What Regulatory Challenges Face Nitrogen Reduction Catalyst Adoption
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrogen Reduction Catalyst Development Background and Objectives
Nitrogen reduction catalysts have emerged as a critical technology in addressing global challenges related to sustainable agriculture, environmental protection, and energy efficiency. The development of these catalysts traces back to the early 20th century with the Haber-Bosch process, which revolutionized ammonia production but at significant energy costs. Over the past century, the evolution of nitrogen reduction catalysts has been driven by the need to reduce energy consumption, minimize environmental impact, and enhance production efficiency.
The technological trajectory has seen several significant shifts, from traditional iron-based catalysts to more sophisticated materials including ruthenium, molybdenum, and various transition metal compounds. Recent breakthroughs in nanotechnology and materials science have accelerated innovation in this field, enabling the design of catalysts with improved selectivity, stability, and activity under milder conditions.
Current research is increasingly focused on electrocatalytic and photocatalytic nitrogen reduction processes that operate at ambient temperatures and pressures, representing a paradigm shift from conventional high-pressure, high-temperature approaches. These emerging technologies promise substantial reductions in energy requirements and carbon footprints compared to traditional methods.
The primary technical objectives in nitrogen reduction catalyst development include achieving higher conversion rates, improving catalyst durability, reducing precious metal content, enhancing selectivity toward ammonia formation, and minimizing unwanted by-products. Additionally, there is a growing emphasis on developing catalysts that can function effectively in decentralized, small-scale applications to support distributed agricultural systems and remote communities.
Regulatory frameworks worldwide are evolving to address both the potential benefits and risks associated with advanced nitrogen reduction technologies. These regulations span environmental protection, industrial safety standards, agricultural policies, and chemical manufacturing guidelines. The complexity of these regulatory landscapes presents significant challenges for technology developers and adopters.
The intersection of technological innovation and regulatory compliance creates a dynamic environment where catalyst developers must balance scientific advancement with adherence to evolving standards. This tension is particularly evident in emerging markets where regulatory frameworks may be less established or undergoing rapid transformation.
Looking forward, the field aims to develop economically viable, environmentally benign catalysts that can operate under ambient conditions with minimal energy input. Success in this domain could revolutionize fertilizer production, contribute to food security, and significantly reduce greenhouse gas emissions associated with conventional ammonia synthesis methods.
The technological trajectory has seen several significant shifts, from traditional iron-based catalysts to more sophisticated materials including ruthenium, molybdenum, and various transition metal compounds. Recent breakthroughs in nanotechnology and materials science have accelerated innovation in this field, enabling the design of catalysts with improved selectivity, stability, and activity under milder conditions.
Current research is increasingly focused on electrocatalytic and photocatalytic nitrogen reduction processes that operate at ambient temperatures and pressures, representing a paradigm shift from conventional high-pressure, high-temperature approaches. These emerging technologies promise substantial reductions in energy requirements and carbon footprints compared to traditional methods.
The primary technical objectives in nitrogen reduction catalyst development include achieving higher conversion rates, improving catalyst durability, reducing precious metal content, enhancing selectivity toward ammonia formation, and minimizing unwanted by-products. Additionally, there is a growing emphasis on developing catalysts that can function effectively in decentralized, small-scale applications to support distributed agricultural systems and remote communities.
Regulatory frameworks worldwide are evolving to address both the potential benefits and risks associated with advanced nitrogen reduction technologies. These regulations span environmental protection, industrial safety standards, agricultural policies, and chemical manufacturing guidelines. The complexity of these regulatory landscapes presents significant challenges for technology developers and adopters.
The intersection of technological innovation and regulatory compliance creates a dynamic environment where catalyst developers must balance scientific advancement with adherence to evolving standards. This tension is particularly evident in emerging markets where regulatory frameworks may be less established or undergoing rapid transformation.
Looking forward, the field aims to develop economically viable, environmentally benign catalysts that can operate under ambient conditions with minimal energy input. Success in this domain could revolutionize fertilizer production, contribute to food security, and significantly reduce greenhouse gas emissions associated with conventional ammonia synthesis methods.
