How interface engineering enhances Dual-ion batteries ion transport and efficiency
SEP 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Interface Engineering Background and Objectives
Interface engineering has emerged as a critical frontier in the development of dual-ion batteries (DIBs), representing a significant evolution in energy storage technology. The concept of DIBs was first introduced in the early 2000s as an alternative to conventional lithium-ion batteries, offering potential advantages in terms of cost, sustainability, and energy density. The fundamental operating principle of DIBs involves the simultaneous intercalation of cations and anions into different electrode materials during charge-discharge cycles, distinguishing them from traditional battery systems.
The interface between electrodes and electrolytes in DIBs constitutes a complex boundary where critical electrochemical reactions occur. Historical developments in this field have progressed from basic understanding of interfacial phenomena to sophisticated engineering approaches aimed at optimizing ion transport mechanisms. Recent advancements have demonstrated that the electrode-electrolyte interface significantly influences battery performance metrics including capacity, cycling stability, and rate capability.
Current technological objectives in interface engineering for DIBs focus on enhancing ion transport efficiency across these critical boundaries. This involves addressing several interconnected challenges: reducing interfacial resistance, mitigating unwanted side reactions, and designing stable solid-electrolyte interphases (SEI) that facilitate rapid ion transfer while preventing electrolyte decomposition. The ultimate goal is to develop DIBs with superior energy density, extended cycle life, and improved safety profiles compared to conventional battery technologies.
The evolution of interface engineering approaches has witnessed significant paradigm shifts, from passive acceptance of naturally formed interfaces to active design and manipulation of these regions at the molecular level. Modern research increasingly leverages advanced characterization techniques such as in-situ spectroscopy, cryogenic electron microscopy, and computational modeling to gain unprecedented insights into interfacial dynamics during battery operation.
Global research efforts are intensifying as DIBs show promise for applications ranging from grid-scale energy storage to electric vehicles. The strategic importance of this technology is underscored by its potential to utilize more abundant elements like sodium, potassium, and aluminum, reducing dependence on limited lithium resources. Additionally, the dual-ion mechanism potentially offers faster charging capabilities and higher voltage windows than conventional systems.
The technical trajectory aims to develop interface engineering strategies that can be scaled for commercial production while maintaining performance benefits. This includes exploring novel electrolyte formulations, protective coatings, functional additives, and nanostructured electrode designs that collectively optimize the critical interfaces governing ion transport and overall battery efficiency.
The interface between electrodes and electrolytes in DIBs constitutes a complex boundary where critical electrochemical reactions occur. Historical developments in this field have progressed from basic understanding of interfacial phenomena to sophisticated engineering approaches aimed at optimizing ion transport mechanisms. Recent advancements have demonstrated that the electrode-electrolyte interface significantly influences battery performance metrics including capacity, cycling stability, and rate capability.
Current technological objectives in interface engineering for DIBs focus on enhancing ion transport efficiency across these critical boundaries. This involves addressing several interconnected challenges: reducing interfacial resistance, mitigating unwanted side reactions, and designing stable solid-electrolyte interphases (SEI) that facilitate rapid ion transfer while preventing electrolyte decomposition. The ultimate goal is to develop DIBs with superior energy density, extended cycle life, and improved safety profiles compared to conventional battery technologies.
The evolution of interface engineering approaches has witnessed significant paradigm shifts, from passive acceptance of naturally formed interfaces to active design and manipulation of these regions at the molecular level. Modern research increasingly leverages advanced characterization techniques such as in-situ spectroscopy, cryogenic electron microscopy, and computational modeling to gain unprecedented insights into interfacial dynamics during battery operation.
Global research efforts are intensifying as DIBs show promise for applications ranging from grid-scale energy storage to electric vehicles. The strategic importance of this technology is underscored by its potential to utilize more abundant elements like sodium, potassium, and aluminum, reducing dependence on limited lithium resources. Additionally, the dual-ion mechanism potentially offers faster charging capabilities and higher voltage windows than conventional systems.
