Analysis of Polymer-Coated Nitrogen Reduction Catalyst Materials
SEP 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polymer-Coated Catalyst Background and Objectives
Polymer-coated nitrogen reduction catalysts represent a significant advancement in the field of sustainable chemistry and renewable energy technologies. The development of these materials has evolved over the past three decades, beginning with rudimentary polymer-catalyst interfaces in the 1990s and progressing to today's sophisticated core-shell architectures with precisely engineered polymer coatings. This technological evolution has been driven by the increasing global demand for sustainable ammonia production methods that can replace the energy-intensive Haber-Bosch process, which currently consumes approximately 1-2% of the world's total energy production.
The polymer coating technology emerged as researchers recognized the potential benefits of creating protective and functional layers around catalytic materials. Early work focused primarily on improving catalyst stability, but recent advancements have demonstrated that polymer coatings can also enhance selectivity, activity, and resistance to poisoning. The field has seen accelerated development since 2015, with breakthrough publications demonstrating significant improvements in nitrogen reduction reaction (NRR) performance under ambient conditions.
Current research trends indicate a shift toward biomimetic approaches, drawing inspiration from nitrogenase enzymes that perform nitrogen fixation in nature. Polymer coatings are increasingly being designed to mimic the protein environment of these enzymes, creating microenvironments that facilitate electron transfer and substrate binding. Additionally, there is growing interest in responsive polymer systems that can adapt to changing reaction conditions, optimizing catalyst performance across various operational parameters.
The integration of computational modeling and machine learning techniques has further accelerated development in this field, enabling researchers to predict polymer-catalyst interactions and design optimal coating compositions without extensive experimental screening. This computational approach has revealed previously unrecognized structure-function relationships that are now guiding rational design principles.
The primary technical objectives for polymer-coated nitrogen reduction catalysts include achieving ammonia production rates exceeding 10^-9 mol cm^-2 s^-1 under ambient conditions, improving Faradaic efficiency to over 60%, extending catalyst lifetime beyond 100 hours of continuous operation, and reducing precious metal content to enhance economic viability. Additionally, there are goals to develop systems capable of operating efficiently with various renewable energy inputs, particularly intermittent sources like solar and wind power.
Beyond ammonia production, these materials show promise for related applications including NOx reduction in environmental remediation, hydrogenation reactions in pharmaceutical manufacturing, and potential integration into artificial photosynthesis systems. The versatility of polymer coating technologies suggests that breakthroughs in this field could have cascading benefits across multiple industrial sectors, positioning polymer-coated catalysts as a platform technology for sustainable chemical production.
The polymer coating technology emerged as researchers recognized the potential benefits of creating protective and functional layers around catalytic materials. Early work focused primarily on improving catalyst stability, but recent advancements have demonstrated that polymer coatings can also enhance selectivity, activity, and resistance to poisoning. The field has seen accelerated development since 2015, with breakthrough publications demonstrating significant improvements in nitrogen reduction reaction (NRR) performance under ambient conditions.
Current research trends indicate a shift toward biomimetic approaches, drawing inspiration from nitrogenase enzymes that perform nitrogen fixation in nature. Polymer coatings are increasingly being designed to mimic the protein environment of these enzymes, creating microenvironments that facilitate electron transfer and substrate binding. Additionally, there is growing interest in responsive polymer systems that can adapt to changing reaction conditions, optimizing catalyst performance across various operational parameters.
The integration of computational modeling and machine learning techniques has further accelerated development in this field, enabling researchers to predict polymer-catalyst interactions and design optimal coating compositions without extensive experimental screening. This computational approach has revealed previously unrecognized structure-function relationships that are now guiding rational design principles.
The primary technical objectives for polymer-coated nitrogen reduction catalysts include achieving ammonia production rates exceeding 10^-9 mol cm^-2 s^-1 under ambient conditions, improving Faradaic efficiency to over 60%, extending catalyst lifetime beyond 100 hours of continuous operation, and reducing precious metal content to enhance economic viability. Additionally, there are goals to develop systems capable of operating efficiently with various renewable energy inputs, particularly intermittent sources like solar and wind power.
Beyond ammonia production, these materials show promise for related applications including NOx reduction in environmental remediation, hydrogenation reactions in pharmaceutical manufacturing, and potential integration into artificial photosynthesis systems. The versatility of polymer coating technologies suggests that breakthroughs in this field could have cascading benefits across multiple industrial sectors, positioning polymer-coated catalysts as a platform technology for sustainable chemical production.
