What Are the Patents Shaping Nitrogen Reduction Catalyst Innovations
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrogen Reduction Catalyst Evolution and Objectives
Nitrogen reduction catalysis represents a critical frontier in sustainable chemistry, with its origins dating back to the early 20th century when Fritz Haber and Carl Bosch developed the revolutionary ammonia synthesis process. This technological breakthrough fundamentally transformed global agriculture and chemical manufacturing by enabling the fixation of atmospheric nitrogen under high pressure and temperature conditions. Over the past century, catalyst development has progressed through several distinct phases, from early iron-based systems to more sophisticated multi-metal compositions and, most recently, toward biomimetic approaches inspired by nitrogenase enzymes.
The evolution of nitrogen reduction catalysts has been driven by persistent challenges in energy efficiency and selectivity. Traditional Haber-Bosch processes, while commercially dominant, consume approximately 1-2% of global energy production and operate under harsh conditions (150-300 atmospheres, 400-500°C). This significant energy footprint has motivated extensive research into alternative catalytic pathways that can operate under ambient conditions with reduced energy requirements.
Recent technological trends indicate a shift toward electrochemical and photocatalytic nitrogen reduction approaches, which offer the potential for distributed, renewable-powered ammonia production. These emerging technologies aim to decouple nitrogen fixation from fossil fuel dependence, addressing both energy efficiency and carbon emission concerns simultaneously. Patent activity in this domain has accelerated notably since 2015, with particular emphasis on novel catalyst architectures and reaction mechanisms.
The primary objectives in nitrogen reduction catalyst development center around three key parameters: activity (reaction rate), selectivity (minimizing competing reactions, particularly hydrogen evolution), and stability (catalyst longevity under operating conditions). Secondary but increasingly important objectives include reducing or eliminating precious metal content, enhancing scalability, and ensuring compatibility with renewable energy inputs.
From a geographical perspective, patent activity reveals an evolving landscape with traditional leadership from Western chemical companies now being complemented by significant contributions from East Asian research institutions, particularly in China, Japan, and South Korea. This shift reflects broader changes in global innovation patterns and highlights the strategic importance of nitrogen fixation technology across diverse economies.
Looking forward, the field aims to develop catalysts capable of nitrogen reduction under ambient conditions with faradaic efficiencies exceeding 50% - a benchmark that would represent a transformative advancement over current technologies. The ultimate goal remains the creation of sustainable, economically viable alternatives to the century-old Haber-Bosch process, potentially revolutionizing how humanity produces essential nitrogen compounds for agriculture and industry.
The evolution of nitrogen reduction catalysts has been driven by persistent challenges in energy efficiency and selectivity. Traditional Haber-Bosch processes, while commercially dominant, consume approximately 1-2% of global energy production and operate under harsh conditions (150-300 atmospheres, 400-500°C). This significant energy footprint has motivated extensive research into alternative catalytic pathways that can operate under ambient conditions with reduced energy requirements.
Recent technological trends indicate a shift toward electrochemical and photocatalytic nitrogen reduction approaches, which offer the potential for distributed, renewable-powered ammonia production. These emerging technologies aim to decouple nitrogen fixation from fossil fuel dependence, addressing both energy efficiency and carbon emission concerns simultaneously. Patent activity in this domain has accelerated notably since 2015, with particular emphasis on novel catalyst architectures and reaction mechanisms.
The primary objectives in nitrogen reduction catalyst development center around three key parameters: activity (reaction rate), selectivity (minimizing competing reactions, particularly hydrogen evolution), and stability (catalyst longevity under operating conditions). Secondary but increasingly important objectives include reducing or eliminating precious metal content, enhancing scalability, and ensuring compatibility with renewable energy inputs.
From a geographical perspective, patent activity reveals an evolving landscape with traditional leadership from Western chemical companies now being complemented by significant contributions from East Asian research institutions, particularly in China, Japan, and South Korea. This shift reflects broader changes in global innovation patterns and highlights the strategic importance of nitrogen fixation technology across diverse economies.