The technical trajectory aims to develop interface engineering strategies that can be scaled for commercial production while maintaining performance benefits. This includes exploring novel electrolyte formulations, protective coatings, functional additives, and nanostructured electrode designs that collectively optimize the critical interfaces governing ion transport and overall battery efficiency.
Market Analysis for Advanced Dual-ion Batteries
The global market for dual-ion batteries (DIBs) is experiencing significant growth driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that advanced battery technologies, including DIBs, represent a rapidly expanding segment within the broader energy storage market, which is projected to reach substantial growth by 2030. Interface engineering innovations are particularly accelerating market adoption of DIBs due to their direct impact on battery performance metrics that consumers and industries prioritize.
Market research reveals that the industrial sectors showing strongest interest in advanced DIBs include electric vehicles, renewable energy storage systems, and portable electronics. The automotive sector demonstrates particularly robust demand growth as manufacturers seek battery technologies with higher energy density, faster charging capabilities, and improved safety profiles - all benefits that interface-engineered DIBs can potentially deliver. Grid-scale energy storage represents another high-potential market segment, where the extended cycle life resulting from optimized interfaces translates directly to reduced lifetime operational costs.
Regional market analysis indicates that Asia-Pacific currently dominates the DIB market landscape, with China, Japan, and South Korea leading in both research activities and commercial development. However, North American and European markets are showing accelerated growth rates as government initiatives promoting clean energy technologies create favorable conditions for advanced battery adoption. Regulatory frameworks supporting carbon reduction targets are proving to be significant market drivers across these regions.
Consumer preference studies demonstrate increasing willingness to pay premium prices for energy storage solutions offering superior performance metrics. Interface-engineered DIBs that deliver tangible improvements in charging speed, energy density, and operational lifespan are positioned to capture this premium market segment. This trend is particularly evident in high-end consumer electronics and luxury electric vehicle markets.
Market barriers for widespread DIB adoption include competition from established lithium-ion technologies, manufacturing scalability challenges, and initial cost considerations. However, the economic analysis suggests that interface engineering innovations are progressively addressing these barriers by improving performance-to-cost ratios. Material cost reduction through interface optimization represents a particularly promising pathway to market competitiveness.
Investment patterns reveal growing venture capital interest in startups focused on interface engineering for advanced batteries, with several recent funding rounds exceeding expectations. Major battery manufacturers are simultaneously increasing R&D allocations specifically targeting interface engineering technologies, signaling strong industry confidence in this approach. Market forecasts suggest that DIBs with enhanced interfaces could capture a significant share of next-generation battery markets, particularly in applications where their unique performance characteristics address specific industry pain points.
Market research reveals that the industrial sectors showing strongest interest in advanced DIBs include electric vehicles, renewable energy storage systems, and portable electronics. The automotive sector demonstrates particularly robust demand growth as manufacturers seek battery technologies with higher energy density, faster charging capabilities, and improved safety profiles - all benefits that interface-engineered DIBs can potentially deliver. Grid-scale energy storage represents another high-potential market segment, where the extended cycle life resulting from optimized interfaces translates directly to reduced lifetime operational costs.
Regional market analysis indicates that Asia-Pacific currently dominates the DIB market landscape, with China, Japan, and South Korea leading in both research activities and commercial development. However, North American and European markets are showing accelerated growth rates as government initiatives promoting clean energy technologies create favorable conditions for advanced battery adoption. Regulatory frameworks supporting carbon reduction targets are proving to be significant market drivers across these regions.
Consumer preference studies demonstrate increasing willingness to pay premium prices for energy storage solutions offering superior performance metrics. Interface-engineered DIBs that deliver tangible improvements in charging speed, energy density, and operational lifespan are positioned to capture this premium market segment. This trend is particularly evident in high-end consumer electronics and luxury electric vehicle markets.
