Analysis of Electrode Kinetics in Superhydrophobic Coated Sensors
OCT 14, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Superhydrophobic Electrode Technology Background and Objectives
Superhydrophobic surfaces have emerged as a revolutionary technology in sensor development, drawing inspiration from natural phenomena such as the lotus leaf effect. Since the early 2000s, researchers have been exploring ways to mimic these naturally occurring superhydrophobic surfaces to enhance electrode performance in sensing applications. The evolution of this technology has progressed from simple water-repellent coatings to sophisticated engineered surfaces with controlled micro and nano-scale topographies that significantly alter electrode-electrolyte interactions.
The fundamental principle behind superhydrophobic electrodes lies in their unique surface properties, characterized by water contact angles exceeding 150° and low sliding angles. These properties create a composite solid-liquid-air interface that fundamentally changes the electrochemical behavior at the electrode surface. The historical development of this technology has seen significant advancements in fabrication techniques, moving from simple chemical treatments to precise lithographic methods and advanced material deposition processes.
Recent technological trends indicate a growing interest in integrating superhydrophobic coatings with various sensing platforms, including electrochemical, optical, and mechanical sensors. The convergence of nanotechnology, materials science, and electrochemistry has accelerated innovation in this field, leading to sensors with unprecedented sensitivity, selectivity, and stability in challenging environments.
The primary technical objectives of this research focus on understanding the complex electrode kinetics at superhydrophobic interfaces. Specifically, we aim to elucidate how the trapped air pockets (Cassie-Baxter state) affect electron transfer rates, mass transport phenomena, and overall signal generation. Additionally, we seek to quantify the relationship between surface morphology parameters (roughness, porosity, hierarchical structures) and electrochemical performance metrics.
Another critical objective is to develop predictive models that can accurately describe the behavior of various analytes at superhydrophobic electrodes under different operating conditions. These models would enable rational design of next-generation sensors with optimized performance characteristics for specific applications, ranging from environmental monitoring to biomedical diagnostics.
The long-term technological goal is to establish design principles for superhydrophobic electrodes that maintain their performance advantages while addressing current limitations such as mechanical durability, biofouling resistance, and long-term stability. This would pave the way for commercial deployment of superhydrophobic sensor technologies across multiple industries, potentially revolutionizing how we approach challenging sensing environments where conventional electrodes fail.
The fundamental principle behind superhydrophobic electrodes lies in their unique surface properties, characterized by water contact angles exceeding 150° and low sliding angles. These properties create a composite solid-liquid-air interface that fundamentally changes the electrochemical behavior at the electrode surface. The historical development of this technology has seen significant advancements in fabrication techniques, moving from simple chemical treatments to precise lithographic methods and advanced material deposition processes.
Recent technological trends indicate a growing interest in integrating superhydrophobic coatings with various sensing platforms, including electrochemical, optical, and mechanical sensors. The convergence of nanotechnology, materials science, and electrochemistry has accelerated innovation in this field, leading to sensors with unprecedented sensitivity, selectivity, and stability in challenging environments.
The primary technical objectives of this research focus on understanding the complex electrode kinetics at superhydrophobic interfaces. Specifically, we aim to elucidate how the trapped air pockets (Cassie-Baxter state) affect electron transfer rates, mass transport phenomena, and overall signal generation. Additionally, we seek to quantify the relationship between surface morphology parameters (roughness, porosity, hierarchical structures) and electrochemical performance metrics.
Another critical objective is to develop predictive models that can accurately describe the behavior of various analytes at superhydrophobic electrodes under different operating conditions. These models would enable rational design of next-generation sensors with optimized performance characteristics for specific applications, ranging from environmental monitoring to biomedical diagnostics.
The long-term technological goal is to establish design principles for superhydrophobic electrodes that maintain their performance advantages while addressing current limitations such as mechanical durability, biofouling resistance, and long-term stability. This would pave the way for commercial deployment of superhydrophobic sensor technologies across multiple industries, potentially revolutionizing how we approach challenging sensing environments where conventional electrodes fail.