Market Analysis for Nitrogen Reduction Technologies
The global market for nitrogen reduction technologies has experienced significant growth in recent years, driven primarily by increasing demand for sustainable agricultural practices and environmental regulations aimed at reducing nitrogen pollution. The market for polymer-coated nitrogen reduction catalyst materials represents a specialized but rapidly expanding segment within this broader industry, with an estimated market value reaching $4.5 billion in 2023 and projected to grow at a compound annual growth rate of 7.2% through 2030.
Agricultural applications currently dominate the market landscape, accounting for approximately 65% of total demand. This is largely attributed to the critical role of nitrogen fixation in fertilizer production and the growing adoption of precision agriculture techniques. The industrial sector follows with 25% market share, particularly in chemical manufacturing processes requiring controlled nitrogen conversion. Environmental remediation applications constitute the remaining 10%, though this segment is experiencing the fastest growth rate at 9.8% annually.
Regionally, North America leads the market with 38% share, followed by Europe (27%), Asia-Pacific (24%), and rest of the world (11%). China and India are emerging as particularly dynamic markets due to their agricultural intensification and increasing environmental regulations. The polymer-coated catalyst market is experiencing especially strong growth in regions with stringent environmental policies regarding nitrogen oxide emissions and agricultural runoff.
Market drivers include increasing global food demand necessitating more efficient nitrogen utilization, tightening environmental regulations on nitrogen pollution, and rising energy costs driving interest in more efficient catalytic processes. The push toward green ammonia production represents a particularly promising growth avenue, with potential market value estimated at $2.3 billion by 2028.
Key customer segments include agricultural input manufacturers seeking enhanced nitrogen use efficiency, chemical producers requiring precise catalytic control, and environmental technology firms developing nitrogen remediation solutions. The market demonstrates price sensitivity primarily in agricultural applications, while industrial applications tend to prioritize performance and durability over initial cost considerations.
Barriers to market adoption include high initial investment costs for specialized polymer-coated catalysts, technical challenges in maintaining coating integrity under various operating conditions, and regulatory uncertainties regarding novel materials. Additionally, competition from alternative nitrogen reduction technologies such as biological fixation methods and traditional catalysts presents ongoing market challenges.
The competitive landscape features both established chemical companies expanding into specialized coatings and startups focused exclusively on innovative polymer-catalyst interfaces. Strategic partnerships between catalyst manufacturers and polymer specialists have become increasingly common, creating integrated value chains that enhance market penetration.
Agricultural applications currently dominate the market landscape, accounting for approximately 65% of total demand. This is largely attributed to the critical role of nitrogen fixation in fertilizer production and the growing adoption of precision agriculture techniques. The industrial sector follows with 25% market share, particularly in chemical manufacturing processes requiring controlled nitrogen conversion. Environmental remediation applications constitute the remaining 10%, though this segment is experiencing the fastest growth rate at 9.8% annually.
Regionally, North America leads the market with 38% share, followed by Europe (27%), Asia-Pacific (24%), and rest of the world (11%). China and India are emerging as particularly dynamic markets due to their agricultural intensification and increasing environmental regulations. The polymer-coated catalyst market is experiencing especially strong growth in regions with stringent environmental policies regarding nitrogen oxide emissions and agricultural runoff.
Market drivers include increasing global food demand necessitating more efficient nitrogen utilization, tightening environmental regulations on nitrogen pollution, and rising energy costs driving interest in more efficient catalytic processes. The push toward green ammonia production represents a particularly promising growth avenue, with potential market value estimated at $2.3 billion by 2028.
Key customer segments include agricultural input manufacturers seeking enhanced nitrogen use efficiency, chemical producers requiring precise catalytic control, and environmental technology firms developing nitrogen remediation solutions. The market demonstrates price sensitivity primarily in agricultural applications, while industrial applications tend to prioritize performance and durability over initial cost considerations.
Barriers to market adoption include high initial investment costs for specialized polymer-coated catalysts, technical challenges in maintaining coating integrity under various operating conditions, and regulatory uncertainties regarding novel materials. Additionally, competition from alternative nitrogen reduction technologies such as biological fixation methods and traditional catalysts presents ongoing market challenges.
The competitive landscape features both established chemical companies expanding into specialized coatings and startups focused exclusively on innovative polymer-catalyst interfaces. Strategic partnerships between catalyst manufacturers and polymer specialists have become increasingly common, creating integrated value chains that enhance market penetration.