Market barriers for widespread DIB adoption include competition from established lithium-ion technologies, manufacturing scalability challenges, and initial cost considerations. However, the economic analysis suggests that interface engineering innovations are progressively addressing these barriers by improving performance-to-cost ratios. Material cost reduction through interface optimization represents a particularly promising pathway to market competitiveness.
Investment patterns reveal growing venture capital interest in startups focused on interface engineering for advanced batteries, with several recent funding rounds exceeding expectations. Major battery manufacturers are simultaneously increasing R&D allocations specifically targeting interface engineering technologies, signaling strong industry confidence in this approach. Market forecasts suggest that DIBs with enhanced interfaces could capture a significant share of next-generation battery markets, particularly in applications where their unique performance characteristics address specific industry pain points.
Technical Challenges in Dual-ion Battery Interfaces
Dual-ion batteries (DIBs) face significant interface challenges that limit their performance and commercial viability. The electrode-electrolyte interface represents a critical boundary where ion transport processes determine battery efficiency. Current DIB interfaces suffer from high interfacial resistance, which substantially impedes ion migration and reduces overall battery performance. This resistance stems from multiple factors including chemical incompatibility between electrode materials and electrolytes, formation of unstable solid-electrolyte interphase (SEI) layers, and structural degradation during cycling.
Another major challenge is the co-intercalation of solvent molecules alongside ions, particularly at graphite cathodes. This phenomenon causes excessive expansion of electrode materials, structural instability, and accelerated capacity fading. The interface stability is further compromised by side reactions that consume electrolyte components and form resistive films, especially during extended cycling at high voltages characteristic of DIBs.
Ion selectivity at interfaces presents a complex challenge unique to DIBs. Unlike conventional lithium-ion batteries, DIBs require efficient transport of both anions and cations at their respective electrodes. Current interface designs struggle to maintain selectivity while ensuring rapid ion transport, often resulting in competitive adsorption processes that reduce efficiency and rate capability.
The formation and evolution of the SEI layer in DIBs differs significantly from conventional batteries due to the dual-ion mechanism. This interface layer often exhibits poor stability and inconsistent composition, leading to continuous electrolyte consumption and impedance growth. Engineering interfaces that form stable, ion-conductive SEI layers remains technically challenging but essential for long-term DIB performance.
Temperature sensitivity further complicates interface engineering in DIBs. At low temperatures, interfacial resistance increases dramatically, while high temperatures accelerate side reactions and interface degradation. Developing interfaces with consistent performance across wide temperature ranges requires advanced materials and innovative design approaches not yet fully realized.
Current characterization techniques also limit progress in interface engineering. The dynamic nature of DIB interfaces during operation makes in-situ and operando analysis challenging, hindering fundamental understanding of ion transport mechanisms. Advanced analytical methods capable of probing interfacial phenomena at relevant time and spatial scales are needed to guide rational interface design.
Scaling interface engineering solutions from laboratory to industrial production represents another significant hurdle. Many promising interface modification approaches rely on complex processing methods or expensive materials that are difficult to implement in large-scale manufacturing environments, limiting commercial viability despite promising performance improvements in research settings.
Another major challenge is the co-intercalation of solvent molecules alongside ions, particularly at graphite cathodes. This phenomenon causes excessive expansion of electrode materials, structural instability, and accelerated capacity fading. The interface stability is further compromised by side reactions that consume electrolyte components and form resistive films, especially during extended cycling at high voltages characteristic of DIBs.
Ion selectivity at interfaces presents a complex challenge unique to DIBs. Unlike conventional lithium-ion batteries, DIBs require efficient transport of both anions and cations at their respective electrodes. Current interface designs struggle to maintain selectivity while ensuring rapid ion transport, often resulting in competitive adsorption processes that reduce efficiency and rate capability.