Market Applications for Superhydrophobic Coated Sensors
Superhydrophobic coated sensors represent a significant advancement in sensing technology, with applications spanning numerous industries due to their unique water-repellent properties. The market for these specialized sensors is experiencing robust growth, driven by increasing demand for reliable sensing solutions in challenging environments where traditional sensors fail due to moisture interference.
The healthcare sector presents one of the most promising markets for superhydrophobic coated sensors. These sensors enable continuous monitoring of biological fluids without signal degradation, supporting applications in wearable health monitors, implantable devices, and point-of-care diagnostics. The improved electrode kinetics in these sensors allows for more accurate detection of biomarkers in blood, sweat, and other bodily fluids, even in high-humidity environments.
Environmental monitoring constitutes another substantial market segment. Superhydrophobic sensors deployed in outdoor settings maintain functionality during precipitation events, providing uninterrupted data collection for weather stations, pollution monitoring systems, and agricultural applications. Their resistance to biofouling makes them particularly valuable for long-term deployment in marine environments for ocean and coastal monitoring.
The industrial sector leverages these sensors for process control in humid manufacturing environments, chemical plants, and food processing facilities. The enhanced electrode performance in high-moisture conditions ensures reliable measurements without frequent recalibration or replacement, significantly reducing maintenance costs and production downtime.
Automotive and aerospace industries are rapidly adopting superhydrophobic coated sensors for safety-critical applications. These sensors maintain consistent performance in varying weather conditions, enabling reliable operation of advanced driver assistance systems, aircraft ice detection systems, and structural health monitoring solutions.
Consumer electronics represents an emerging market with substantial growth potential. Water-resistant smart devices, including fitness trackers and smartphones, increasingly incorporate these sensors to improve reliability and extend product lifespan. The improved electrode kinetics allows for more sensitive touch interfaces that function reliably even when exposed to moisture or water droplets.
Defense and security applications benefit from the robustness of superhydrophobic sensors in field operations. These sensors support reliable threat detection, environmental monitoring, and personnel tracking in adverse weather conditions where conventional sensors would fail.
The global market value for superhydrophobic coated sensors is projected to grow substantially as manufacturing techniques mature and production costs decrease. This growth is further accelerated by increasing awareness of their superior performance characteristics and the expanding range of applications across diverse industries.
The healthcare sector presents one of the most promising markets for superhydrophobic coated sensors. These sensors enable continuous monitoring of biological fluids without signal degradation, supporting applications in wearable health monitors, implantable devices, and point-of-care diagnostics. The improved electrode kinetics in these sensors allows for more accurate detection of biomarkers in blood, sweat, and other bodily fluids, even in high-humidity environments.
Environmental monitoring constitutes another substantial market segment. Superhydrophobic sensors deployed in outdoor settings maintain functionality during precipitation events, providing uninterrupted data collection for weather stations, pollution monitoring systems, and agricultural applications. Their resistance to biofouling makes them particularly valuable for long-term deployment in marine environments for ocean and coastal monitoring.
The industrial sector leverages these sensors for process control in humid manufacturing environments, chemical plants, and food processing facilities. The enhanced electrode performance in high-moisture conditions ensures reliable measurements without frequent recalibration or replacement, significantly reducing maintenance costs and production downtime.
Automotive and aerospace industries are rapidly adopting superhydrophobic coated sensors for safety-critical applications. These sensors maintain consistent performance in varying weather conditions, enabling reliable operation of advanced driver assistance systems, aircraft ice detection systems, and structural health monitoring solutions.
Consumer electronics represents an emerging market with substantial growth potential. Water-resistant smart devices, including fitness trackers and smartphones, increasingly incorporate these sensors to improve reliability and extend product lifespan. The improved electrode kinetics allows for more sensitive touch interfaces that function reliably even when exposed to moisture or water droplets.