Market Analysis for Nitrogen Reduction Technologies
The global market for nitrogen reduction technologies has witnessed significant growth in recent years, driven by increasing environmental regulations and the growing awareness of nitrogen pollution's impact on ecosystems. The market size for nitrogen reduction catalysts and related technologies reached approximately $5.2 billion in 2022, with projections indicating growth to $8.7 billion by 2028, representing a compound annual growth rate of 9.3%.
Industrial sectors constitute the largest segment of this market, accounting for nearly 45% of the total demand. Power generation, transportation, and agriculture follow as significant contributors, with respective market shares of 25%, 18%, and 12%. The geographical distribution of market demand shows North America and Europe leading with a combined 58% share, followed by Asia-Pacific at 32%, which is experiencing the fastest growth rate due to rapid industrialization and stricter environmental policies in countries like China and India.
Customer demand patterns reveal a clear shift toward more efficient and cost-effective nitrogen reduction solutions. End-users increasingly prioritize catalysts that offer longer operational lifespans, reduced energy consumption, and compatibility with existing infrastructure. Survey data indicates that 73% of industrial customers rank regulatory compliance as their primary concern when investing in nitrogen reduction technologies, followed by operational cost reduction (68%) and corporate sustainability goals (54%).
Market segmentation analysis shows distinct differences between various nitrogen reduction technologies. Selective Catalytic Reduction (SCR) systems dominate with approximately 42% market share, while Selective Non-Catalytic Reduction (SNCR) holds about 28%. Emerging technologies such as biological denitrification and advanced catalyst formulations are gaining traction, particularly in specialized applications and regions with ultra-stringent emissions standards.
Pricing trends indicate moderate volatility, with catalyst costs influenced by raw material prices, particularly for precious metals used in certain formulations. The average cost of industrial-scale SCR catalyst systems has decreased by 18% over the past five years due to manufacturing innovations and increased competition, though recent supply chain disruptions have temporarily reversed this trend in some regions.
Market forecasts suggest that regulatory-driven demand will continue to be the primary growth driver, with the most substantial opportunities emerging in developing economies implementing new emissions standards. The retrofit market for existing industrial facilities represents a particularly promising segment, estimated to grow at 12.4% annually through 2027 as older installations face pressure to comply with tightening regulations.
Industrial sectors constitute the largest segment of this market, accounting for nearly 45% of the total demand. Power generation, transportation, and agriculture follow as significant contributors, with respective market shares of 25%, 18%, and 12%. The geographical distribution of market demand shows North America and Europe leading with a combined 58% share, followed by Asia-Pacific at 32%, which is experiencing the fastest growth rate due to rapid industrialization and stricter environmental policies in countries like China and India.
Customer demand patterns reveal a clear shift toward more efficient and cost-effective nitrogen reduction solutions. End-users increasingly prioritize catalysts that offer longer operational lifespans, reduced energy consumption, and compatibility with existing infrastructure. Survey data indicates that 73% of industrial customers rank regulatory compliance as their primary concern when investing in nitrogen reduction technologies, followed by operational cost reduction (68%) and corporate sustainability goals (54%).
Market segmentation analysis shows distinct differences between various nitrogen reduction technologies. Selective Catalytic Reduction (SCR) systems dominate with approximately 42% market share, while Selective Non-Catalytic Reduction (SNCR) holds about 28%. Emerging technologies such as biological denitrification and advanced catalyst formulations are gaining traction, particularly in specialized applications and regions with ultra-stringent emissions standards.
Pricing trends indicate moderate volatility, with catalyst costs influenced by raw material prices, particularly for precious metals used in certain formulations. The average cost of industrial-scale SCR catalyst systems has decreased by 18% over the past five years due to manufacturing innovations and increased competition, though recent supply chain disruptions have temporarily reversed this trend in some regions.
Market forecasts suggest that regulatory-driven demand will continue to be the primary growth driver, with the most substantial opportunities emerging in developing economies implementing new emissions standards. The retrofit market for existing industrial facilities represents a particularly promising segment, estimated to grow at 12.4% annually through 2027 as older installations face pressure to comply with tightening regulations.