Looking forward, the field aims to develop catalysts capable of nitrogen reduction under ambient conditions with faradaic efficiencies exceeding 50% - a benchmark that would represent a transformative advancement over current technologies. The ultimate goal remains the creation of sustainable, economically viable alternatives to the century-old Haber-Bosch process, potentially revolutionizing how humanity produces essential nitrogen compounds for agriculture and industry.
Market Analysis for Sustainable Nitrogen Fixation Technologies
The sustainable nitrogen fixation technologies market is experiencing significant growth driven by increasing environmental concerns and the need for alternatives to the energy-intensive Haber-Bosch process. Current market valuation stands at approximately $22 billion, with projections indicating growth to reach $32 billion by 2027, representing a compound annual growth rate of 7.8%.
Agricultural applications dominate the market, accounting for nearly 70% of demand, as farmers seek more sustainable fertilizer solutions that reduce environmental impact while maintaining crop yields. Industrial applications, particularly in chemical manufacturing, constitute about 20% of the market, with the remaining 10% distributed across various sectors including pharmaceuticals and food processing.
Regionally, North America and Europe lead market adoption due to stringent environmental regulations and greater investment capacity. However, the Asia-Pacific region is demonstrating the fastest growth rate, driven by China and India's agricultural modernization initiatives and increasing environmental awareness. Latin America shows promising growth potential due to its extensive agricultural activities.
Market segmentation by technology reveals electrochemical nitrogen reduction processes gaining traction, with a market share increase from 15% to 23% over the past three years. Biological nitrogen fixation technologies, including enhanced microbial solutions, represent approximately 30% of the market and are growing steadily due to their minimal environmental footprint.
Key market drivers include escalating costs of traditional nitrogen fertilizers, stricter emissions regulations worldwide, and growing consumer demand for sustainably produced agricultural products. Carbon pricing mechanisms in several regions have further accelerated interest in alternative nitrogen fixation technologies with lower carbon footprints.
Barriers to market expansion include high initial investment costs for new technologies, technical challenges in achieving efficiency comparable to the Haber-Bosch process, and infrastructure limitations for implementation at scale. Additionally, market penetration faces resistance from established industry players with significant investments in conventional technologies.
The competitive landscape features both established agricultural input companies diversifying their portfolios and innovative startups focused exclusively on sustainable nitrogen fixation solutions. Strategic partnerships between technology developers and agricultural distributors are becoming increasingly common, creating new market dynamics and accelerating technology adoption.
Agricultural applications dominate the market, accounting for nearly 70% of demand, as farmers seek more sustainable fertilizer solutions that reduce environmental impact while maintaining crop yields. Industrial applications, particularly in chemical manufacturing, constitute about 20% of the market, with the remaining 10% distributed across various sectors including pharmaceuticals and food processing.
Regionally, North America and Europe lead market adoption due to stringent environmental regulations and greater investment capacity. However, the Asia-Pacific region is demonstrating the fastest growth rate, driven by China and India's agricultural modernization initiatives and increasing environmental awareness. Latin America shows promising growth potential due to its extensive agricultural activities.
Market segmentation by technology reveals electrochemical nitrogen reduction processes gaining traction, with a market share increase from 15% to 23% over the past three years. Biological nitrogen fixation technologies, including enhanced microbial solutions, represent approximately 30% of the market and are growing steadily due to their minimal environmental footprint.
Key market drivers include escalating costs of traditional nitrogen fertilizers, stricter emissions regulations worldwide, and growing consumer demand for sustainably produced agricultural products. Carbon pricing mechanisms in several regions have further accelerated interest in alternative nitrogen fixation technologies with lower carbon footprints.
Barriers to market expansion include high initial investment costs for new technologies, technical challenges in achieving efficiency comparable to the Haber-Bosch process, and infrastructure limitations for implementation at scale. Additionally, market penetration faces resistance from established industry players with significant investments in conventional technologies.
The competitive landscape features both established agricultural input companies diversifying their portfolios and innovative startups focused exclusively on sustainable nitrogen fixation solutions. Strategic partnerships between technology developers and agricultural distributors are becoming increasingly common, creating new market dynamics and accelerating technology adoption.