Defense and security applications benefit from the robustness of superhydrophobic sensors in field operations. These sensors support reliable threat detection, environmental monitoring, and personnel tracking in adverse weather conditions where conventional sensors would fail.
The global market value for superhydrophobic coated sensors is projected to grow substantially as manufacturing techniques mature and production costs decrease. This growth is further accelerated by increasing awareness of their superior performance characteristics and the expanding range of applications across diverse industries.
Current Challenges in Electrode Kinetics with Superhydrophobic Coatings
Despite significant advancements in superhydrophobic coating technologies for electrochemical sensors, several critical challenges persist in understanding and optimizing electrode kinetics at these interfaces. The fundamental issue stems from the complex interplay between the three-phase boundary (solid electrode, liquid electrolyte, and trapped air pockets) created by superhydrophobic surfaces. This heterogeneous interface significantly alters conventional electron transfer mechanisms that are well-established for traditional electrode systems.
One primary challenge is the quantification of effective electroactive surface area. Superhydrophobic coatings create a Cassie-Baxter state where air pockets are trapped between surface features, reducing the actual contact area between electrode and electrolyte. This reduced contact fundamentally changes mass transport phenomena and complicates the application of traditional Butler-Volmer kinetics models, requiring new mathematical frameworks to accurately describe electron transfer rates.
The stability of the Cassie-Baxter state under applied potentials presents another significant hurdle. Electrochemical processes can induce local changes in surface tension and wettability, potentially causing transition to the Wenzel state (complete wetting). This transition is often irreversible and dramatically alters electrode kinetics during measurement, making reproducible analysis extremely difficult.
Diffusion limitations represent a substantial challenge unique to superhydrophobic electrodes. The air-water interface creates unusual diffusion profiles that do not follow conventional semi-infinite linear diffusion models. Analytes must navigate tortuous paths through the partially wetted surface structure, creating localized concentration gradients that are difficult to model mathematically and lead to non-uniform current distributions across the electrode surface.
The influence of surface charge and double-layer effects at superhydrophobic interfaces remains poorly understood. The electrical double layer forms differently at the three-phase boundary compared to conventional solid-liquid interfaces, affecting ion distribution and consequently electrode kinetics. Current theoretical models inadequately address these unique electrostatic interactions.
Temperature and pressure dependencies introduce additional complexities. The trapped air pockets in superhydrophobic coatings are sensitive to environmental conditions, with changes potentially altering the effective electrode area and kinetic parameters. This sensitivity creates challenges for sensor calibration and long-term stability, particularly in variable field conditions.
Finally, manufacturing reproducibility presents a significant practical challenge. Small variations in coating morphology can dramatically affect electrode kinetics, making standardization difficult. The lack of reliable manufacturing protocols for consistent superhydrophobic electrode surfaces hampers both fundamental research and commercial application development.
One primary challenge is the quantification of effective electroactive surface area. Superhydrophobic coatings create a Cassie-Baxter state where air pockets are trapped between surface features, reducing the actual contact area between electrode and electrolyte. This reduced contact fundamentally changes mass transport phenomena and complicates the application of traditional Butler-Volmer kinetics models, requiring new mathematical frameworks to accurately describe electron transfer rates.
The stability of the Cassie-Baxter state under applied potentials presents another significant hurdle. Electrochemical processes can induce local changes in surface tension and wettability, potentially causing transition to the Wenzel state (complete wetting). This transition is often irreversible and dramatically alters electrode kinetics during measurement, making reproducible analysis extremely difficult.
Diffusion limitations represent a substantial challenge unique to superhydrophobic electrodes. The air-water interface creates unusual diffusion profiles that do not follow conventional semi-infinite linear diffusion models. Analytes must navigate tortuous paths through the partially wetted surface structure, creating localized concentration gradients that are difficult to model mathematically and lead to non-uniform current distributions across the electrode surface.