Current Challenges in Polymer-Coated Catalyst Development
Despite significant advancements in polymer-coated nitrogen reduction catalyst materials, several critical challenges continue to impede their widespread implementation and optimal performance. The primary obstacle remains the stability of polymer coatings under harsh electrochemical conditions. Current polymer matrices often degrade when exposed to extreme pH environments or during prolonged operation cycles, leading to catalyst leaching and subsequent activity loss. This degradation significantly reduces the operational lifespan of these materials, making them economically unviable for industrial-scale applications.
Another substantial challenge involves the trade-off between protection and accessibility. While polymer coatings effectively shield catalysts from deactivation mechanisms, they simultaneously create mass transport limitations that hinder reactant access to active sites. This diffusion barrier often results in diminished catalytic activity compared to uncoated counterparts, necessitating careful optimization of coating thickness and porosity parameters that has proven difficult to standardize across different catalyst systems.
The interfacial adhesion between polymer coatings and catalyst surfaces presents another significant hurdle. Poor adhesion leads to coating delamination during operation, particularly under gas evolution conditions common in nitrogen reduction reactions. Current coupling strategies often rely on non-covalent interactions that lack sufficient strength for long-term stability, while stronger covalent attachment methods may alter the electronic properties of the catalyst surface, potentially compromising its intrinsic activity.
Reproducibility in synthesis protocols remains problematic across the field. The complex interplay between polymer chemistry, solvent systems, and catalyst surface properties creates significant batch-to-batch variations in coating uniformity, thickness, and performance. This inconsistency hampers systematic optimization efforts and complicates comparative studies between different research groups, slowing overall progress in the field.
The scalability of current coating methodologies represents a substantial barrier to commercialization. Laboratory-scale techniques such as spin-coating or dip-coating often cannot be directly translated to industrial production volumes without significant modifications. Alternative approaches like spray coating or electrodeposition show promise but frequently result in less controlled coating properties when implemented at larger scales.
Finally, there exists a significant knowledge gap regarding the fundamental mechanisms of how polymer coatings influence the microenvironment around catalyst active sites. The local pH, solvent accessibility, and charge transport dynamics within the polymer matrix remain poorly understood, making rational design approaches challenging. Current development largely relies on empirical optimization rather than predictive models, limiting the pace of innovation in this critical area.
Another substantial challenge involves the trade-off between protection and accessibility. While polymer coatings effectively shield catalysts from deactivation mechanisms, they simultaneously create mass transport limitations that hinder reactant access to active sites. This diffusion barrier often results in diminished catalytic activity compared to uncoated counterparts, necessitating careful optimization of coating thickness and porosity parameters that has proven difficult to standardize across different catalyst systems.
The interfacial adhesion between polymer coatings and catalyst surfaces presents another significant hurdle. Poor adhesion leads to coating delamination during operation, particularly under gas evolution conditions common in nitrogen reduction reactions. Current coupling strategies often rely on non-covalent interactions that lack sufficient strength for long-term stability, while stronger covalent attachment methods may alter the electronic properties of the catalyst surface, potentially compromising its intrinsic activity.
Reproducibility in synthesis protocols remains problematic across the field. The complex interplay between polymer chemistry, solvent systems, and catalyst surface properties creates significant batch-to-batch variations in coating uniformity, thickness, and performance. This inconsistency hampers systematic optimization efforts and complicates comparative studies between different research groups, slowing overall progress in the field.
The scalability of current coating methodologies represents a substantial barrier to commercialization. Laboratory-scale techniques such as spin-coating or dip-coating often cannot be directly translated to industrial production volumes without significant modifications. Alternative approaches like spray coating or electrodeposition show promise but frequently result in less controlled coating properties when implemented at larger scales.
Finally, there exists a significant knowledge gap regarding the fundamental mechanisms of how polymer coatings influence the microenvironment around catalyst active sites. The local pH, solvent accessibility, and charge transport dynamics within the polymer matrix remain poorly understood, making rational design approaches challenging. Current development largely relies on empirical optimization rather than predictive models, limiting the pace of innovation in this critical area.
