through improved brand reputation and readiness for future regulatory frameworks, creating market incentives for continued innovation in this space.
Production processes for these electrolytes typically involve energy-intensive synthesis methods and hazardous chemical reactions, resulting in considerable carbon emissions. For every kilogram of conventional electrolyte produced, approximately 5-7 kg of CO2 equivalent is released into the atmosphere, significantly impacting the overall carbon footprint of battery manufacturing operations.
The toxicity profile of traditional electrolyte components presents additional environmental challenges. Fluorinated compounds can persist in the environment for extended periods, potentially contaminating water sources and soil when improperly disposed. Studies indicate that leaching of these materials from discarded batteries can lead to bioaccumulation in aquatic organisms and disrupt local ecosystems, with potential long-term consequences for biodiversity.
Recent sustainability assessments have highlighted the end-of-life management challenges associated with electrolyte materials. The difficulty in separating and recovering electrolytes from spent batteries results in significant material loss and prevents the establishment of closed-loop recycling systems. This inefficiency undermines circular economy principles and contributes to the growing electronic waste problem globally.
Alternative green electrolyte formulations show promising sustainability improvements. Bio-derived solvents and water-based electrolytes can reduce environmental impact by up to 40% compared to conventional options, while maintaining acceptable electrochemical performance. Ionic liquids, despite their higher production costs, offer extended cycle life that may offset their initial environmental footprint through longer battery service life.
The sustainability profile of electrolyte materials directly influences regulatory compliance and market acceptance. As environmental regulations become increasingly stringent worldwide, manufacturers face growing pressure to adopt greener formulations. Companies pioneering sustainable electrolyte technologies gain competitive advantages through improved brand reputation and readiness for future regulatory frameworks, creating market incentives for continued innovation in this space.
Scalability and Manufacturing Considerations for Advanced Electrolytes
The scaling of advanced electrolyte formulations for dual-ion batteries (DIBs) presents significant manufacturing challenges that directly impact their commercial viability. Current laboratory-scale electrolyte preparation methods often involve precise mixing of multiple components under controlled environments, which becomes increasingly complex when transitioning to industrial production. The consistency of electrolyte quality across large batches remains a critical concern, as minor variations can significantly affect battery performance and longevity.
Material sourcing represents another key consideration, particularly for specialized additives that enhance electrolyte stability and performance. Many high-performance electrolyte components face supply chain constraints or high costs when required at commercial scales. This necessitates either securing reliable supply channels or developing alternative formulations using more readily available materials without compromising performance.
Manufacturing processes must also address safety concerns inherent to electrolyte production. Many electrolyte components are flammable, volatile, or environmentally sensitive, requiring specialized handling equipment and safety protocols. These requirements add complexity and cost to manufacturing operations, potentially limiting production capacity and increasing unit costs.
Quality control systems for advanced electrolytes require sophisticated analytical techniques to ensure consistent composition and purity. Implementing these quality measures at production scale demands significant investment in analytical equipment and expertise. The development of streamlined quality control protocols that can be efficiently integrated into manufacturing workflows remains an ongoing challenge.
Environmental considerations also impact scalability, as regulations increasingly restrict certain solvents and additives commonly used in high-performance electrolytes. Manufacturers must balance performance requirements with environmental compliance, potentially necessitating reformulation or additional processing steps to minimize environmental impact.
Cost-effectiveness ultimately determines commercial viability. While laboratory-scale electrolytes can utilize expensive components to achieve optimal performance, commercial applications require careful cost-benefit analysis. Manufacturing processes must be optimized to reduce waste, energy consumption, and processing time while maintaining quality standards.
Recent innovations in continuous flow manufacturing and automated mixing systems show promise for addressing these scalability challenges. These approaches enable more precise control over mixing parameters and can significantly increase production throughput while maintaining consistency. Additionally, the development of pre-formulated electrolyte concentrates that can be diluted on-site may offer logistical advantages for global distribution and implementation.
Material sourcing represents another key consideration, particularly for specialized additives that enhance electrolyte stability and performance. Many high-performance electrolyte components face supply chain constraints or high costs when required at commercial scales. This necessitates either securing reliable supply channels or developing alternative formulations using more readily available materials without compromising performance.
Manufacturing processes must also address safety concerns inherent to electrolyte production. Many electrolyte components are flammable, volatile, or environmentally sensitive, requiring specialized handling equipment and safety protocols. These requirements add complexity and cost to manufacturing operations, potentially limiting production capacity and increasing unit costs.
Quality control systems for advanced electrolytes require sophisticated analytical techniques to ensure consistent composition and purity. Implementing these quality measures at production scale demands significant investment in analytical equipment and expertise. The development of streamlined quality control protocols that can be efficiently integrated into manufacturing workflows remains an ongoing challenge.
Environmental considerations also impact scalability, as regulations increasingly restrict certain solvents and additives commonly used in high-performance electrolytes. Manufacturers must balance performance requirements with environmental compliance, potentially necessitating reformulation or additional processing steps to minimize environmental impact.
Cost-effectiveness ultimately determines commercial viability. While laboratory-scale electrolytes can utilize expensive components to achieve optimal performance, commercial applications require careful cost-benefit analysis. Manufacturing processes must be optimized to reduce waste, energy consumption, and processing time while maintaining quality standards.
Recent innovations in continuous flow manufacturing and automated mixing systems show promise for addressing these scalability challenges. These approaches enable more precise control over mixing parameters and can significantly increase production throughput while maintaining consistency. Additionally, the development of pre-formulated electrolyte concentrates that can be diluted on-site may offer logistical advantages for global distribution and implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!