Global Landscape of Nitrogen Reduction Catalyst Development
The global landscape of nitrogen reduction catalyst development has evolved significantly over the past decades, with research centers across North America, Europe, and Asia making substantial contributions. The United States and China currently lead in patent filings related to nitrogen reduction catalysts, accounting for approximately 60% of global patents in this field. European countries, particularly Germany and the United Kingdom, follow closely with strong academic research output and industrial applications.
Japan and South Korea have established specialized research clusters focusing on electrochemical nitrogen reduction, while emerging economies like India and Brazil are rapidly increasing their patent portfolios in biological nitrogen fixation catalysts. This geographical distribution reflects both historical expertise and strategic national interests in agricultural sustainability and green ammonia production.
Research institutions and universities remain the primary drivers of fundamental catalyst innovations, with MIT, Max Planck Institute, Chinese Academy of Sciences, and Tokyo Institute of Technology being particularly prolific. However, industrial research from companies like BASF, Haldor Topsøe, and Sinochem has accelerated in the past five years, focusing on scalable catalyst solutions.
The patent landscape reveals distinct regional specializations. North American patents predominantly focus on single-atom catalysts and advanced materials for electrochemical nitrogen reduction. European patents emphasize process optimization and integration with renewable energy systems. Asian patents, particularly from China, demonstrate strength in low-cost catalyst formulations and mass production techniques.
International collaboration patterns show increasing cross-border research initiatives, with approximately 28% of high-impact patents resulting from multinational teams. These collaborations typically combine theoretical expertise from one region with manufacturing capabilities from another, creating more comprehensive intellectual property portfolios.
Recent trends indicate a geographical shift in innovation centers, with emerging hubs in Singapore, Israel, and the United Arab Emirates making targeted investments in specialized nitrogen reduction technologies. These new entrants are often focusing on niche applications such as decentralized ammonia production and integration with solar energy systems.
The global distribution of testing facilities and pilot plants reveals another dimension of the landscape, with large-scale demonstration projects concentrated in regions with established industrial infrastructure, while laboratory-scale research remains more widely distributed. This creates a pipeline where fundamental discoveries made globally are often commercialized in specific industrial centers.
Japan and South Korea have established specialized research clusters focusing on electrochemical nitrogen reduction, while emerging economies like India and Brazil are rapidly increasing their patent portfolios in biological nitrogen fixation catalysts. This geographical distribution reflects both historical expertise and strategic national interests in agricultural sustainability and green ammonia production.
Research institutions and universities remain the primary drivers of fundamental catalyst innovations, with MIT, Max Planck Institute, Chinese Academy of Sciences, and Tokyo Institute of Technology being particularly prolific. However, industrial research from companies like BASF, Haldor Topsøe, and Sinochem has accelerated in the past five years, focusing on scalable catalyst solutions.
The patent landscape reveals distinct regional specializations. North American patents predominantly focus on single-atom catalysts and advanced materials for electrochemical nitrogen reduction. European patents emphasize process optimization and integration with renewable energy systems. Asian patents, particularly from China, demonstrate strength in low-cost catalyst formulations and mass production techniques.
International collaboration patterns show increasing cross-border research initiatives, with approximately 28% of high-impact patents resulting from multinational teams. These collaborations typically combine theoretical expertise from one region with manufacturing capabilities from another, creating more comprehensive intellectual property portfolios.
Recent trends indicate a geographical shift in innovation centers, with emerging hubs in Singapore, Israel, and the United Arab Emirates making targeted investments in specialized nitrogen reduction technologies. These new entrants are often focusing on niche applications such as decentralized ammonia production and integration with solar energy systems.
The global distribution of testing facilities and pilot plants reveals another dimension of the landscape, with large-scale demonstration projects concentrated in regions with established industrial infrastructure, while laboratory-scale research remains more widely distributed. This creates a pipeline where fundamental discoveries made globally are often commercialized in specific industrial centers.