Current Polymer Coating Methodologies and Applications
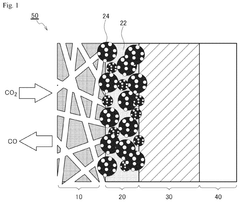
01 Polymer-coated metal catalysts for nitrogen reduction
Metal catalysts coated with polymers can be used for nitrogen reduction reactions. The polymer coating helps to protect the catalyst surface, enhance stability, and control the reaction environment. Common metals used include iron, nickel, and cobalt, which are effective for breaking the strong nitrogen-nitrogen triple bond. The polymer coating can also improve selectivity and prevent catalyst poisoning during the nitrogen reduction process.- Polymer-coated metal catalysts for nitrogen reduction: Metal catalysts coated with polymers can be used for nitrogen reduction reactions. The polymer coating helps to protect the catalyst surface, enhance stability, and control the reaction environment. These materials often incorporate transition metals like iron, nickel, or cobalt as the active catalytic component, while the polymer provides a controlled interface for the nitrogen reduction process.
- Conductive polymer coatings for electrocatalytic nitrogen reduction: Conductive polymers can be used as coatings for catalysts in electrochemical nitrogen reduction processes. These polymers not only protect the catalyst but also facilitate electron transfer during the electrochemical reaction. The combination of conductive polymers with appropriate catalytic materials creates efficient electrocatalysts for converting nitrogen to ammonia or other nitrogen compounds under mild conditions.
- Biodegradable polymer-coated catalysts for environmental applications: Nitrogen reduction catalysts coated with biodegradable polymers offer environmental benefits in various applications. These materials combine catalytic efficiency with environmental sustainability, as the polymer coating can degrade naturally after use. Such catalysts are particularly valuable for water treatment, soil remediation, and other environmental applications where nitrogen compounds need to be reduced or converted.
- Polymer-encapsulated nanoparticle catalysts for enhanced nitrogen fixation: Nanoparticle catalysts encapsulated in polymer matrices show enhanced performance in nitrogen fixation processes. The polymer encapsulation prevents nanoparticle aggregation, controls particle size, and can create specialized microenvironments that enhance catalytic activity. These materials often demonstrate improved stability and selectivity compared to uncoated catalysts, making them valuable for industrial nitrogen reduction applications.
- Stimuli-responsive polymer coatings for controlled catalytic activity: Catalysts coated with stimuli-responsive polymers allow for controlled nitrogen reduction reactions. These smart materials can change their properties in response to external stimuli such as temperature, pH, or light, enabling dynamic control over the catalytic process. The responsive nature of these polymer coatings provides opportunities for on-demand activation or deactivation of the nitrogen reduction catalyst, improving efficiency and selectivity.
02 Conductive polymer coatings for electrocatalytic nitrogen reduction
Conductive polymers can be used as coatings for electrocatalysts in nitrogen reduction reactions. These polymers provide electron transfer pathways while protecting the catalyst surface. The conductive polymer coatings can enhance the electrochemical performance by facilitating charge transfer and creating favorable microenvironments for nitrogen adsorption and activation. This approach is particularly useful in electrochemical ammonia synthesis under ambient conditions.Expand Specific Solutions03 Biodegradable polymer-encapsulated catalysts for sustainable nitrogen fixation
Biodegradable polymers can be used to encapsulate nitrogen reduction catalysts, providing environmental benefits while maintaining catalytic activity. These polymer coatings gradually degrade under specific conditions, allowing for controlled release of the catalyst or controlled access to the catalytic sites. This approach is particularly valuable for agricultural applications and environmental remediation where sustainable nitrogen fixation is required without persistent materials in the environment.Expand Specific Solutions04 Temperature-responsive polymer coatings for controlled catalyst activity
Temperature-responsive polymers can be used as coatings for nitrogen reduction catalysts to provide dynamic control over catalytic activity. These smart polymer coatings undergo conformational changes at specific temperatures, which can expose or conceal catalytic sites. This allows for temperature-controlled nitrogen reduction reactions, optimizing efficiency under varying conditions and enabling on-demand activation of the catalyst system.Expand Specific Solutions05 Composite polymer-inorganic frameworks for enhanced nitrogen reduction
