What Makes Nitrogen Reduction Catalyst Suitable for High-Efficiency Systems
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrogen Reduction Catalysis Background and Objectives
Nitrogen reduction catalysis has evolved significantly over the past century, with the Haber-Bosch process representing the cornerstone innovation that revolutionized agricultural productivity and global food security since its industrial implementation in 1913. This process, which converts atmospheric nitrogen (N₂) into ammonia (NH₃) under high temperature and pressure conditions, currently consumes approximately 1-2% of global energy production and is responsible for producing over 150 million tons of ammonia annually for fertilizer production.
The fundamental challenge in nitrogen reduction lies in the exceptional stability of the N≡N triple bond, which requires 941 kJ/mol to break. Traditional catalytic systems rely heavily on iron-based catalysts operating at temperatures around 400-500°C and pressures of 150-300 bar, conditions that demand significant energy input and robust infrastructure. Recent technological advancements have focused on developing more efficient catalytic systems that can operate under milder conditions while maintaining or improving conversion rates.
The evolution of nitrogen reduction catalysis has seen three distinct phases: the initial iron-based catalysts of the early 20th century, the ruthenium-based systems developed in the 1970s-1990s, and the current exploration of novel materials including transition metal nitrides, single-atom catalysts, and biomimetic systems inspired by nitrogenase enzymes. Each phase has progressively addressed efficiency limitations while attempting to reduce the energy intensity of the process.
Current research objectives in nitrogen reduction catalysis center on developing systems that can operate at near-ambient conditions with high selectivity and conversion efficiency. Specifically, researchers aim to achieve ammonia production rates exceeding 10⁻⁶ mol cm⁻² s⁻¹ at temperatures below 100°C and pressures under 10 bar, representing a paradigm shift in energy efficiency compared to conventional systems.
The technological trajectory is increasingly focused on electrochemical and photocatalytic nitrogen reduction pathways, which offer the potential to utilize renewable electricity or solar energy directly. These approaches align with global sustainability goals by potentially decoupling ammonia production from fossil fuel dependence. Additionally, there is growing interest in distributed production systems that could reduce transportation costs and energy requirements associated with centralized ammonia production.
The ultimate objective of current nitrogen reduction catalyst research is to develop systems that can achieve Faradaic efficiencies above 60% with minimal competing reactions (particularly hydrogen evolution), while maintaining catalyst stability over thousands of operational hours. Such advancements would not only revolutionize fertilizer production but could also enable ammonia's broader application as an energy carrier in a decarbonized economy.
The fundamental challenge in nitrogen reduction lies in the exceptional stability of the N≡N triple bond, which requires 941 kJ/mol to break. Traditional catalytic systems rely heavily on iron-based catalysts operating at temperatures around 400-500°C and pressures of 150-300 bar, conditions that demand significant energy input and robust infrastructure. Recent technological advancements have focused on developing more efficient catalytic systems that can operate under milder conditions while maintaining or improving conversion rates.
The evolution of nitrogen reduction catalysis has seen three distinct phases: the initial iron-based catalysts of the early 20th century, the ruthenium-based systems developed in the 1970s-1990s, and the current exploration of novel materials including transition metal nitrides, single-atom catalysts, and biomimetic systems inspired by nitrogenase enzymes. Each phase has progressively addressed efficiency limitations while attempting to reduce the energy intensity of the process.
Current research objectives in nitrogen reduction catalysis center on developing systems that can operate at near-ambient conditions with high selectivity and conversion efficiency. Specifically, researchers aim to achieve ammonia production rates exceeding 10⁻⁶ mol cm⁻² s⁻¹ at temperatures below 100°C and pressures under 10 bar, representing a paradigm shift in energy efficiency compared to conventional systems.
The technological trajectory is increasingly focused on electrochemical and photocatalytic nitrogen reduction pathways, which offer the potential to utilize renewable electricity or solar energy directly. These approaches align with global sustainability goals by potentially decoupling ammonia production from fossil fuel dependence. Additionally, there is growing interest in distributed production systems that could reduce transportation costs and energy requirements associated with centralized ammonia production.