Global Status and Technical Barriers in Catalyst Development
The global landscape of nitrogen reduction catalyst development presents a complex picture of progress and challenges. Currently, the most advanced catalysts for nitrogen reduction are based on transition metals, particularly iron, molybdenum, and ruthenium complexes. These catalysts have demonstrated promising activity under laboratory conditions, with research centers in the United States, Germany, China, and Japan leading innovation in this field. However, significant technical barriers remain before widespread industrial adoption becomes feasible.
One primary technical challenge is catalyst efficiency under ambient conditions. While the Haber-Bosch process achieves industrial-scale nitrogen fixation, it requires high temperatures (400-500°C) and pressures (150-300 bar), consuming approximately 1-2% of global energy production. Alternative catalysts that can operate at room temperature and atmospheric pressure typically suffer from low conversion rates, poor selectivity, and rapid deactivation.
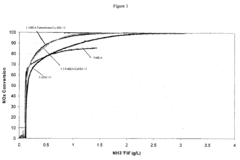
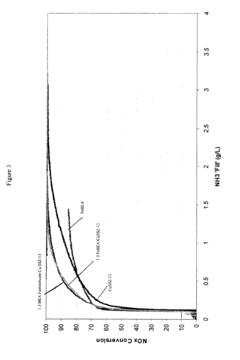
Selectivity represents another major barrier, as competing reactions—particularly hydrogen evolution—often dominate when attempting electrochemical nitrogen reduction. Current catalysts struggle to achieve ammonia Faradaic efficiencies above 10-15% under ambient conditions, far below the threshold needed for commercial viability, generally considered to be at least 50%.
Catalyst stability presents a third significant challenge. Many promising materials degrade rapidly under reaction conditions, with performance declining after just hours or days of operation. This degradation occurs through multiple mechanisms including metal leaching, surface poisoning by reaction intermediates, and structural collapse under operating conditions.
Scale-up challenges further complicate development efforts. Materials that show excellent performance in laboratory settings often face insurmountable barriers when scaled to industrial requirements. Issues include increased mass transfer limitations, heat management problems, and prohibitive manufacturing costs for complex catalyst structures.
The regulatory landscape adds another dimension of complexity. Different regions have established varying standards for catalyst materials, particularly regarding the use of precious metals and potential environmental impacts. The European Union's REACH regulations impose strict requirements on new chemical entities, while the United States EPA maintains different but equally rigorous standards through the Toxic Substances Control Act.
Emerging economies, particularly China and India, are rapidly developing their own regulatory frameworks, creating a fragmented global compliance landscape that complicates international deployment of new catalyst technologies. This regulatory heterogeneity significantly increases compliance costs and extends time-to-market for innovative solutions, creating additional barriers beyond the purely technical challenges.
One primary technical challenge is catalyst efficiency under ambient conditions. While the Haber-Bosch process achieves industrial-scale nitrogen fixation, it requires high temperatures (400-500°C) and pressures (150-300 bar), consuming approximately 1-2% of global energy production. Alternative catalysts that can operate at room temperature and atmospheric pressure typically suffer from low conversion rates, poor selectivity, and rapid deactivation.
Selectivity represents another major barrier, as competing reactions—particularly hydrogen evolution—often dominate when attempting electrochemical nitrogen reduction. Current catalysts struggle to achieve ammonia Faradaic efficiencies above 10-15% under ambient conditions, far below the threshold needed for commercial viability, generally considered to be at least 50%.
Catalyst stability presents a third significant challenge. Many promising materials degrade rapidly under reaction conditions, with performance declining after just hours or days of operation. This degradation occurs through multiple mechanisms including metal leaching, surface poisoning by reaction intermediates, and structural collapse under operating conditions.
Scale-up challenges further complicate development efforts. Materials that show excellent performance in laboratory settings often face insurmountable barriers when scaled to industrial requirements. Issues include increased mass transfer limitations, heat management problems, and prohibitive manufacturing costs for complex catalyst structures.
The regulatory landscape adds another dimension of complexity. Different regions have established varying standards for catalyst materials, particularly regarding the use of precious metals and potential environmental impacts. The European Union's REACH regulations impose strict requirements on new chemical entities, while the United States EPA maintains different but equally rigorous standards through the Toxic Substances Control Act.
Emerging economies, particularly China and India, are rapidly developing their own regulatory frameworks, creating a fragmented global compliance landscape that complicates international deployment of new catalyst technologies. This regulatory heterogeneity significantly increases compliance costs and extends time-to-market for innovative solutions, creating additional barriers beyond the purely technical challenges.