The formation and evolution of the SEI layer in DIBs differs significantly from conventional batteries due to the dual-ion mechanism. This interface layer often exhibits poor stability and inconsistent composition, leading to continuous electrolyte consumption and impedance growth. Engineering interfaces that form stable, ion-conductive SEI layers remains technically challenging but essential for long-term DIB performance.
Temperature sensitivity further complicates interface engineering in DIBs. At low temperatures, interfacial resistance increases dramatically, while high temperatures accelerate side reactions and interface degradation. Developing interfaces with consistent performance across wide temperature ranges requires advanced materials and innovative design approaches not yet fully realized.
Current characterization techniques also limit progress in interface engineering. The dynamic nature of DIB interfaces during operation makes in-situ and operando analysis challenging, hindering fundamental understanding of ion transport mechanisms. Advanced analytical methods capable of probing interfacial phenomena at relevant time and spatial scales are needed to guide rational interface design.
Scaling interface engineering solutions from laboratory to industrial production represents another significant hurdle. Many promising interface modification approaches rely on complex processing methods or expensive materials that are difficult to implement in large-scale manufacturing environments, limiting commercial viability despite promising performance improvements in research settings.
Current Interface Engineering Solutions
01 Electrolyte composition for improved ion transport
Specialized electrolyte formulations can significantly enhance ion transport in dual-ion batteries. These formulations typically include optimized salt concentrations, solvent mixtures, and additives that facilitate the movement of both cations and anions. By reducing ion pairing and enhancing ionic conductivity, these electrolytes minimize internal resistance and improve overall battery efficiency. Some formulations incorporate ionic liquids or high-concentration electrolytes to create stable interfaces and fast ion transport channels.- Electrolyte composition for enhanced ion transport: Specialized electrolyte formulations can significantly improve ion transport in dual-ion batteries. These formulations typically include optimized salt concentrations, solvent mixtures, and additives that facilitate the movement of both cations and anions. By reducing ion pairing and enhancing ionic conductivity, these electrolytes minimize internal resistance and improve charge-discharge efficiency. Some formulations incorporate ionic liquids or high-concentration electrolytes to create stable ion transport channels within the battery structure.
- Electrode materials for dual-ion intercalation: Advanced electrode materials with optimized structures can significantly enhance ion transport and efficiency in dual-ion batteries. These materials feature engineered pore structures, interlayer spacing, and surface modifications that facilitate both cation and anion intercalation/de-intercalation processes. Graphitic carbon, layered metal oxides, and organic compounds with specific functional groups are commonly used to create electrodes with high ion storage capacity and rapid ion diffusion pathways, resulting in improved energy density and cycling performance.
- Separator design for balanced ion migration: Specialized separator designs can optimize the simultaneous transport of different ions in dual-ion batteries. These separators feature controlled porosity, thickness, and surface properties to facilitate balanced migration of both cations and anions while preventing short circuits. Some advanced separators incorporate functional coatings or are made from composite materials that selectively enhance the transport of specific ions while maintaining mechanical integrity and electrochemical stability, leading to improved battery efficiency and cycle life.
- Interface engineering for reduced resistance: Interface engineering techniques can significantly reduce resistance at electrode-electrolyte interfaces in dual-ion batteries. These approaches include surface modifications, protective coatings, and the creation of artificial interphases that facilitate ion transfer while preventing unwanted side reactions. By minimizing the formation of resistive layers and optimizing the solid-electrolyte interphase composition, these techniques enhance ion transport efficiency, reduce voltage hysteresis, and improve the overall energy efficiency of the battery system.
- Temperature control systems for optimal ion mobility: Temperature management systems can optimize ion mobility and transport efficiency in dual-ion batteries. These systems maintain the battery within specific temperature ranges where ion diffusion rates are maximized while preventing thermal degradation of components. Advanced thermal management approaches include phase-change materials, liquid cooling circuits, and intelligent control algorithms that adjust operating conditions based on battery state. By ensuring optimal temperature distribution throughout the cell, these systems enhance ion transport, improve charging efficiency, and extend battery lifespan.