The influence of surface charge and double-layer effects at superhydrophobic interfaces remains poorly understood. The electrical double layer forms differently at the three-phase boundary compared to conventional solid-liquid interfaces, affecting ion distribution and consequently electrode kinetics. Current theoretical models inadequately address these unique electrostatic interactions.
Temperature and pressure dependencies introduce additional complexities. The trapped air pockets in superhydrophobic coatings are sensitive to environmental conditions, with changes potentially altering the effective electrode area and kinetic parameters. This sensitivity creates challenges for sensor calibration and long-term stability, particularly in variable field conditions.
Finally, manufacturing reproducibility presents a significant practical challenge. Small variations in coating morphology can dramatically affect electrode kinetics, making standardization difficult. The lack of reliable manufacturing protocols for consistent superhydrophobic electrode surfaces hampers both fundamental research and commercial application development.
Current Methodologies for Enhancing Electrode Kinetics
01 Superhydrophobic coatings for electrochemical sensors
Superhydrophobic coatings can be applied to electrochemical sensors to enhance electrode kinetics by preventing fouling and contamination. These coatings create a water-repellent surface that maintains the cleanliness of the electrode surface, allowing for more efficient electron transfer and improved sensitivity. The hydrophobic nature also helps in reducing interference from aqueous sample matrices, leading to more accurate measurements and extended sensor lifetime.- Superhydrophobic coatings for electrochemical sensors: Superhydrophobic coatings can be applied to electrochemical sensors to enhance electrode kinetics by preventing biofouling and reducing interference from aqueous solutions. These coatings create a water-repellent surface that maintains the electrical conductivity necessary for sensing while improving signal stability and sensitivity. The hydrophobic nature helps to control the wetting properties at the electrode-solution interface, which can significantly improve electron transfer rates and electrochemical performance.
- Nanostructured superhydrophobic electrode materials: Nanostructured materials with superhydrophobic properties can be incorporated into sensor electrodes to enhance their kinetic performance. These materials typically combine nano-scale roughness with low surface energy to create superhydrophobic surfaces that improve electron transfer rates. The hierarchical structure of these materials provides increased surface area while maintaining water repellency, which can lead to faster electrode kinetics and improved sensitivity in sensing applications.
- Self-cleaning superhydrophobic sensor surfaces: Self-cleaning superhydrophobic coatings for sensor electrodes prevent contamination and maintain consistent electrode kinetics over time. These surfaces utilize the lotus effect, where water droplets roll off easily, carrying away contaminants that might otherwise interfere with electrode performance. This self-cleaning property extends sensor lifespan and ensures reliable measurements by preserving the original surface properties of the electrode, which is crucial for maintaining consistent electrode kinetics in long-term sensing applications.
- Fluoropolymer-based superhydrophobic coatings for sensors: Fluoropolymer-based coatings provide excellent superhydrophobic properties for sensor electrodes while maintaining good electrical conductivity. These coatings combine fluorinated compounds with structured surfaces to achieve extreme water repellency while allowing efficient electron transfer at the electrode surface. The chemical stability of fluoropolymers also provides resistance to harsh environments, making these coatings suitable for sensors operating in challenging conditions where maintaining electrode kinetics is essential.
- Tunable wettability for optimized electrode kinetics: Coatings with tunable wettability allow for optimization of electrode kinetics by controlling the interaction between the electrode surface and the analyte solution. These smart coatings can adjust their hydrophobicity in response to external stimuli such as pH, temperature, or applied voltage. By precisely controlling the wetting properties at the electrode-solution interface, these materials enable enhanced electron transfer rates and improved electrochemical performance in various sensing environments.