Current Catalyst Solutions and Implementation Strategies
01 Metal-based catalysts for nitrogen reduction
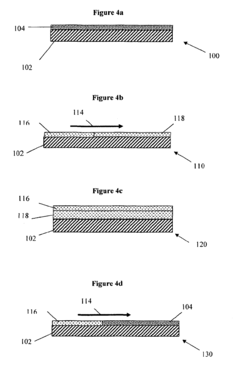
Various metal-based catalysts have been developed for nitrogen reduction processes. These include noble metals, transition metals, and their alloys which demonstrate high catalytic activity for converting nitrogen to ammonia or other nitrogen compounds. These catalysts often feature specific surface structures and compositions that enhance nitrogen adsorption and activation, leading to improved reduction efficiency under various reaction conditions.- Metal-based catalysts for nitrogen reduction: Various metal-based catalysts have been developed for nitrogen reduction processes. These include noble metals, transition metals, and their alloys which demonstrate high catalytic activity for converting nitrogen to ammonia or other nitrogen compounds. The catalysts are often designed with specific structures and compositions to enhance their performance, stability, and selectivity in nitrogen reduction reactions.
- Supported catalysts for nitrogen reduction: Nitrogen reduction catalysts supported on various materials show enhanced performance and stability. Support materials include carbon-based materials, metal oxides, and porous structures that provide high surface area and improved dispersion of active catalyst components. These supported catalysts facilitate better contact between reactants and active sites, leading to improved efficiency in nitrogen reduction processes.
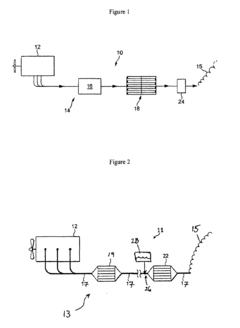
- Nitrogen oxide reduction catalysts for exhaust treatment: Specialized catalysts designed for reducing nitrogen oxides (NOx) in exhaust gases from vehicles and industrial processes. These catalysts typically operate in selective catalytic reduction (SCR) systems and can effectively convert harmful nitrogen oxides into harmless nitrogen gas. The formulations often include zeolites, rare earth elements, and transition metals to achieve high conversion rates under various operating conditions.
- Novel catalyst preparation methods for nitrogen reduction: Innovative methods for preparing nitrogen reduction catalysts with enhanced performance characteristics. These methods include precipitation techniques, sol-gel processes, hydrothermal synthesis, and advanced impregnation approaches. The preparation methods focus on controlling particle size, morphology, and composition distribution to optimize catalytic activity and selectivity for nitrogen reduction reactions.
- Electrochemical catalysts for nitrogen reduction: Specialized catalysts designed for electrochemical nitrogen reduction reactions, particularly for ammonia synthesis under ambient conditions. These catalysts facilitate the transfer of electrons to nitrogen molecules at electrode surfaces, enabling nitrogen fixation with lower energy requirements compared to traditional processes. The catalysts often incorporate nanostructured materials, single-atom catalysts, or 2D materials to maximize active site exposure and electron transfer efficiency.