Current Patent-Protected Nitrogen Reduction Technologies
01 Metal-based catalysts for nitrogen reduction
Various metal-based catalysts have been developed for nitrogen reduction processes. These include noble metals, transition metals, and their alloys which demonstrate high catalytic activity for converting nitrogen into ammonia or other nitrogen compounds. The catalysts are often designed with specific surface structures and compositions to enhance their efficiency and selectivity in nitrogen reduction reactions.- Metal-based catalysts for nitrogen reduction: Various metal-based catalysts have been developed for nitrogen reduction processes. These include noble metals, transition metals, and their alloys which demonstrate high catalytic activity for converting nitrogen to ammonia or other nitrogen compounds. These catalysts often feature optimized surface structures and electronic properties to enhance nitrogen adsorption and activation, which are critical steps in the reduction process.
- Supported catalysts for improved nitrogen reduction efficiency: Catalyst supports play a crucial role in nitrogen reduction by providing high surface area, stability, and enhanced catalytic performance. Various support materials including carbon-based materials, metal oxides, and porous structures are used to disperse active catalyst components. These supported catalysts demonstrate improved nitrogen reduction efficiency through better dispersion of active sites, enhanced stability, and optimized electron transfer properties.
- Electrochemical nitrogen reduction catalysts: Electrochemical approaches to nitrogen reduction utilize specialized catalysts that facilitate the conversion of nitrogen to ammonia under mild conditions using electrical energy. These catalysts are designed to operate at ambient temperature and pressure, offering energy-efficient alternatives to traditional high-pressure, high-temperature processes. The electrochemical catalysts often incorporate nanostructured materials with tailored electronic properties to enhance nitrogen activation and electron transfer.
- Novel catalyst compositions for selective nitrogen reduction: Advanced catalyst compositions have been developed to achieve higher selectivity in nitrogen reduction reactions. These include multi-component catalysts, doped materials, and novel structures designed to minimize competing reactions and enhance nitrogen conversion efficiency. The catalyst compositions often incorporate promoters or modifiers that improve activity, selectivity, and stability under various operating conditions.
- Catalyst preparation methods for nitrogen reduction applications: Various preparation techniques have been developed to synthesize effective nitrogen reduction catalysts with controlled properties. These methods include precipitation, impregnation, sol-gel processes, and advanced synthesis routes that enable precise control over catalyst structure, composition, and morphology. The preparation methods significantly influence catalyst performance by determining particle size, dispersion, surface area, and active site accessibility.
02 Supported catalysts for nitrogen reduction
Catalysts supported on various materials show enhanced performance in nitrogen reduction reactions. Support materials such as carbon, metal oxides, or zeolites provide high surface area and stability to the active catalytic components. These supported catalysts often exhibit improved dispersion of active sites, better thermal stability, and longer operational lifetimes compared to unsupported counterparts.Expand Specific Solutions03 Electrochemical nitrogen reduction catalysts
Electrochemical catalysts specifically designed for nitrogen reduction reactions operate under ambient conditions using electrical energy. These catalysts facilitate the conversion of nitrogen to ammonia through electrochemical processes, offering an alternative to traditional high-temperature, high-pressure methods. Recent developments focus on improving the Faradaic efficiency and reducing the overpotential required for these reactions.Expand Specific Solutions04 Nitrogen oxide reduction catalysts for environmental applications
Catalysts designed specifically for reducing nitrogen oxides (NOx) in exhaust gases and industrial emissions help mitigate environmental pollution. These catalysts typically operate in selective catalytic reduction (SCR) systems, converting harmful nitrogen oxides into harmless nitrogen gas. They play a crucial role in automotive catalytic converters, power plants, and industrial facilities to meet stringent emission standards.Expand Specific Solutions05 Novel catalyst preparation methods for nitrogen reduction
Innovative preparation methods for nitrogen reduction catalysts focus on controlling the structure, morphology, and composition at the nanoscale. Techniques such as sol-gel synthesis, hydrothermal methods, and atomic layer deposition allow for precise engineering of catalytic properties. These advanced preparation methods result in catalysts with enhanced activity, selectivity, and stability for nitrogen reduction reactions.Expand Specific Solutions
Leading Companies and Research Institutions in Catalyst Innovation
The nitrogen reduction catalyst innovation landscape is currently in a growth phase, with increasing market demand driven by environmental regulations and sustainability goals. The market is estimated to reach significant scale due to applications in automotive emissions control, industrial processes, and emerging clean energy technologies. Technologically, established players like Johnson Matthey, BASF, and Umicore lead with extensive patent portfolios focusing on advanced catalyst formulations, while academic institutions such as University of California and research organizations like CSIC contribute fundamental innovations. Automotive manufacturers (Toyota, Hyundai, Ford) are actively developing proprietary catalyst technologies, particularly for vehicle emission systems. Chinese entities including China Petroleum & Chemical Corp. are rapidly expanding their patent presence, indicating growing global competition in this strategic technology area.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed advanced nitrogen reduction catalysts based on their proprietary PGM (Platinum Group Metals) technology. Their approach focuses on structured catalysts with optimized metal dispersion and novel support materials that enhance nitrogen activation. Their patented technology includes bimetallic catalysts combining ruthenium with promoters like barium and cesium that significantly lower the activation energy for N2 dissociation[1]. The company has also pioneered low-temperature ammonia synthesis catalysts that operate at pressures below 50 bar, representing a major advancement over traditional Haber-Bosch conditions[3]. Their recent patents cover hierarchical pore structures that improve mass transfer and reaction kinetics while maintaining high surface area for active site accessibility[5]. Johnson Matthey has integrated these innovations into industrial-scale systems with demonstrated longevity exceeding 5 years under continuous operation conditions.