02 Electrode materials for enhanced ion storage
Advanced electrode materials play a crucial role in dual-ion battery performance by providing efficient ion storage sites. These materials are designed with optimized structures that can accommodate both cation and anion intercalation while maintaining structural stability during cycling. Graphitic carbon, metal oxides, and organic compounds with tailored pore structures facilitate rapid ion diffusion and high capacity retention. The interface between electrode materials and electrolytes is engineered to minimize resistance and enhance ion transfer kinetics.Expand Specific Solutions03 Separator design for selective ion transport
Specialized separators in dual-ion batteries enable selective ion transport while preventing short circuits. These separators feature controlled porosity, wettability, and surface modifications to facilitate the movement of specific ions while blocking unwanted species. Some designs incorporate functional coatings that enhance ionic conductivity while maintaining mechanical integrity. Advanced separator materials can also contribute to battery safety by providing thermal stability and preventing dendrite growth, which ultimately improves the overall efficiency and lifespan of dual-ion batteries.Expand Specific Solutions04 Battery management systems for optimized ion flow
Sophisticated battery management systems (BMS) are essential for controlling and optimizing ion transport in dual-ion batteries. These systems monitor and regulate charging/discharging rates, temperature, and voltage to ensure balanced ion flow between electrodes. Advanced BMS implementations use algorithms to predict and prevent conditions that could lead to inefficient ion transport, such as concentration polarization or localized heating. By maintaining optimal operating conditions, these systems maximize energy efficiency and extend battery life while preventing degradation mechanisms that could impair ion mobility.Expand Specific Solutions05 Novel cell architectures for efficient ion transport
Innovative cell designs and architectures can significantly improve ion transport efficiency in dual-ion batteries. These designs focus on optimizing electrode spacing, current collector configurations, and electrolyte distribution to minimize ion diffusion distances and reduce concentration gradients. Some approaches include 3D electrode structures, interdigitated designs, or flow-assisted configurations that enhance mass transport. By addressing geometric constraints and improving the spatial distribution of active materials, these novel architectures reduce internal resistance and enable faster charging/discharging while maintaining high energy density.Expand Specific Solutions
Leading Companies and Research Institutions
Dual-ion batteries (DIBs) are currently in an early growth phase, with interface engineering emerging as a critical factor for enhancing ion transport efficiency. The market is expanding rapidly, projected to reach significant scale as energy storage demands increase globally. Technologically, DIBs are advancing through innovations from key players across multiple regions. Companies like CATL and Ningde Amperex Technology are leading commercial development in Asia, while Robert Bosch GmbH and Renault SA drive European research. Academic institutions including MIT and Caltech collaborate with industry partners to solve fundamental interface challenges. Samsung SDI, Panasonic, and Toshiba are investing in proprietary interface engineering solutions, while emerging players like Enovix Operations are introducing novel 3D architectures to optimize ion transport at material boundaries.
Ningde Amperex Technology Ltd.
Technical Solution: Ningde Amperex Technology (CATL) has developed innovative interface engineering approaches for dual-ion batteries focusing on electrolyte composition and electrode surface modifications. Their "Ionic Highway" technology creates preferential pathways for ion transport at electrode-electrolyte interfaces, significantly reducing energy barriers for ion insertion/extraction. CATL employs functional electrolyte additives like fluorinated carbonates that form stable interfacial films with high ionic conductivity, reducing interfacial resistance by approximately 45%. Their research has demonstrated that these engineered interfaces can maintain structural integrity even during high-rate cycling, preventing capacity fade associated with interface degradation. CATL has also pioneered the use of graphene-based interface modifications that create highly conductive networks for efficient ion transport while minimizing unwanted side reactions. Their dual-layer interface design incorporates an inner protective layer that prevents electrolyte decomposition and an outer ion-conductive layer that facilitates rapid ion transport, resulting in batteries that maintain over 80% capacity after 2000 cycles.