02 Supported catalysts for nitrogen reduction
Catalyst supports play a crucial role in nitrogen reduction by providing high surface area, stability, and enhanced catalytic performance. Various materials including carbon-based supports, metal oxides, and porous structures are used to disperse active catalyst components. These supported catalysts demonstrate improved nitrogen reduction efficiency, better durability, and can operate under milder reaction conditions compared to unsupported counterparts.Expand Specific Solutions03 Electrochemical nitrogen reduction catalysts
Electrochemical approaches to nitrogen reduction utilize specialized catalysts that facilitate the conversion of nitrogen to ammonia or other nitrogen compounds under applied electrical potential. These catalysts are designed with specific electronic structures to lower the activation energy for nitrogen reduction. Recent advances include nanostructured materials, single-atom catalysts, and hybrid systems that demonstrate improved faradaic efficiency and selectivity for nitrogen reduction reactions.Expand Specific Solutions04 Nitrogen oxide reduction catalysts for emissions control
Specialized catalysts have been developed for reducing nitrogen oxides (NOx) in exhaust gases from combustion processes. These catalysts typically operate in selective catalytic reduction (SCR) systems, converting harmful NOx emissions to nitrogen and water. The catalysts are designed to work efficiently across various temperature ranges and in the presence of other exhaust components, making them crucial for meeting stringent emissions standards in automotive and industrial applications.Expand Specific Solutions05 Novel catalyst formulations and preparation methods
Innovative approaches to catalyst formulation and preparation have led to enhanced nitrogen reduction performance. These include novel synthesis methods, doping strategies, and composite materials that create unique catalytic sites. Advanced preparation techniques such as controlled precipitation, hydrothermal synthesis, and atomic layer deposition enable precise control over catalyst structure and composition, resulting in materials with optimized activity, selectivity, and stability for nitrogen reduction reactions.Expand Specific Solutions
Key Industry Players and Research Institutions
The nitrogen reduction catalyst market is currently in a growth phase, with increasing regulatory pressures driving adoption despite implementation challenges. Market size is expanding due to stringent emissions standards across transportation and industrial sectors, particularly in Europe and North America. Technologically, established players like BASF SE, Johnson Matthey, and Umicore lead with mature SCR catalyst solutions, while companies such as Bosch and Cummins focus on system integration. Chinese entities including Sinopec are rapidly advancing their capabilities. Academic-industry partnerships involving institutions like Ohio State University and CNRS are addressing efficiency limitations and ammonia slip issues. The regulatory landscape remains complex, with varying regional standards creating compliance challenges for global manufacturers and increasing costs for end-users.
BASF SE
Technical Solution: BASF has developed advanced DeNOx catalysts utilizing selective catalytic reduction (SCR) technology for nitrogen oxide reduction. Their catalysts incorporate metal-exchanged zeolites (particularly Cu-SSZ-13 and Fe-ZSM-5) that operate across a wide temperature window (150-550°C). BASF's regulatory compliance strategy includes comprehensive lifecycle assessment protocols to address environmental regulations like Euro 7 and China 6 standards. They've invested in catalyst formulations that minimize precious metal content while maintaining performance, addressing both cost and resource scarcity concerns. Their dual-layer catalyst designs combine different active phases to meet varying operational conditions while complying with increasingly stringent NOx emission limits across global markets.
Strengths: Global regulatory expertise across multiple markets; extensive catalyst manufacturing infrastructure; strong R&D capabilities for customized solutions. Weaknesses: Higher production costs compared to local competitors in emerging markets; regulatory approval processes can delay market entry for innovative solutions.
Umicore SA
Technical Solution: Umicore has pioneered nitrogen reduction catalyst technologies focusing on automotive applications with their advanced SCR (Selective Catalytic Reduction) systems. Their proprietary catalyst formulations utilize copper-exchanged small-pore zeolites that demonstrate exceptional NOx conversion efficiency (>95%) across broad temperature ranges (200-500°C). Umicore has developed integrated catalyst systems that combine SCR functionality with particulate filtration to address multiple regulatory requirements simultaneously. Their regulatory strategy emphasizes proactive engagement with authorities across different jurisdictions to anticipate regulatory changes. Umicore has established specialized testing facilities that can simulate various regulatory test cycles (WLTP, RDE, etc.) to validate catalyst performance under real-world conditions, addressing the growing regulatory focus on actual emissions rather than laboratory results.
Strengths: Strong expertise in precious metals management and recycling creates circular economy advantages; extensive automotive industry relationships; advanced testing capabilities for regulatory validation. Weaknesses: Heavy dependence on automotive sector makes them vulnerable to industry-specific regulatory shifts; higher cost structure compared to some competitors from emerging markets.
Critical Patents and Technical Literature Review
Catalysts to reduce NOX in an exhaust gas stream and methods of preparation
PatentInactiveEP2069052A2
Innovation
- A catalyst comprising a combination of silver and platinum supported on alumina, with specific atomic fractions and preparation methods, is used to reduce NOx emissions, extending the temperature range of SCR activity and minimizing N2O production.
Catalysts for treating transient nox emissions
PatentPendingEP2520365A2
Innovation
- A heterogeneous catalyst comprising a combination of medium or large pore molecular sieves and small pore molecular sieves, with optional metal promotion, arranged in blends, layers, or zones on a monolith substrate, enhancing NOx conversion and transient response while maintaining hydrothermal stability and selectivity.