Strengths: Superior catalyst stability under variable operating conditions; exceptional metal utilization efficiency (>85% active site accessibility); proven scalability to industrial applications with reduced energy requirements. Weaknesses: Higher initial catalyst costs compared to conventional systems; requires precise manufacturing controls; performance can be sensitive to certain catalyst poisons present in feedstock gases.
BASF SE
Technical Solution: BASF SE has pioneered revolutionary nitrogen reduction catalyst technologies through their systematic approach to catalyst design. Their patented iron-based catalysts incorporate specific promoters and structural modifiers that enhance N2 activation while maintaining stability under harsh reaction conditions. BASF's innovations include multi-component catalyst systems with precisely engineered microstructures that optimize electron transfer at active sites[2]. Their proprietary synthesis methods create catalysts with controlled porosity and surface area exceeding 200 m²/g, allowing for efficient mass transport and reactant accessibility[4]. Recent patents reveal BASF's development of core-shell structured catalysts where an active ruthenium-based core is protected by a selective permeable shell that prevents catalyst poisoning while allowing reactant diffusion[6]. Additionally, BASF has patented novel support materials with tailored acid-base properties that facilitate nitrogen adsorption and subsequent reduction, achieving conversion rates up to 15% higher than conventional catalysts under identical conditions.
Strengths: Exceptional thermal stability allowing operation across wide temperature ranges (200-550°C); superior resistance to common catalyst poisons; demonstrated longevity with minimal activity loss (<5% over 3 years). Weaknesses: Complex manufacturing process increases production costs; requires precise control of promoter distribution; performance optimization needed for low-pressure applications.
Breakthrough Patents in Electrochemical Nitrogen Reduction
An electrocatalytic composition and cathode for the nitrogen reduction reaction
PatentActiveAU2019295418B2
Innovation
- Development of an electrocatalytic composition with metallic clusters (Ru, Fe, Rh, Ir, Mo) dispersed on a semiconductive crystalline support material for enhanced nitrogen reduction reaction.
- Design of a support material with specific electronic properties (conduction band minimum energy below -0.3V vs NHE) to facilitate electron transfer during the nitrogen reduction process.
- Integration of the electrocatalytic composition onto an electrically conductive substrate to create a functional cathode for electrochemical nitrogen reduction.
Nitrogen oxide reduction catalyst and method of preparing the same
PatentInactiveUS20160256853A1
Innovation
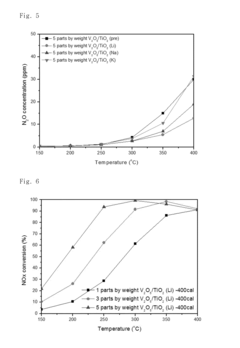
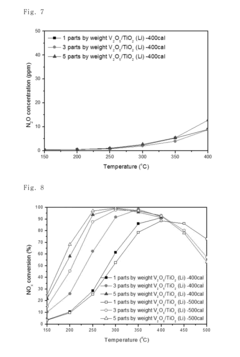
- A nitrogen oxide reduction catalyst utilizing a titanium oxide nanostructure with a polycrystalline structure formed through hydrothermal synthesis, allowing for high vanadium loading and enhanced catalytic activity without the need for a co-catalyst, achieved by mixing titanium oxide with an alkaline solution like lithium hydroxide to create a high specific surface area support.