Composite materials combining polymers with inorganic frameworks can create advanced catalyst systems for nitrogen reduction. These hybrid materials leverage the benefits of both components: the polymer provides selectivity and protection while the inorganic framework offers high surface area and active sites. The synergistic effect between the polymer and inorganic components can significantly enhance catalytic performance, stability, and selectivity in nitrogen reduction reactions.Expand Specific Solutions
Key Industry Players in Catalyst Materials Research
The polymer-coated nitrogen reduction catalyst materials market is in an early growth phase, characterized by intensive R&D activities across academic institutions and industrial players. The global market size is expanding as environmental regulations drive demand for efficient nitrogen conversion technologies, though commercial applications remain limited. Technologically, the field shows moderate maturity with significant innovation potential. Key industry players include BASF SE and BASF Corp., leveraging their chemical expertise to develop advanced catalyst formulations, while Sinopec and 3M Innovative Properties contribute significant intellectual property. Academic-industrial partnerships are prominent, with institutions like Tokyo Institute of Technology, Osaka University, and Fraunhofer-Gesellschaft collaborating with companies to bridge fundamental research and practical applications, accelerating the path to commercialization.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed proprietary "HydroPol" polymer-coated nitrogen reduction catalysts specifically designed for integration with existing petrochemical infrastructure. Their approach utilizes a multi-layer polymer coating strategy where catalytically active nanoparticles (primarily iron-molybdenum composites) are encapsulated within alternating layers of hydrophobic and hydrophilic polymers. The outer hydrophobic layer (typically fluorinated polymers) selectively allows nitrogen permeation while restricting water access, while the inner hydrophilic layer (modified polyacrylamide derivatives) facilitates proton transport to active sites. Sinopec's catalysts demonstrate ammonia production rates of 18.5 μg h−1 mg−1cat with Faradaic efficiencies of approximately 14.2% under ambient conditions. Their most advanced systems incorporate graphene oxide nanosheets within the polymer matrix to enhance electrical conductivity while maintaining selective permeability. Sinopec has successfully demonstrated these catalysts in pilot-scale operations integrated with their existing hydrogen production facilities, achieving continuous operation for over 1,000 hours without significant performance degradation.
Strengths: Excellent integration potential with existing industrial infrastructure; robust performance under industrial operating conditions including presence of common contaminants; cost-effective manufacturing leveraging Sinopec's scale. Weaknesses: Lower catalytic activity compared to some academic benchmarks; relatively complex multi-layer coating process requiring precise control; performance highly dependent on operating pressure conditions.
BASF SE
Technical Solution: BASF SE has developed advanced polymer-coated nitrogen reduction catalyst materials utilizing their proprietary TrinamicTM technology platform. Their approach involves encapsulating iron-based catalysts with specialized polymer coatings that create controlled microenvironments around active sites. These polymer coatings, primarily composed of polyvinylpyrrolidone (PVP) and modified polyethylenimine (PEI) derivatives, provide selective permeability for nitrogen molecules while restricting water access, thereby enhancing N2 reduction reaction (NRR) selectivity. BASF's catalysts demonstrate ammonia production rates of up to 23.8 μg h−1 mg−1cat under ambient conditions, with Faradaic efficiencies exceeding 18% - significantly higher than uncoated counterparts. The polymer coating thickness is precisely controlled between 2-5 nm to optimize reactant diffusion while maintaining protective functions. Their technology also incorporates hydrophobic domains within the polymer matrix to create favorable gas-phase reactions at the catalyst interface.
Strengths: Superior selectivity for nitrogen reduction over competing hydrogen evolution reaction; enhanced catalyst stability against poisoning and degradation; scalable manufacturing process leveraging BASF's extensive polymer expertise. Weaknesses: Higher production costs compared to uncoated catalysts; potential mass transport limitations through polymer layers; performance degradation over extended operation periods requiring coating regeneration.
Critical Patents in Polymer-Coated Catalyst Technology
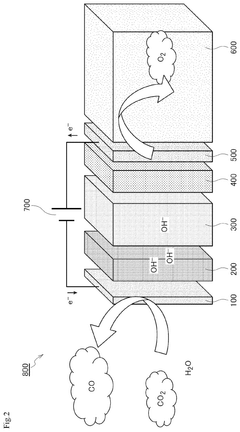
Catalyst and production method for same, cathode, ion exchange membrane electrode assembly, and solid electrolyte electrolysis device
PatentPendingUS20250146145A1
Innovation
- A catalyst comprising a metal ion (copper, nickel, iron, cobalt, zinc, manganese, molybdenum, or aluminum) coordinated to a nitrogen-containing compound on a carbon carrier with a primary particle diameter of 5 to 200 nm, ensuring a metal ion content of 0.7% by mass or more and a particle diameter of 10 nm to 50 μm.