The ultimate objective of current nitrogen reduction catalyst research is to develop systems that can achieve Faradaic efficiencies above 60% with minimal competing reactions (particularly hydrogen evolution), while maintaining catalyst stability over thousands of operational hours. Such advancements would not only revolutionize fertilizer production but could also enable ammonia's broader application as an energy carrier in a decarbonized economy.
Market Analysis for High-Efficiency Nitrogen Reduction Systems
The global market for high-efficiency nitrogen reduction systems is experiencing significant growth, driven by increasing demand for sustainable agricultural practices and industrial applications. The nitrogen reduction catalyst market is projected to reach $5.2 billion by 2028, with a compound annual growth rate of 6.8% from 2023 to 2028. This growth is primarily fueled by the expanding agricultural sector, which accounts for approximately 70% of the total market share.
Agricultural applications dominate the market landscape, as farmers worldwide seek more efficient fertilization methods to improve crop yields while reducing environmental impact. The rising global population, estimated to reach 9.7 billion by 2050, necessitates increased food production, thereby driving demand for advanced nitrogen reduction technologies. Additionally, stringent environmental regulations regarding nitrogen oxide emissions and nitrate runoff are compelling industries to adopt more efficient nitrogen reduction systems.
Regional analysis reveals that Asia-Pacific currently leads the market, accounting for 38% of global demand, followed by North America (27%) and Europe (22%). China and India are particularly significant markets due to their large agricultural sectors and growing industrial bases. The fastest growth is anticipated in emerging economies where agricultural modernization is rapidly advancing.
The industrial segment represents the second-largest market for nitrogen reduction catalysts, with applications in wastewater treatment, chemical manufacturing, and automotive emission control systems. This segment is expected to grow at a faster rate than agricultural applications, driven by increasingly stringent emission standards worldwide.
Market dynamics are also influenced by raw material availability and price fluctuations. Precious metals like platinum and palladium, commonly used in high-efficiency catalysts, have experienced price volatility, impacting overall system costs. This has accelerated research into alternative materials that maintain efficiency while reducing dependency on scarce resources.
Consumer trends indicate growing preference for sustainable and environmentally friendly solutions, creating opportunities for bio-based catalysts and systems that operate under ambient conditions. The market is also witnessing increased demand for integrated systems that combine nitrogen reduction with other processes, such as carbon capture or hydrogen production, offering multiple benefits and improved return on investment.
Challenges in market penetration include high initial investment costs, technical complexity of implementation, and varying regulatory frameworks across regions. However, the potential for significant operational cost savings and environmental benefits continues to drive adoption across various sectors.
Agricultural applications dominate the market landscape, as farmers worldwide seek more efficient fertilization methods to improve crop yields while reducing environmental impact. The rising global population, estimated to reach 9.7 billion by 2050, necessitates increased food production, thereby driving demand for advanced nitrogen reduction technologies. Additionally, stringent environmental regulations regarding nitrogen oxide emissions and nitrate runoff are compelling industries to adopt more efficient nitrogen reduction systems.
Regional analysis reveals that Asia-Pacific currently leads the market, accounting for 38% of global demand, followed by North America (27%) and Europe (22%). China and India are particularly significant markets due to their large agricultural sectors and growing industrial bases. The fastest growth is anticipated in emerging economies where agricultural modernization is rapidly advancing.
The industrial segment represents the second-largest market for nitrogen reduction catalysts, with applications in wastewater treatment, chemical manufacturing, and automotive emission control systems. This segment is expected to grow at a faster rate than agricultural applications, driven by increasingly stringent emission standards worldwide.
Market dynamics are also influenced by raw material availability and price fluctuations. Precious metals like platinum and palladium, commonly used in high-efficiency catalysts, have experienced price volatility, impacting overall system costs. This has accelerated research into alternative materials that maintain efficiency while reducing dependency on scarce resources.