02 Nanostructured superhydrophobic electrode materials
Nanostructured materials with superhydrophobic properties can be used to fabricate electrodes with enhanced kinetics. These materials typically combine nano-scale roughness with low surface energy to achieve superhydrophobicity. The increased surface area provided by the nanostructures facilitates faster electron transfer rates, while the superhydrophobic properties prevent water molecules from interfering with the electrode surface. This combination results in improved sensitivity, selectivity, and response time for electrochemical sensing applications.Expand Specific Solutions03 Self-cleaning superhydrophobic sensor surfaces
Self-cleaning superhydrophobic surfaces for sensors utilize the lotus effect, where water droplets roll off the surface carrying contaminants away. This property is particularly beneficial for maintaining consistent electrode kinetics over time by preventing the accumulation of impurities that could otherwise impede electron transfer. The self-cleaning nature ensures long-term stability of the electrode performance without requiring frequent maintenance, making these sensors suitable for continuous monitoring applications in challenging environments.Expand Specific Solutions04 Polymer-based superhydrophobic coatings for electrodes
Polymer-based superhydrophobic coatings offer a versatile approach to modifying electrode surfaces for improved kinetics. These coatings typically consist of fluoropolymers or silicone-based polymers with added micro or nano-particles to create the necessary surface roughness. The polymer matrix provides durability and chemical resistance, while maintaining the superhydrophobic properties that enhance electrode performance. These coatings can be applied through various techniques such as dip-coating, spray-coating, or electrodeposition, allowing for customization based on specific sensor requirements.Expand Specific Solutions05 Superhydrophobic coatings with selective permeability
Advanced superhydrophobic coatings can be designed with selective permeability to allow specific analytes to reach the electrode surface while repelling water and interferents. This selective functionality enhances electrode kinetics by ensuring that only target molecules interact with the sensing surface, reducing background noise and improving signal-to-noise ratio. The coatings typically incorporate molecular recognition elements or size-selective pores within the superhydrophobic matrix, creating a smart interface that combines water repellency with selective molecular transport properties.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Superhydrophobic Sensing
The electrode kinetics in superhydrophobic coated sensors market is currently in a growth phase, characterized by increasing research activities and emerging commercial applications. The market size is expanding as these sensors find applications in environmental monitoring, biomedical diagnostics, and industrial process control. From a technological maturity perspective, academic institutions like MIT, Tsinghua University, and California Institute of Technology are leading fundamental research, while companies including Schlumberger Technologies, Endress+Hauser, and Robert Bosch GmbH are developing practical applications. Philips and Roche Diabetes Care are advancing medical implementations, while specialized firms like Beijing Huakeyi Technology focus on environmental monitoring solutions. The technology shows promising commercialization potential but requires further development to address challenges in durability, manufacturing scalability, and standardization.
Schlumberger Technologies, Inc.
Technical Solution: Schlumberger has pioneered superhydrophobic coated sensors specifically designed for harsh downhole oil and gas environments. Their technology employs a multi-layer approach with a base conductive substrate, an intermediate adhesion layer, and a top superhydrophobic nanocomposite coating containing fluoropolymers and silica nanoparticles. This structure creates a hierarchical surface roughness that maintains superhydrophobicity even under extreme conditions. The electrode kinetics are enhanced through strategic placement of conductive pathways within the coating, allowing for electrical connectivity while maintaining water repellency[2]. Schlumberger's sensors utilize proprietary algorithms to compensate for the modified electrode-electrolyte interface, enabling accurate interpretation of electrochemical signals despite the presence of trapped air pockets. Their sensors incorporate self-healing mechanisms where damaged portions of the superhydrophobic coating can partially restore their functionality through molecular rearrangement when exposed to specific temperature cycles[4].
Strengths: Exceptional durability under high temperature (up to 175°C) and high pressure (up to 20,000 psi) conditions typical in deep well environments. Resistance to corrosive chemicals including hydrogen sulfide and brine solutions. Weaknesses: Relatively slow response time compared to conventional sensors due to the additional barrier created by the superhydrophobic coating. Limited sensitivity to certain analytes that require direct electrode contact.