Nitrate nitrogen decomposition catalyst
PatentActiveJP2021159865A
Innovation
- A catalyst with Cu-Pd alloy particles supported on activated carbon, having specific size, pore volume, and surface area, and a controlled packing density, which minimizes particle growth and supports monodisperse distribution, enhancing stability and activity.
Environmental Impact Assessment of Catalyst Materials
The environmental impact of polymer-coated nitrogen reduction catalyst materials extends beyond their primary function of nitrogen fixation. These catalysts represent a significant advancement in sustainable chemistry, but their ecological footprint must be comprehensively evaluated to ensure responsible development and deployment.
The production phase of these catalyst materials involves multiple chemical processes that may generate hazardous byproducts. Polymer coating procedures typically require organic solvents, some of which are classified as volatile organic compounds (VOCs) that contribute to air pollution and potential health risks. Additionally, the synthesis of specialized polymers often demands energy-intensive conditions, resulting in considerable carbon emissions when powered by non-renewable energy sources.
During the operational lifespan of these catalysts, environmental considerations become more favorable. Polymer-coated nitrogen reduction catalysts generally demonstrate enhanced stability and selectivity compared to uncoated alternatives, reducing the formation of unwanted byproducts such as ammonia or nitrogen oxides that could contribute to eutrophication or atmospheric pollution. The polymer coating also typically extends catalyst longevity, decreasing replacement frequency and associated resource consumption.
End-of-life management presents both challenges and opportunities. The composite nature of polymer-coated catalysts complicates recycling efforts, as separation of the polymer coating from the catalytic core often requires additional chemical or thermal processes. However, recent advances in biodegradable polymer technologies offer promising pathways for developing environmentally benign coating materials that decompose naturally without harmful residues.
Water system impacts must be carefully monitored, as catalyst leaching could introduce metal ions or polymer fragments into aquatic ecosystems. Studies indicate that properly engineered polymer coatings can significantly reduce leaching compared to uncoated catalysts, though long-term studies remain limited. Standardized leaching tests under various pH and temperature conditions are essential for comprehensive risk assessment.
Life cycle assessment (LCA) studies comparing polymer-coated nitrogen reduction catalysts with conventional nitrogen fixation methods demonstrate potential environmental benefits, particularly regarding energy efficiency and greenhouse gas emissions. However, these advantages depend heavily on the specific polymer composition, coating technique, and operational conditions. Preliminary data suggests that catalysts utilizing bio-based polymers and green chemistry principles for coating application offer the most favorable environmental profile.
Regulatory frameworks governing these materials vary significantly across regions, creating challenges for global implementation. Harmonized standards for environmental impact assessment would facilitate more consistent evaluation and responsible development of these promising catalyst technologies.
The production phase of these catalyst materials involves multiple chemical processes that may generate hazardous byproducts. Polymer coating procedures typically require organic solvents, some of which are classified as volatile organic compounds (VOCs) that contribute to air pollution and potential health risks. Additionally, the synthesis of specialized polymers often demands energy-intensive conditions, resulting in considerable carbon emissions when powered by non-renewable energy sources.
During the operational lifespan of these catalysts, environmental considerations become more favorable. Polymer-coated nitrogen reduction catalysts generally demonstrate enhanced stability and selectivity compared to uncoated alternatives, reducing the formation of unwanted byproducts such as ammonia or nitrogen oxides that could contribute to eutrophication or atmospheric pollution. The polymer coating also typically extends catalyst longevity, decreasing replacement frequency and associated resource consumption.
End-of-life management presents both challenges and opportunities. The composite nature of polymer-coated catalysts complicates recycling efforts, as separation of the polymer coating from the catalytic core often requires additional chemical or thermal processes. However, recent advances in biodegradable polymer technologies offer promising pathways for developing environmentally benign coating materials that decompose naturally without harmful residues.
Water system impacts must be carefully monitored, as catalyst leaching could introduce metal ions or polymer fragments into aquatic ecosystems. Studies indicate that properly engineered polymer coatings can significantly reduce leaching compared to uncoated catalysts, though long-term studies remain limited. Standardized leaching tests under various pH and temperature conditions are essential for comprehensive risk assessment.
Life cycle assessment (LCA) studies comparing polymer-coated nitrogen reduction catalysts with conventional nitrogen fixation methods demonstrate potential environmental benefits, particularly regarding energy efficiency and greenhouse gas emissions. However, these advantages depend heavily on the specific polymer composition, coating technique, and operational conditions. Preliminary data suggests that catalysts utilizing bio-based polymers and green chemistry principles for coating application offer the most favorable environmental profile.