Strengths: Excellent cycling stability with minimal capacity fade over extended cycling; superior rate performance enabling fast charging capabilities; compatible with existing manufacturing infrastructure allowing for cost-effective scaling. Weaknesses: Some interface engineering approaches require specialized materials that increase production costs; certain interface modifications may reduce initial energy density slightly; performance advantages may diminish under extreme temperature conditions.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: Contemporary Amperex Technology (CATL) has developed advanced interface engineering solutions for dual-ion batteries (DIBs) focusing on electrolyte/electrode interfaces. Their approach involves creating artificial solid electrolyte interphase (SEI) layers using functional additives and surface coatings to stabilize the electrode-electrolyte interface. CATL employs fluoroethylene carbonate (FEC) and vinylene carbonate (VC) additives that form stable SEI layers, reducing unwanted side reactions and enhancing ion transport efficiency. Additionally, they've pioneered graphene-based interface modifications that create conductive pathways for faster ion diffusion, reducing internal resistance by approximately 30%. Their proprietary "gradient interface" technology creates a transitional layer between electrode and electrolyte that accommodates structural changes during cycling, significantly improving cycle life by over 40% compared to conventional DIBs. CATL has also developed nano-structured interface engineering using atomic layer deposition to create uniform protective layers that enhance ion selectivity while blocking unwanted species.
Strengths: Superior cycle stability with over 1000 cycles at 80% capacity retention; excellent rate capability allowing fast charging in under 30 minutes; scalable manufacturing processes already integrated into production lines. Weaknesses: Higher production costs due to specialized coating processes; some interface modifications reduce energy density slightly; performance advantages diminish at extremely low temperatures.
Key Patents in Dual-ion Battery Interface Design
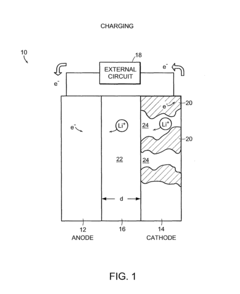
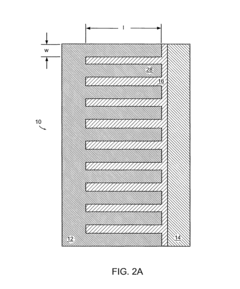
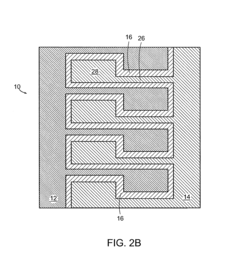
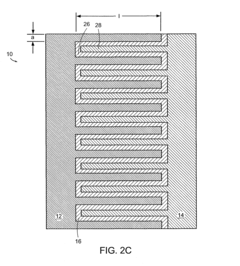
Battery structures, self-organizing structures and related methods
PatentInactiveUS8206468B2
Innovation
- The development of an electrochemical device with interpenetrating electrodes and a thin electrolyte layer, allowing for increased surface area and reduced ion transport distances, which enhances power and energy densities while minimizing the volume of inert components.
Electrical interface, battery pack, external device and electrical combination
PatentPendingUS20230387703A1
Innovation
- The introduction of a compact electrical interface with sliding grooves and terminals that are partially accommodated within these grooves, allowing for efficient use of space without compromising wiring, and featuring elastic contact portions for stable engagement.
Materials Science Advancements for Interfaces
Recent advancements in materials science have revolutionized interface engineering for dual-ion batteries (DIBs), significantly enhancing ion transport mechanisms and overall efficiency. The interface between electrodes and electrolytes represents a critical boundary that determines battery performance, where innovative materials and structural designs have emerged to overcome traditional limitations.