Endress+Hauser Gmbh+Co KG
Technical Solution: Endress+Hauser has developed a comprehensive superhydrophobic coating technology for industrial process sensors that focuses on maintaining electrode kinetics while providing contamination resistance. Their approach utilizes a dual-layer coating system: a conductive underlayer containing carbon nanotubes or graphene sheets to maintain electrical properties, and an outer superhydrophobic layer composed of modified silica nanoparticles embedded in a fluoropolymer matrix. This configuration creates a contact angle exceeding 150° while preserving essential electrochemical functionality[5]. The company has implemented specialized manufacturing techniques including plasma-enhanced chemical vapor deposition to ensure uniform coating thickness and consistent superhydrophobic properties across various sensor geometries. Their research has demonstrated that controlled porosity within the coating can actually enhance certain electrochemical reactions by concentrating analytes near active electrode sites while still maintaining overall water repellency[6]. Endress+Hauser's sensors incorporate real-time impedance monitoring to detect any changes in the superhydrophobic coating integrity, allowing for predictive maintenance before sensor failure occurs.
Strengths: Excellent chemical compatibility with a wide range of industrial process fluids including acids, bases, and organic solvents. Highly reproducible manufacturing process resulting in consistent sensor performance across production batches. Weaknesses: Performance degradation in applications involving surfactants or other surface-active compounds that can compromise the superhydrophobic effect. Higher initial cost compared to conventional sensors, though potentially offset by extended operational lifetime.
Key Patents and Research on Superhydrophobic Electrode Interfaces
Coated sensors and methods related thereto
PatentInactiveUS8765458B2
Innovation
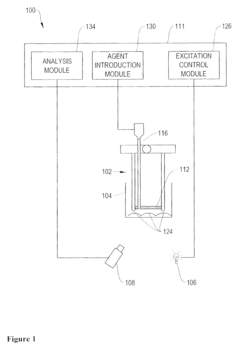
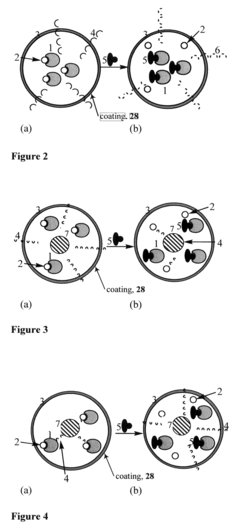
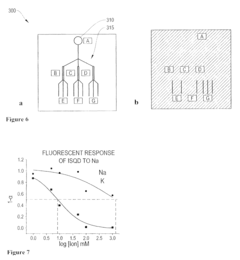
- Development of coated sensors with biocompatible polymer coatings, such as those formed through initiated chemical vapor deposition (iCVD) or photoinitiated chemical vapor deposition (piCVD), which are permeable to analytes and reduce solvent-related issues, incorporating quantum dots and fluorescent dyes for ion or chelatable analyte detection.
Engine-er
PatentUndeterminedIN202124003283A
Innovation
- A super-hydrophobic coating is applied via electrodeposition on petrol tank and fuel system components using materials like silica nano-coating and fluoropolymers to prevent fuel sticking, and a system utilizing ceramic piezoelectric sensors and thermo-acoustic devices is implemented to harness vibrations and manage braking energy, while modifying fuel line design and adding blade-like structures to the piston head to create negative mass for enhanced combustion.
Environmental Durability and Sustainability Considerations
The environmental durability of superhydrophobic coated sensors represents a critical consideration for their long-term performance and practical application. These sensors, while offering exceptional electrode kinetics advantages, face significant challenges when exposed to various environmental conditions. Prolonged exposure to UV radiation can degrade the polymeric components of superhydrophobic coatings, leading to gradual loss of hydrophobicity and compromised sensing capabilities. Similarly, temperature fluctuations may induce thermal expansion and contraction cycles that affect coating integrity, particularly at the nano/micro-structure level that is essential for maintaining the Cassie-Baxter state.