Consumer trends indicate growing preference for sustainable and environmentally friendly solutions, creating opportunities for bio-based catalysts and systems that operate under ambient conditions. The market is also witnessing increased demand for integrated systems that combine nitrogen reduction with other processes, such as carbon capture or hydrogen production, offering multiple benefits and improved return on investment.
Challenges in market penetration include high initial investment costs, technical complexity of implementation, and varying regulatory frameworks across regions. However, the potential for significant operational cost savings and environmental benefits continues to drive adoption across various sectors.
Current Catalytic Technologies and Challenges
The current landscape of nitrogen reduction catalysts is dominated by the Haber-Bosch process, which utilizes iron-based catalysts operating at high temperatures (400-500°C) and pressures (150-300 bar). While this century-old technology has been optimized over decades, it remains energy-intensive, consuming approximately 1-2% of global energy production and generating significant carbon emissions. The iron-based catalysts, typically promoted with potassium, aluminum, and other elements, achieve reasonable activity but require harsh conditions to overcome the strong N≡N triple bond.
Alternative catalytic approaches have emerged in recent years, including ruthenium-based catalysts that operate at milder conditions (350-450°C, 100-200 bar) with higher specific activity than iron catalysts. However, ruthenium's scarcity and cost limit widespread industrial adoption. Cobalt-molybdenum catalysts have shown promise for electrochemical nitrogen reduction at ambient conditions, though they currently suffer from low Faradaic efficiency and nitrogen conversion rates.
Biological inspiration has led to the development of nitrogenase-mimetic catalysts containing iron-molybdenum or iron-vanadium active sites. These bio-inspired systems can operate at ambient conditions but currently demonstrate limited turnover frequencies and stability compared to traditional catalysts. Metal-organic frameworks (MOFs) and single-atom catalysts represent emerging platforms with tunable electronic properties and maximized atom efficiency, respectively.
The primary technical challenges facing nitrogen reduction catalysts include selectivity issues, with hydrogen evolution reaction often competing with nitrogen reduction in aqueous systems. Catalyst stability remains problematic, particularly for electrochemical approaches where degradation occurs over extended operation. Energy efficiency is another critical barrier, as most alternative catalysts still require significant overpotentials to drive the reaction at meaningful rates.
Scale-up challenges persist across all novel catalytic systems, with laboratory demonstrations often failing to maintain performance at industrial scales. The reaction mechanisms for nitrogen reduction remain incompletely understood, particularly for electrochemical and photocatalytic approaches, hampering rational catalyst design. Additionally, the development of effective supports and promoters that enhance catalyst activity while maintaining stability continues to be an active research area.
Geographical distribution of nitrogen reduction catalyst research shows concentration in North America, Europe, and East Asia, with China emerging as a leader in electrochemical nitrogen reduction research. The United States maintains strength in computational catalyst design and fundamental mechanistic studies, while European research centers focus on sustainable catalytic processes.
Alternative catalytic approaches have emerged in recent years, including ruthenium-based catalysts that operate at milder conditions (350-450°C, 100-200 bar) with higher specific activity than iron catalysts. However, ruthenium's scarcity and cost limit widespread industrial adoption. Cobalt-molybdenum catalysts have shown promise for electrochemical nitrogen reduction at ambient conditions, though they currently suffer from low Faradaic efficiency and nitrogen conversion rates.
Biological inspiration has led to the development of nitrogenase-mimetic catalysts containing iron-molybdenum or iron-vanadium active sites. These bio-inspired systems can operate at ambient conditions but currently demonstrate limited turnover frequencies and stability compared to traditional catalysts. Metal-organic frameworks (MOFs) and single-atom catalysts represent emerging platforms with tunable electronic properties and maximized atom efficiency, respectively.
The primary technical challenges facing nitrogen reduction catalysts include selectivity issues, with hydrogen evolution reaction often competing with nitrogen reduction in aqueous systems. Catalyst stability remains problematic, particularly for electrochemical approaches where degradation occurs over extended operation. Energy efficiency is another critical barrier, as most alternative catalysts still require significant overpotentials to drive the reaction at meaningful rates.