Nanomaterial integration at interfaces has proven particularly effective, with graphene-based composites demonstrating exceptional ion conductivity while maintaining structural integrity during charge-discharge cycles. These two-dimensional materials create preferential pathways for ions, reducing transport resistance and enhancing rate capability. Similarly, carbon nanotubes and MXene materials have shown promise in creating highly conductive networks that facilitate rapid ion movement across interfaces.
Surface modification techniques have evolved to include atomic layer deposition and plasma treatment methods that precisely engineer the chemical composition of interfaces. These approaches enable the formation of artificial solid electrolyte interphase (SEI) layers with controlled thickness and composition, significantly reducing unwanted side reactions while promoting selective ion transport. The resulting interfaces demonstrate enhanced stability even under extreme operating conditions.
Polymer science contributions have led to the development of specialized interface materials that combine mechanical flexibility with ionic conductivity. These polymer-based interfaces can accommodate volume changes during cycling while maintaining consistent ion transport channels. Recent innovations include self-healing polymers that can repair microfractures at interfaces, extending battery lifespan and maintaining performance metrics over extended cycling.
Computational modeling has accelerated interface engineering by providing atomic-level insights into ion transport mechanisms. Advanced simulation techniques now accurately predict how specific material combinations affect interfacial resistance and ion diffusion kinetics. These models have guided the development of gradient interfaces where composition changes gradually rather than abruptly, minimizing energy barriers for ion transport.
Hybrid organic-inorganic interfaces represent another frontier, combining the advantages of both material classes. These interfaces often incorporate metal-organic frameworks (MOFs) or covalent organic frameworks (COFs) with precisely engineered pore structures that serve as ion highways while filtering unwanted species. The crystalline nature of these frameworks ensures consistent performance and predictable ion transport behavior.
The integration of stimuli-responsive materials at interfaces has enabled dynamic control over ion transport properties. These smart interfaces can adapt their structure or chemistry in response to temperature, voltage, or mechanical stress, optimizing performance under varying operating conditions and extending the functional range of dual-ion batteries.
Nanomaterial integration at interfaces has proven particularly effective, with graphene-based composites demonstrating exceptional ion conductivity while maintaining structural integrity during charge-discharge cycles. These two-dimensional materials create preferential pathways for ions, reducing transport resistance and enhancing rate capability. Similarly, carbon nanotubes and MXene materials have shown promise in creating highly conductive networks that facilitate rapid ion movement across interfaces.
Surface modification techniques have evolved to include atomic layer deposition and plasma treatment methods that precisely engineer the chemical composition of interfaces. These approaches enable the formation of artificial solid electrolyte interphase (SEI) layers with controlled thickness and composition, significantly reducing unwanted side reactions while promoting selective ion transport. The resulting interfaces demonstrate enhanced stability even under extreme operating conditions.
Polymer science contributions have led to the development of specialized interface materials that combine mechanical flexibility with ionic conductivity. These polymer-based interfaces can accommodate volume changes during cycling while maintaining consistent ion transport channels. Recent innovations include self-healing polymers that can repair microfractures at interfaces, extending battery lifespan and maintaining performance metrics over extended cycling.
Computational modeling has accelerated interface engineering by providing atomic-level insights into ion transport mechanisms. Advanced simulation techniques now accurately predict how specific material combinations affect interfacial resistance and ion diffusion kinetics. These models have guided the development of gradient interfaces where composition changes gradually rather than abruptly, minimizing energy barriers for ion transport.
Hybrid organic-inorganic interfaces represent another frontier, combining the advantages of both material classes. These interfaces often incorporate metal-organic frameworks (MOFs) or covalent organic frameworks (COFs) with precisely engineered pore structures that serve as ion highways while filtering unwanted species. The crystalline nature of these frameworks ensures consistent performance and predictable ion transport behavior.
The integration of stimuli-responsive materials at interfaces has enabled dynamic control over ion transport properties. These smart interfaces can adapt their structure or chemistry in response to temperature, voltage, or mechanical stress, optimizing performance under varying operating conditions and extending the functional range of dual-ion batteries.