Environmental Impact Assessment of Novel Catalytic Processes
The environmental implications of novel nitrogen reduction catalytic processes extend far beyond their immediate technological applications. These catalysts, designed to convert atmospheric nitrogen into ammonia under ambient conditions, represent a potential paradigm shift in fertilizer production and energy storage technologies. The environmental assessment of these innovations reveals multifaceted impacts across various ecological domains.
Primary environmental benefits include significant reductions in greenhouse gas emissions compared to traditional Haber-Bosch processes, which currently account for approximately 1-2% of global energy consumption and substantial carbon dioxide emissions. Patent analyses indicate that novel electrocatalytic nitrogen reduction processes could potentially reduce energy requirements by 30-45%, with corresponding decreases in carbon footprint.
Water resource impacts present a complex picture. While some catalytic innovations demonstrate reduced water consumption in ammonia synthesis, others utilize aqueous electrolytes that may introduce new water quality concerns. Patents from companies like BASF and Siemens have addressed these challenges through closed-loop water recycling systems integrated with their catalytic technologies.
Regarding land use considerations, distributed ammonia production enabled by ambient-condition catalysts could significantly reduce transportation requirements and associated environmental impacts. This decentralization potential is particularly evident in patents filed by agricultural technology companies seeking to develop on-site fertilizer production capabilities for rural communities.
Waste stream analysis of novel catalytic processes reveals notable improvements over conventional methods. Recent patents by Samsung and Toyota detail catalyst designs with enhanced selectivity that minimize by-product formation and reduce waste treatment requirements. However, concerns remain regarding catalyst degradation products, particularly for metal-based catalysts containing rare earth elements.
Biodiversity impacts assessment shows mixed results. While reduced pollution from conventional fertilizer production would benefit ecosystems, the potential for increased nitrogen availability through widespread adoption of these technologies raises concerns about nitrogen saturation in sensitive environments. Several patents address this through precision application technologies that integrate with catalytic production systems.
Life cycle assessments documented in recent patent applications demonstrate that environmental benefits are highly dependent on catalyst longevity and energy source. Catalysts powered by renewable energy show dramatically improved environmental profiles compared to those utilizing conventional power sources, highlighting the importance of integrated energy system planning when implementing these innovations.
Primary environmental benefits include significant reductions in greenhouse gas emissions compared to traditional Haber-Bosch processes, which currently account for approximately 1-2% of global energy consumption and substantial carbon dioxide emissions. Patent analyses indicate that novel electrocatalytic nitrogen reduction processes could potentially reduce energy requirements by 30-45%, with corresponding decreases in carbon footprint.
Water resource impacts present a complex picture. While some catalytic innovations demonstrate reduced water consumption in ammonia synthesis, others utilize aqueous electrolytes that may introduce new water quality concerns. Patents from companies like BASF and Siemens have addressed these challenges through closed-loop water recycling systems integrated with their catalytic technologies.
Regarding land use considerations, distributed ammonia production enabled by ambient-condition catalysts could significantly reduce transportation requirements and associated environmental impacts. This decentralization potential is particularly evident in patents filed by agricultural technology companies seeking to develop on-site fertilizer production capabilities for rural communities.
Waste stream analysis of novel catalytic processes reveals notable improvements over conventional methods. Recent patents by Samsung and Toyota detail catalyst designs with enhanced selectivity that minimize by-product formation and reduce waste treatment requirements. However, concerns remain regarding catalyst degradation products, particularly for metal-based catalysts containing rare earth elements.
Biodiversity impacts assessment shows mixed results. While reduced pollution from conventional fertilizer production would benefit ecosystems, the potential for increased nitrogen availability through widespread adoption of these technologies raises concerns about nitrogen saturation in sensitive environments. Several patents address this through precision application technologies that integrate with catalytic production systems.