Scale-up challenges persist across all novel catalytic systems, with laboratory demonstrations often failing to maintain performance at industrial scales. The reaction mechanisms for nitrogen reduction remain incompletely understood, particularly for electrochemical and photocatalytic approaches, hampering rational catalyst design. Additionally, the development of effective supports and promoters that enhance catalyst activity while maintaining stability continues to be an active research area.
Geographical distribution of nitrogen reduction catalyst research shows concentration in North America, Europe, and East Asia, with China emerging as a leader in electrochemical nitrogen reduction research. The United States maintains strength in computational catalyst design and fundamental mechanistic studies, while European research centers focus on sustainable catalytic processes.
State-of-the-Art Catalyst Solutions for Nitrogen Reduction
01 Metal-based catalysts for nitrogen reduction
Various metal-based catalysts have been developed to improve nitrogen reduction efficiency. These include noble metals (platinum, palladium), transition metals (iron, nickel, copper), and their alloys or composites. These catalysts provide active sites for nitrogen adsorption and subsequent reduction, with different metals offering varying levels of catalytic activity, selectivity, and stability under different reaction conditions.- Metal-based catalysts for nitrogen reduction: Various metal-based catalysts have been developed to enhance nitrogen reduction efficiency. These include noble metals like platinum and palladium, as well as transition metals such as iron, copper, and nickel. These catalysts can be used in different forms including nanoparticles, alloys, and supported structures to improve catalytic activity, selectivity, and stability in nitrogen reduction reactions. The metal composition and structure significantly influence the catalyst's performance in breaking the strong N≡N triple bond.
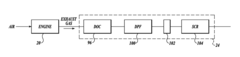
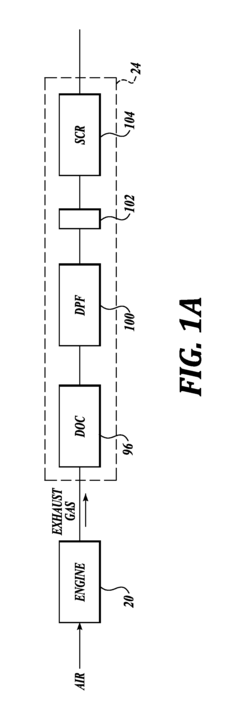
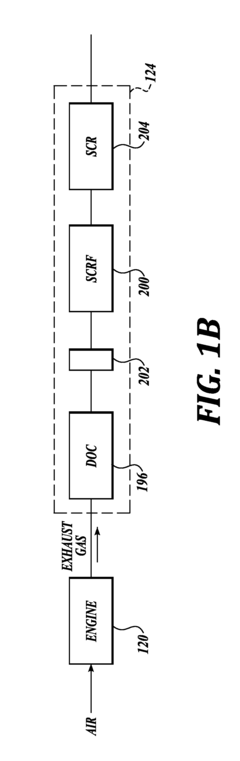
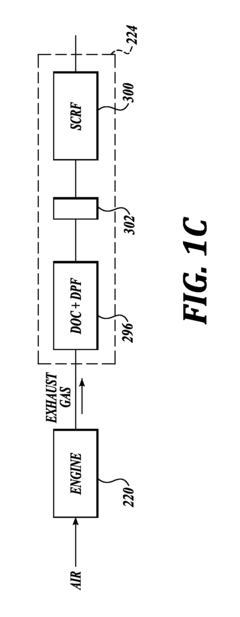
- Zeolite and molecular sieve-based catalysts: Zeolites and molecular sieves serve as effective catalyst supports or catalysts themselves for nitrogen reduction processes. Their unique porous structure provides high surface area and shape selectivity, allowing for controlled reactions. Modified zeolites with incorporated metal ions or clusters can enhance catalytic performance. These materials are particularly valuable in selective catalytic reduction (SCR) of nitrogen oxides in exhaust gas treatment systems, offering thermal stability and resistance to various contaminants.