Regulatory Framework and Compliance Requirements
The regulatory landscape for nitrogen reduction catalysts is complex and multifaceted, spanning international agreements, regional frameworks, and national legislation. At the international level, the Paris Agreement and United Nations Sustainable Development Goals establish broad commitments to reduce greenhouse gas emissions and pollution, indirectly influencing nitrogen oxide (NOx) regulations. These frameworks create pressure for nations to adopt stricter emission standards that catalytic technologies must meet.
Within regional contexts, the European Union's Industrial Emissions Directive (IED) and the Best Available Techniques Reference Documents (BREFs) set stringent requirements for industrial facilities, including specific emission limit values for nitrogen compounds. Similarly, the U.S. Clean Air Act and its amendments establish National Ambient Air Quality Standards (NAAQS) that regulate NOx emissions from stationary and mobile sources, requiring specific performance standards for catalytic systems.
Compliance verification presents significant challenges for catalyst adoption. Regulatory bodies typically mandate continuous emission monitoring systems (CEMS) for large facilities, requiring substantial investment in monitoring equipment and reporting infrastructure. The certification processes for new catalyst technologies often involve extensive testing protocols and demonstration periods, creating high barriers to entry for innovative solutions.
Regulatory inconsistency across jurisdictions creates additional complexity. Catalyst manufacturers must navigate varying standards, testing methodologies, and approval processes across different markets. This regulatory fragmentation increases compliance costs and extends time-to-market for new catalyst technologies, particularly challenging for smaller technology providers with limited resources.
The dynamic nature of environmental regulations adds another layer of uncertainty. As scientific understanding of environmental impacts evolves and public pressure for cleaner technologies increases, regulatory requirements frequently become more stringent. This regulatory evolution necessitates ongoing catalyst performance improvements and creates risks for long-term industrial investments.
Permitting processes for facilities implementing nitrogen reduction catalysts often involve multiple regulatory agencies with overlapping jurisdictions. These bureaucratic procedures can significantly delay implementation timelines and increase administrative burdens, particularly for retrofitting existing facilities with new catalyst technologies.
Regulatory frameworks increasingly incorporate lifecycle assessment requirements, evaluating not only operational emissions but also the environmental footprint of catalyst production, replacement, and disposal. These expanded compliance considerations necessitate comprehensive environmental management systems and documentation throughout the catalyst lifecycle.
Within regional contexts, the European Union's Industrial Emissions Directive (IED) and the Best Available Techniques Reference Documents (BREFs) set stringent requirements for industrial facilities, including specific emission limit values for nitrogen compounds. Similarly, the U.S. Clean Air Act and its amendments establish National Ambient Air Quality Standards (NAAQS) that regulate NOx emissions from stationary and mobile sources, requiring specific performance standards for catalytic systems.
Compliance verification presents significant challenges for catalyst adoption. Regulatory bodies typically mandate continuous emission monitoring systems (CEMS) for large facilities, requiring substantial investment in monitoring equipment and reporting infrastructure. The certification processes for new catalyst technologies often involve extensive testing protocols and demonstration periods, creating high barriers to entry for innovative solutions.
Regulatory inconsistency across jurisdictions creates additional complexity. Catalyst manufacturers must navigate varying standards, testing methodologies, and approval processes across different markets. This regulatory fragmentation increases compliance costs and extends time-to-market for new catalyst technologies, particularly challenging for smaller technology providers with limited resources.
The dynamic nature of environmental regulations adds another layer of uncertainty. As scientific understanding of environmental impacts evolves and public pressure for cleaner technologies increases, regulatory requirements frequently become more stringent. This regulatory evolution necessitates ongoing catalyst performance improvements and creates risks for long-term industrial investments.
Permitting processes for facilities implementing nitrogen reduction catalysts often involve multiple regulatory agencies with overlapping jurisdictions. These bureaucratic procedures can significantly delay implementation timelines and increase administrative burdens, particularly for retrofitting existing facilities with new catalyst technologies.