Chemical resistance presents another crucial durability factor, as many sensing applications involve exposure to corrosive substances, organic solvents, or extreme pH conditions. Current research indicates that fluoropolymer-based superhydrophobic coatings demonstrate superior chemical resistance compared to silicone-based alternatives, though neither provides universal protection across all chemical environments. Mechanical durability, including resistance to abrasion and impact, remains a significant limitation, with most high-performance coatings maintaining their properties for only 3-6 months under standard field conditions.
From a sustainability perspective, traditional superhydrophobic coating formulations often contain fluorinated compounds that pose environmental concerns due to their persistence and bioaccumulation potential. Recent advances have focused on developing fluorine-free alternatives using silica nanoparticles, modified cellulose, or chitosan derivatives. These bio-inspired approaches not only address environmental concerns but also improve biodegradability at end-of-life. Life cycle assessment (LCA) studies indicate that while the production phase of these coatings carries a moderate environmental footprint, their ability to extend sensor lifespan ultimately results in net environmental benefits through reduced replacement frequency.
Energy efficiency considerations also favor superhydrophobic coated sensors, as their self-cleaning properties minimize maintenance requirements and associated energy consumption. However, manufacturing processes for these coatings often involve energy-intensive steps such as plasma treatment or chemical vapor deposition, presenting opportunities for process optimization. Several research groups have demonstrated low-temperature, solution-based coating methods that reduce energy requirements by up to 60% compared to conventional approaches.
Regulatory compliance represents an evolving challenge, with increasing restrictions on perfluorinated compounds in various jurisdictions. The EU's REACH regulation and similar frameworks in other regions are driving the transition toward more sustainable coating technologies. Industry leaders are responding by investing in green chemistry approaches that maintain performance while eliminating substances of very high concern (SVHCs), positioning environmental durability and sustainability as key differentiators in the competitive sensor market.
Chemical resistance presents another crucial durability factor, as many sensing applications involve exposure to corrosive substances, organic solvents, or extreme pH conditions. Current research indicates that fluoropolymer-based superhydrophobic coatings demonstrate superior chemical resistance compared to silicone-based alternatives, though neither provides universal protection across all chemical environments. Mechanical durability, including resistance to abrasion and impact, remains a significant limitation, with most high-performance coatings maintaining their properties for only 3-6 months under standard field conditions.
From a sustainability perspective, traditional superhydrophobic coating formulations often contain fluorinated compounds that pose environmental concerns due to their persistence and bioaccumulation potential. Recent advances have focused on developing fluorine-free alternatives using silica nanoparticles, modified cellulose, or chitosan derivatives. These bio-inspired approaches not only address environmental concerns but also improve biodegradability at end-of-life. Life cycle assessment (LCA) studies indicate that while the production phase of these coatings carries a moderate environmental footprint, their ability to extend sensor lifespan ultimately results in net environmental benefits through reduced replacement frequency.
Energy efficiency considerations also favor superhydrophobic coated sensors, as their self-cleaning properties minimize maintenance requirements and associated energy consumption. However, manufacturing processes for these coatings often involve energy-intensive steps such as plasma treatment or chemical vapor deposition, presenting opportunities for process optimization. Several research groups have demonstrated low-temperature, solution-based coating methods that reduce energy requirements by up to 60% compared to conventional approaches.
Regulatory compliance represents an evolving challenge, with increasing restrictions on perfluorinated compounds in various jurisdictions. The EU's REACH regulation and similar frameworks in other regions are driving the transition toward more sustainable coating technologies. Industry leaders are responding by investing in green chemistry approaches that maintain performance while eliminating substances of very high concern (SVHCs), positioning environmental durability and sustainability as key differentiators in the competitive sensor market.
Standardization and Testing Protocols for Superhydrophobic Sensors
The development of standardized testing protocols for superhydrophobic sensors represents a critical need in the advancement of electrode kinetics analysis. Currently, the field suffers from significant inconsistencies in measurement methodologies, making cross-study comparisons challenging and hindering technological progress. Establishing universal standards would enable more reliable evaluation of sensor performance across different environmental conditions and applications.