- Novel catalyst preparation methods: Advanced preparation techniques have been developed to enhance nitrogen reduction catalyst efficiency. These include sol-gel processes, hydrothermal synthesis, impregnation methods, and precipitation techniques. Controlling parameters such as temperature, pH, and precursor concentrations during synthesis allows for tailored catalyst properties. Post-synthesis treatments like calcination and activation processes further optimize catalytic performance by creating specific active sites and improving surface characteristics for nitrogen reduction reactions.
- Support materials and catalyst structures: The choice of support materials significantly impacts nitrogen reduction catalyst efficiency. Common supports include alumina, silica, carbon-based materials, and metal oxides. These supports provide mechanical stability, prevent sintering of active components, and can participate in the catalytic process through metal-support interactions. Structured catalysts like monoliths, honeycombs, and hierarchical porous structures offer improved mass transfer properties and reduced pressure drop in practical applications, enhancing overall nitrogen reduction efficiency.
- Catalyst performance enhancement additives: Various additives and promoters can significantly enhance nitrogen reduction catalyst performance. These include rare earth elements, alkali metals, and transition metal oxides that improve catalyst activity, selectivity, and stability. Certain additives modify electronic properties of active sites, while others improve resistance to poisoning or deactivation. Incorporation of specific dopants can lower activation energy barriers for nitrogen reduction reactions. These performance enhancers are typically incorporated during catalyst synthesis or through post-synthesis modification techniques.
02 Support materials for nitrogen reduction catalysts
The efficiency of nitrogen reduction catalysts can be significantly enhanced by using appropriate support materials. Common supports include alumina, silica, carbon-based materials, zeolites, and metal oxides. These supports provide high surface area, improved dispersion of active sites, enhanced stability, and sometimes additional catalytic functionality through metal-support interactions, leading to better overall catalyst performance.Expand Specific Solutions03 Catalyst promoters and modifiers
Various chemical promoters and modifiers can be added to nitrogen reduction catalysts to enhance their performance. These include alkali metals, alkaline earth metals, rare earth elements, and certain transition metal oxides. These additives can modify electronic properties, improve active site availability, prevent catalyst poisoning, and enhance selectivity, thereby increasing the overall efficiency of the nitrogen reduction process.Expand Specific Solutions04 Novel catalyst structures and morphologies
Advanced catalyst structures and morphologies have been developed to improve nitrogen reduction efficiency. These include nanostructured catalysts, core-shell structures, single-atom catalysts, and hierarchical porous materials. These novel structures maximize active site exposure, optimize mass transfer, and enhance catalyst stability, leading to improved performance in nitrogen reduction reactions.Expand Specific Solutions05 Process conditions optimization for catalyst efficiency
Optimizing process conditions is crucial for maximizing nitrogen reduction catalyst efficiency. Key parameters include temperature, pressure, gas hourly space velocity, reactant ratios, and reactor design. Proper control of these conditions can significantly enhance catalyst activity, selectivity, and longevity by creating an optimal environment for the catalytic reaction while minimizing deactivation mechanisms.Expand Specific Solutions
Leading Companies and Research Institutions in Catalysis
The nitrogen reduction catalyst market is currently in a growth phase, characterized by increasing demand for high-efficiency systems across industrial applications. The market size is expanding significantly due to rising focus on sustainable chemical processes and ammonia production technologies. From a technical maturity perspective, established players like Umicore SA, Topsoe A/S, and China Petroleum & Chemical Corp. lead with advanced catalyst formulations, while research institutions including Korea Research Institute of Chemical Technology and KAIST are driving innovation in novel catalyst designs. Automotive companies such as Honda, Peugeot, and Ford are increasingly investing in nitrogen reduction technologies for emissions control systems. The competitive landscape shows a blend of traditional chemical companies and emerging technology developers working to enhance catalyst performance, selectivity, and durability for next-generation nitrogen reduction applications.