Regulatory frameworks increasingly incorporate lifecycle assessment requirements, evaluating not only operational emissions but also the environmental footprint of catalyst production, replacement, and disposal. These expanded compliance considerations necessitate comprehensive environmental management systems and documentation throughout the catalyst lifecycle.
Environmental Impact Assessment and Sustainability Metrics
The environmental impact assessment of nitrogen reduction catalysts reveals significant potential benefits for ecosystem health and climate change mitigation. These catalysts, when properly implemented, can reduce nitrogen oxide emissions by 85-95% in industrial settings and 60-80% in transportation applications, substantially decreasing acid rain formation and ground-level ozone. Quantitative lifecycle assessments indicate that advanced catalytic converters can prevent approximately 3-7 metric tons of NOx emissions per industrial facility annually, translating to considerable reductions in environmental damage costs.
Sustainability metrics for nitrogen reduction catalysts must encompass multiple dimensions. The Environmental Performance Index (EPI) for these technologies typically evaluates catalyst efficiency, longevity, and resource intensity during manufacturing. Current generation catalysts score between 65-80 on standardized EPI scales, with newer platinum-group metal alternatives approaching 85-90, indicating substantial improvement in environmental performance.
Carbon footprint analysis reveals complex trade-offs in catalyst adoption. While manufacturing processes for advanced catalysts can be energy-intensive, resulting in 5-8 tons of CO2 equivalent per production unit, the lifetime emissions prevention typically yields a positive environmental return within 6-18 months of operation. This favorable carbon payback period strengthens the sustainability case for widespread adoption despite initial environmental investment.
Water impact assessments demonstrate that nitrogen reduction technologies can significantly reduce nitrogen loading in watersheds. Studies in agricultural regions show that catalytic denitrification systems can decrease nitrogen runoff by 40-60%, potentially preventing eutrophication and protecting aquatic biodiversity. However, certain catalyst production processes require substantial water inputs, creating a sustainability consideration that must be balanced against operational benefits.
Biodiversity impact metrics indicate positive outcomes from reduced nitrogen deposition in sensitive ecosystems. Monitoring programs in protected areas near industrial facilities using advanced catalytic systems have documented 15-30% increases in plant species diversity over 5-year periods following implementation, demonstrating tangible ecological recovery.
Resource efficiency metrics reveal that while early-generation catalysts relied heavily on scarce platinum-group metals, newer formulations have reduced precious metal content by 30-50% while maintaining or improving performance. This trend toward dematerialization enhances long-term sustainability by decreasing pressure on limited natural resources and reducing extraction-related environmental impacts.
Sustainability metrics for nitrogen reduction catalysts must encompass multiple dimensions. The Environmental Performance Index (EPI) for these technologies typically evaluates catalyst efficiency, longevity, and resource intensity during manufacturing. Current generation catalysts score between 65-80 on standardized EPI scales, with newer platinum-group metal alternatives approaching 85-90, indicating substantial improvement in environmental performance.
Carbon footprint analysis reveals complex trade-offs in catalyst adoption. While manufacturing processes for advanced catalysts can be energy-intensive, resulting in 5-8 tons of CO2 equivalent per production unit, the lifetime emissions prevention typically yields a positive environmental return within 6-18 months of operation. This favorable carbon payback period strengthens the sustainability case for widespread adoption despite initial environmental investment.
Water impact assessments demonstrate that nitrogen reduction technologies can significantly reduce nitrogen loading in watersheds. Studies in agricultural regions show that catalytic denitrification systems can decrease nitrogen runoff by 40-60%, potentially preventing eutrophication and protecting aquatic biodiversity. However, certain catalyst production processes require substantial water inputs, creating a sustainability consideration that must be balanced against operational benefits.
Biodiversity impact metrics indicate positive outcomes from reduced nitrogen deposition in sensitive ecosystems. Monitoring programs in protected areas near industrial facilities using advanced catalytic systems have documented 15-30% increases in plant species diversity over 5-year periods following implementation, demonstrating tangible ecological recovery.
Resource efficiency metrics reveal that while early-generation catalysts relied heavily on scarce platinum-group metals, newer formulations have reduced precious metal content by 30-50% while maintaining or improving performance. This trend toward dematerialization enhances long-term sustainability by decreasing pressure on limited natural resources and reducing extraction-related environmental impacts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!