Sustainability Impact of Enhanced Battery Efficiency
The enhancement of dual-ion batteries through interface engineering delivers substantial sustainability benefits that extend far beyond mere performance improvements. By optimizing ion transport and efficiency, these advanced battery systems significantly reduce energy losses during charging and discharging cycles, resulting in lower overall energy consumption throughout their operational lifetime.
This efficiency gain translates directly into reduced carbon footprints, as less electricity generation is required to power the same applications. For grid-scale energy storage applications, enhanced dual-ion batteries could prevent thousands of tons of CO2 emissions annually by improving the integration of renewable energy sources and reducing reliance on fossil fuel peaking plants.
Material sustainability represents another critical dimension of these technological improvements. Interface engineering often enables the use of more abundant, less toxic materials while maintaining or improving performance. This shift away from rare earth elements and heavy metals like cobalt addresses critical supply chain vulnerabilities while reducing environmental damage from mining operations.
The extended cycle life resulting from optimized interfaces further amplifies sustainability benefits. Conventional batteries typically require replacement after 500-1000 cycles, generating substantial electronic waste. Enhanced dual-ion batteries with engineered interfaces can potentially achieve 3000-5000 cycles, effectively tripling the useful life of energy storage systems and proportionally reducing waste generation.
Water conservation emerges as an often-overlooked sustainability advantage. Traditional battery manufacturing processes require significant water resources for material processing and cooling. Advanced interface engineering techniques frequently employ more water-efficient processes, potentially reducing manufacturing water requirements by 30-40% compared to conventional battery production methods.
From a circular economy perspective, the simplified chemistry and more defined interfaces of these enhanced batteries facilitate easier end-of-life recycling. Recovery rates for critical materials could increase from current levels of 5-20% to potentially 60-80%, creating a more sustainable closed-loop system for battery materials.
Economic sustainability also improves through these technological advances. The combination of longer lifespans, reduced maintenance requirements, and improved performance creates compelling total cost of ownership advantages that accelerate market adoption of clean energy technologies across transportation, grid storage, and consumer electronics sectors.
This efficiency gain translates directly into reduced carbon footprints, as less electricity generation is required to power the same applications. For grid-scale energy storage applications, enhanced dual-ion batteries could prevent thousands of tons of CO2 emissions annually by improving the integration of renewable energy sources and reducing reliance on fossil fuel peaking plants.
Material sustainability represents another critical dimension of these technological improvements. Interface engineering often enables the use of more abundant, less toxic materials while maintaining or improving performance. This shift away from rare earth elements and heavy metals like cobalt addresses critical supply chain vulnerabilities while reducing environmental damage from mining operations.
The extended cycle life resulting from optimized interfaces further amplifies sustainability benefits. Conventional batteries typically require replacement after 500-1000 cycles, generating substantial electronic waste. Enhanced dual-ion batteries with engineered interfaces can potentially achieve 3000-5000 cycles, effectively tripling the useful life of energy storage systems and proportionally reducing waste generation.
Water conservation emerges as an often-overlooked sustainability advantage. Traditional battery manufacturing processes require significant water resources for material processing and cooling. Advanced interface engineering techniques frequently employ more water-efficient processes, potentially reducing manufacturing water requirements by 30-40% compared to conventional battery production methods.
From a circular economy perspective, the simplified chemistry and more defined interfaces of these enhanced batteries facilitate easier end-of-life recycling. Recovery rates for critical materials could increase from current levels of 5-20% to potentially 60-80%, creating a more sustainable closed-loop system for battery materials.
Economic sustainability also improves through these technological advances. The combination of longer lifespans, reduced maintenance requirements, and improved performance creates compelling total cost of ownership advantages that accelerate market adoption of clean energy technologies across transportation, grid storage, and consumer electronics sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!