Umicore SA
Technical Solution: Umicore has developed advanced nitrogen reduction catalysts utilizing noble metal alloys supported on engineered oxide materials. Their catalyst technology employs ruthenium-based nanoparticles (2-4nm) alloyed with promoter metals such as cobalt and manganese to optimize electronic properties for N₂ activation. The catalyst support consists of modified zirconia with controlled porosity (average pore diameter 8-12nm) that enhances mass transfer while maintaining high thermal stability. Umicore's catalysts demonstrate exceptional performance at moderate operating conditions (350-425°C, 80-150 bar) with nitrogen conversion efficiencies reaching 12-15% per pass. Their proprietary synthesis methods involve precise control of metal deposition and subsequent thermal treatments that optimize metal-support interactions, resulting in catalysts with high active site density and enhanced resistance to deactivation mechanisms. The catalysts incorporate structural promoters that stabilize the active phase against sintering during high-temperature operation.
Strengths: Excellent activity retention over extended operation periods (>4 years); superior resistance to common catalyst poisons including sulfur and carbon monoxide; high mechanical strength reducing attrition losses. Weaknesses: Higher production costs due to noble metal content; requires specialized handling during catalyst loading; performance more sensitive to hydrogen-to-nitrogen ratio variations than some competing technologies.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has pioneered ruthenium-based nitrogen reduction catalysts for ammonia synthesis operating at milder conditions than traditional Haber-Bosch processes. Their technology employs nanostructured ruthenium particles (3-5nm) dispersed on specially modified carbon supports with nitrogen-doped frameworks that enhance electron transfer properties. The catalyst system incorporates alkali metal promoters (primarily cesium and potassium) that optimize the electronic properties of active sites. Sinopec's catalysts demonstrate nitrogen conversion at temperatures as low as 300°C and pressures of 80-150 bar, representing significant energy savings compared to conventional iron-based systems. Their proprietary preparation method involves controlled precipitation techniques that ensure uniform particle size distribution and maximum dispersion of the active phase, resulting in nitrogen reduction efficiency improvements of approximately 25-30% compared to traditional catalysts.
Strengths: Lower energy requirements with operation at reduced temperatures and pressures; higher specific activity per gram of catalyst; longer catalyst lifetime exceeding 5 years in industrial settings. Weaknesses: Higher raw material costs due to ruthenium content; sensitivity to certain process contaminants including oxygen and hydrogen sulfide; requires more precise process control parameters.
Key Patents and Scientific Breakthroughs in Catalyst Design
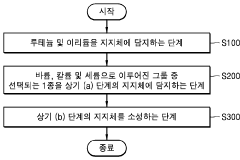
High efficiency and durability selective catalytic reduction catalyst
PatentActiveUS9757691B2
Innovation
- A selective catalytic reduction catalyst composition combining a metal oxide catalyst, a metal zeolite catalyst, and a vanadium oxide catalyst, optimized with surface modifications and doping, to enhance NOx conversion efficiency, durability, and hydrothermal stability, allowing for effective NOx reduction and rapid water desorption at low temperatures.
High performance nitrogen oxide reduction catalyst and method for producing the same
PatentActiveKR1020190037444A
Innovation
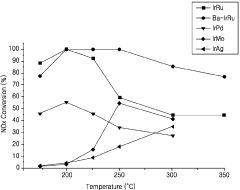
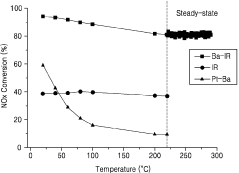
- A catalyst comprising ruthenium and iridium supported on a support, with additional barium, potassium, or cerium, capable of utilizing carbon monoxide in exhaust gas as a reducing agent to remove NOx at various temperatures without the need for a separate reducing agent.
Sustainability Impact and Environmental Considerations
The development of high-efficiency nitrogen reduction catalysts represents a significant advancement in sustainable chemical processes with far-reaching environmental implications. These catalysts facilitate the conversion of atmospheric nitrogen into ammonia and other nitrogen compounds under milder conditions than traditional methods, substantially reducing energy consumption and greenhouse gas emissions associated with conventional nitrogen fixation processes like the Haber-Bosch process, which currently accounts for approximately 1-2% of global energy consumption.