Regulatory frameworks governing these materials vary significantly across regions, creating challenges for global implementation. Harmonized standards for environmental impact assessment would facilitate more consistent evaluation and responsible development of these promising catalyst technologies.
Scalability and Industrial Implementation Strategies
The scalability of polymer-coated nitrogen reduction catalyst materials represents a critical challenge for transitioning from laboratory success to industrial implementation. Current production methods typically involve batch processes that yield gram-scale quantities, which are insufficient for commercial applications requiring kilogram to ton-scale production. To address this gap, several promising scale-up strategies have emerged in recent years.
Continuous flow synthesis methods offer significant advantages over traditional batch processes, enabling consistent coating quality and thickness while reducing production time by up to 70%. These systems can be designed with in-line monitoring capabilities to ensure real-time quality control during the polymer coating process, maintaining catalyst performance even at larger scales.
Modular manufacturing approaches present another viable pathway, where production units can be replicated and operated in parallel to achieve desired output volumes. This strategy allows for incremental capacity expansion without requiring complete redesign of production facilities, reducing capital investment risks. Several pilot plants utilizing this approach have demonstrated successful scale-up factors of 100-500x from laboratory scale.
Cost considerations remain paramount for industrial implementation. Economic analyses indicate that polymer coating adds approximately 15-30% to catalyst production costs, necessitating optimization of coating materials and processes. Recent innovations in polymer precursor synthesis have reduced raw material costs by up to 40%, improving the economic viability of these catalysts for large-scale nitrogen reduction applications.
Regulatory and safety frameworks must also be established for industrial implementation. Polymer-coated catalysts introduce unique considerations regarding handling, storage, and disposal. Industry-academic consortia have begun developing standardized protocols for safety assessment and quality control, which will facilitate regulatory approval and industrial adoption.
Integration with existing industrial infrastructure represents another key implementation strategy. Retrofit solutions that incorporate polymer-coated catalysts into conventional nitrogen processing facilities can significantly reduce implementation barriers. Engineering studies suggest that many existing plants can be modified to accommodate these advanced catalysts with modifications to reactor design, temperature control systems, and separation processes.
Long-term stability under industrial conditions remains an ongoing research focus. Accelerated aging tests simulating industrial environments have identified polymer degradation mechanisms that must be addressed for commercial viability. Recent developments in cross-linking techniques and stabilizing additives have extended catalyst lifetimes by 300-400%, approaching the durability requirements for industrial implementation.
Continuous flow synthesis methods offer significant advantages over traditional batch processes, enabling consistent coating quality and thickness while reducing production time by up to 70%. These systems can be designed with in-line monitoring capabilities to ensure real-time quality control during the polymer coating process, maintaining catalyst performance even at larger scales.
Modular manufacturing approaches present another viable pathway, where production units can be replicated and operated in parallel to achieve desired output volumes. This strategy allows for incremental capacity expansion without requiring complete redesign of production facilities, reducing capital investment risks. Several pilot plants utilizing this approach have demonstrated successful scale-up factors of 100-500x from laboratory scale.
Cost considerations remain paramount for industrial implementation. Economic analyses indicate that polymer coating adds approximately 15-30% to catalyst production costs, necessitating optimization of coating materials and processes. Recent innovations in polymer precursor synthesis have reduced raw material costs by up to 40%, improving the economic viability of these catalysts for large-scale nitrogen reduction applications.
Regulatory and safety frameworks must also be established for industrial implementation. Polymer-coated catalysts introduce unique considerations regarding handling, storage, and disposal. Industry-academic consortia have begun developing standardized protocols for safety assessment and quality control, which will facilitate regulatory approval and industrial adoption.
Integration with existing industrial infrastructure represents another key implementation strategy. Retrofit solutions that incorporate polymer-coated catalysts into conventional nitrogen processing facilities can significantly reduce implementation barriers. Engineering studies suggest that many existing plants can be modified to accommodate these advanced catalysts with modifications to reactor design, temperature control systems, and separation processes.
Long-term stability under industrial conditions remains an ongoing research focus. Accelerated aging tests simulating industrial environments have identified polymer degradation mechanisms that must be addressed for commercial viability. Recent developments in cross-linking techniques and stabilizing additives have extended catalyst lifetimes by 300-400%, approaching the durability requirements for industrial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!