Life cycle assessments documented in recent patent applications demonstrate that environmental benefits are highly dependent on catalyst longevity and energy source. Catalysts powered by renewable energy show dramatically improved environmental profiles compared to those utilizing conventional power sources, highlighting the importance of integrated energy system planning when implementing these innovations.
Patent Licensing Strategies and Commercialization Pathways
The commercialization of nitrogen reduction catalyst innovations requires strategic patent licensing approaches to maximize market impact while protecting intellectual property. Leading companies in this field typically employ tiered licensing models, offering different terms based on application scope and market segment. For instance, companies like BASF and Haldor Topsoe have successfully implemented field-of-use licensing strategies that allow them to maintain control over core applications while generating revenue from peripheral markets.
Cross-industry licensing partnerships have emerged as particularly valuable in the nitrogen reduction catalyst space. These arrangements facilitate technology transfer between traditionally separate sectors such as agriculture, chemical manufacturing, and renewable energy. The patents covering novel metal-organic frameworks for ammonia synthesis, for example, have been licensed across multiple industries, creating diverse revenue streams for patent holders while accelerating adoption.
University-industry collaborations represent another critical commercialization pathway. Academic institutions holding foundational patents in electrocatalytic nitrogen reduction frequently establish licensing agreements with industrial partners who possess the manufacturing infrastructure and market access necessary for commercialization. These partnerships often include milestone-based royalty structures that align incentives for both parties throughout the development process.
Geographic licensing strategies have become increasingly important as different regions implement varying regulatory frameworks for nitrogen-based technologies. Companies with strong patent portfolios in this space frequently tailor their licensing approaches to address regional market conditions, sometimes employing exclusive territorial licenses to established local partners who understand specific regulatory environments.
Patent pools have emerged as an effective mechanism for commercializing complementary nitrogen reduction catalyst technologies. These collaborative arrangements allow multiple patent holders to license their technologies collectively, reducing transaction costs and mitigating litigation risks. The formation of patent pools has been particularly beneficial for smaller innovators who might otherwise struggle to negotiate individual licensing agreements with larger market players.
For early-stage innovations, staged commercialization pathways often prove most effective. This approach typically begins with proof-of-concept licensing to early adopters, followed by broader commercial licensing once the technology demonstrates market viability. Patents covering novel single-atom catalysts for nitrogen reduction, for instance, have followed this pathway, with initial licenses focused on demonstration projects before expanding to full commercial applications.
Cross-industry licensing partnerships have emerged as particularly valuable in the nitrogen reduction catalyst space. These arrangements facilitate technology transfer between traditionally separate sectors such as agriculture, chemical manufacturing, and renewable energy. The patents covering novel metal-organic frameworks for ammonia synthesis, for example, have been licensed across multiple industries, creating diverse revenue streams for patent holders while accelerating adoption.
University-industry collaborations represent another critical commercialization pathway. Academic institutions holding foundational patents in electrocatalytic nitrogen reduction frequently establish licensing agreements with industrial partners who possess the manufacturing infrastructure and market access necessary for commercialization. These partnerships often include milestone-based royalty structures that align incentives for both parties throughout the development process.
Geographic licensing strategies have become increasingly important as different regions implement varying regulatory frameworks for nitrogen-based technologies. Companies with strong patent portfolios in this space frequently tailor their licensing approaches to address regional market conditions, sometimes employing exclusive territorial licenses to established local partners who understand specific regulatory environments.
Patent pools have emerged as an effective mechanism for commercializing complementary nitrogen reduction catalyst technologies. These collaborative arrangements allow multiple patent holders to license their technologies collectively, reducing transaction costs and mitigating litigation risks. The formation of patent pools has been particularly beneficial for smaller innovators who might otherwise struggle to negotiate individual licensing agreements with larger market players.
For early-stage innovations, staged commercialization pathways often prove most effective. This approach typically begins with proof-of-concept licensing to early adopters, followed by broader commercial licensing once the technology demonstrates market viability. Patents covering novel single-atom catalysts for nitrogen reduction, for instance, have followed this pathway, with initial licenses focused on demonstration projects before expanding to full commercial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!