When evaluating the sustainability impact of nitrogen reduction catalysts, life cycle assessment (LCA) reveals significant environmental benefits. Advanced catalysts can reduce the carbon footprint of nitrogen fixation by up to 30-40% compared to conventional methods, primarily through lower operating temperatures and pressures. This translates to substantial reductions in fossil fuel consumption and associated emissions across the ammonia production value chain.
Water conservation represents another critical environmental consideration. High-efficiency catalysts often enable electrochemical nitrogen reduction reactions that can operate in aqueous environments, potentially reducing water consumption compared to traditional processes that require extensive cooling systems. Some emerging catalyst designs incorporate water recycling mechanisms, further enhancing their sustainability profile.
Resource efficiency is markedly improved through the use of advanced nitrogen reduction catalysts. By enabling reactions at ambient conditions, these systems reduce material degradation and extend equipment lifespan. Additionally, recent innovations have focused on developing catalysts from earth-abundant elements rather than precious metals, addressing concerns about resource depletion and extraction impacts.
Biodiversity protection emerges as an indirect benefit of improved nitrogen fixation technologies. By enabling more precise and efficient nitrogen utilization in agricultural applications, these catalysts can help reduce nitrogen runoff that contributes to eutrophication of water bodies and subsequent ecosystem disruption. Studies indicate that optimized nitrogen management enabled by these technologies could reduce harmful algal blooms by 15-25% in affected watersheds.
Circular economy principles are increasingly incorporated into catalyst design, with significant research directed toward catalyst recovery and regeneration. Some next-generation systems demonstrate up to 90% catalyst recovery rates, dramatically reducing waste and the need for continuous catalyst replacement. This approach aligns with global sustainability frameworks and supports the transition toward closed-loop industrial systems.
The regulatory landscape surrounding nitrogen reduction technologies continues to evolve, with environmental standards increasingly favoring low-impact solutions. Catalysts that minimize byproduct formation, particularly nitrous oxide (a potent greenhouse gas), are gaining preferential status in emerging environmental policies across major industrial economies.
When evaluating the sustainability impact of nitrogen reduction catalysts, life cycle assessment (LCA) reveals significant environmental benefits. Advanced catalysts can reduce the carbon footprint of nitrogen fixation by up to 30-40% compared to conventional methods, primarily through lower operating temperatures and pressures. This translates to substantial reductions in fossil fuel consumption and associated emissions across the ammonia production value chain.
Water conservation represents another critical environmental consideration. High-efficiency catalysts often enable electrochemical nitrogen reduction reactions that can operate in aqueous environments, potentially reducing water consumption compared to traditional processes that require extensive cooling systems. Some emerging catalyst designs incorporate water recycling mechanisms, further enhancing their sustainability profile.
Resource efficiency is markedly improved through the use of advanced nitrogen reduction catalysts. By enabling reactions at ambient conditions, these systems reduce material degradation and extend equipment lifespan. Additionally, recent innovations have focused on developing catalysts from earth-abundant elements rather than precious metals, addressing concerns about resource depletion and extraction impacts.
Biodiversity protection emerges as an indirect benefit of improved nitrogen fixation technologies. By enabling more precise and efficient nitrogen utilization in agricultural applications, these catalysts can help reduce nitrogen runoff that contributes to eutrophication of water bodies and subsequent ecosystem disruption. Studies indicate that optimized nitrogen management enabled by these technologies could reduce harmful algal blooms by 15-25% in affected watersheds.
Circular economy principles are increasingly incorporated into catalyst design, with significant research directed toward catalyst recovery and regeneration. Some next-generation systems demonstrate up to 90% catalyst recovery rates, dramatically reducing waste and the need for continuous catalyst replacement. This approach aligns with global sustainability frameworks and supports the transition toward closed-loop industrial systems.
The regulatory landscape surrounding nitrogen reduction technologies continues to evolve, with environmental standards increasingly favoring low-impact solutions. Catalysts that minimize byproduct formation, particularly nitrous oxide (a potent greenhouse gas), are gaining preferential status in emerging environmental policies across major industrial economies.