Testing protocols must address multiple parameters simultaneously, including contact angle measurement, sliding angle assessment, and durability under various conditions. The International Electrotechnical Commission (IEC) and the American Society for Testing and Materials (ASTM) have begun preliminary work on standardization, but comprehensive protocols specific to superhydrophobic electrochemical sensors remain underdeveloped.
A robust standardization framework should incorporate electrode kinetics parameters such as electron transfer rates, diffusion coefficients, and reaction mechanisms at superhydrophobic interfaces. These measurements require specialized techniques including electrochemical impedance spectroscopy (EIS), cyclic voltammetry under controlled humidity, and chronoamperometry with precise temperature regulation.
Environmental testing represents another crucial aspect of standardization. Protocols must specify testing under varying humidity levels (30-95%), temperature ranges (-20°C to 80°C), and exposure to common contaminants. The durability of superhydrophobic coatings should be evaluated through accelerated aging tests, including UV exposure, chemical resistance, and mechanical abrasion cycles.
Reproducibility challenges demand particular attention in protocol development. Inter-laboratory validation studies have shown variance exceeding 15% in electrode kinetics measurements on identical superhydrophobic surfaces, highlighting the need for detailed procedural specifications. Calibration standards and reference materials specifically designed for superhydrophobic electrodes would significantly improve measurement consistency.
Data reporting formats constitute another essential component of standardization efforts. Comprehensive protocols should mandate reporting of coating composition, fabrication methods, surface roughness parameters, and detailed electrochemical characterization. Statistical analysis requirements, including minimum sample sizes and appropriate significance testing, would enhance the reliability of published results.
Implementation of these standardized protocols would accelerate commercialization by providing manufacturers with clear performance benchmarks and regulatory compliance pathways. Additionally, standardization would facilitate more meaningful academic research by enabling direct comparison between different superhydrophobic coating technologies and their effects on electrode kinetics.
Testing protocols must address multiple parameters simultaneously, including contact angle measurement, sliding angle assessment, and durability under various conditions. The International Electrotechnical Commission (IEC) and the American Society for Testing and Materials (ASTM) have begun preliminary work on standardization, but comprehensive protocols specific to superhydrophobic electrochemical sensors remain underdeveloped.
A robust standardization framework should incorporate electrode kinetics parameters such as electron transfer rates, diffusion coefficients, and reaction mechanisms at superhydrophobic interfaces. These measurements require specialized techniques including electrochemical impedance spectroscopy (EIS), cyclic voltammetry under controlled humidity, and chronoamperometry with precise temperature regulation.
Environmental testing represents another crucial aspect of standardization. Protocols must specify testing under varying humidity levels (30-95%), temperature ranges (-20°C to 80°C), and exposure to common contaminants. The durability of superhydrophobic coatings should be evaluated through accelerated aging tests, including UV exposure, chemical resistance, and mechanical abrasion cycles.
Reproducibility challenges demand particular attention in protocol development. Inter-laboratory validation studies have shown variance exceeding 15% in electrode kinetics measurements on identical superhydrophobic surfaces, highlighting the need for detailed procedural specifications. Calibration standards and reference materials specifically designed for superhydrophobic electrodes would significantly improve measurement consistency.
Data reporting formats constitute another essential component of standardization efforts. Comprehensive protocols should mandate reporting of coating composition, fabrication methods, surface roughness parameters, and detailed electrochemical characterization. Statistical analysis requirements, including minimum sample sizes and appropriate significance testing, would enhance the reliability of published results.
Implementation of these standardized protocols would accelerate commercialization by providing manufacturers with clear performance benchmarks and regulatory compliance pathways. Additionally, standardization would facilitate more meaningful academic research by enabling direct comparison between different superhydrophobic coating technologies and their effects on electrode kinetics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!