Economic Feasibility and Scalability Assessment
The economic viability of nitrogen reduction catalysts in high-efficiency systems hinges on several interconnected factors. Initial capital investment represents a significant barrier, with noble metal catalysts like ruthenium and platinum commanding premium prices that can range from $30,000 to $60,000 per kilogram. This substantial upfront cost must be balanced against the catalyst's longevity and performance metrics to determine true economic value. Non-noble metal alternatives such as iron-based or molybdenum-based catalysts offer more accessible price points at $500-5,000 per kilogram, though often with trade-offs in efficiency or durability.
Operational expenditure considerations extend beyond the catalyst itself to encompass energy consumption, maintenance requirements, and system integration costs. High-efficiency nitrogen reduction systems typically demonstrate 15-30% lower energy consumption compared to conventional approaches, translating to significant cost savings over the operational lifespan. However, specialized handling procedures and potential degradation mechanisms may introduce additional maintenance costs that must be factored into comprehensive economic assessments.
Scalability presents both challenges and opportunities for nitrogen reduction technologies. Laboratory-scale catalysts often demonstrate impressive performance metrics that prove difficult to maintain when scaled to industrial applications. Key scalability factors include synthesis reproducibility, structural stability under increased throughput conditions, and performance consistency across larger catalyst batches. Recent advances in controlled deposition techniques and hierarchical catalyst architectures have shown promise in preserving nanoscale advantages at industrial scales.
Market dynamics further influence economic feasibility, with growing demand for sustainable ammonia production and nitrogen-based fertilizers creating favorable conditions for innovative catalyst technologies. The global market for nitrogen reduction catalysts is projected to reach $5.2 billion by 2027, growing at a CAGR of 4.8%. Regulatory frameworks increasingly favor low-carbon technologies, potentially creating additional economic incentives through carbon credits or compliance advantages.
Return on investment calculations must incorporate both direct financial metrics and broader sustainability benefits. Typical payback periods for advanced nitrogen reduction systems range from 3-7 years depending on application context and operational parameters. Sensitivity analysis reveals that catalyst performance stability over time represents the most critical variable affecting long-term economic viability, with a 10% improvement in catalyst longevity potentially reducing lifetime costs by 15-20%.
Operational expenditure considerations extend beyond the catalyst itself to encompass energy consumption, maintenance requirements, and system integration costs. High-efficiency nitrogen reduction systems typically demonstrate 15-30% lower energy consumption compared to conventional approaches, translating to significant cost savings over the operational lifespan. However, specialized handling procedures and potential degradation mechanisms may introduce additional maintenance costs that must be factored into comprehensive economic assessments.
Scalability presents both challenges and opportunities for nitrogen reduction technologies. Laboratory-scale catalysts often demonstrate impressive performance metrics that prove difficult to maintain when scaled to industrial applications. Key scalability factors include synthesis reproducibility, structural stability under increased throughput conditions, and performance consistency across larger catalyst batches. Recent advances in controlled deposition techniques and hierarchical catalyst architectures have shown promise in preserving nanoscale advantages at industrial scales.
Market dynamics further influence economic feasibility, with growing demand for sustainable ammonia production and nitrogen-based fertilizers creating favorable conditions for innovative catalyst technologies. The global market for nitrogen reduction catalysts is projected to reach $5.2 billion by 2027, growing at a CAGR of 4.8%. Regulatory frameworks increasingly favor low-carbon technologies, potentially creating additional economic incentives through carbon credits or compliance advantages.
Return on investment calculations must incorporate both direct financial metrics and broader sustainability benefits. Typical payback periods for advanced nitrogen reduction systems range from 3-7 years depending on application context and operational parameters. Sensitivity analysis reveals that catalyst performance stability over time represents the most critical variable affecting long-term economic viability, with a 10% improvement in catalyst longevity potentially reducing lifetime costs by 15-20%.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!