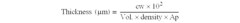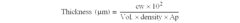The Strategic Importance of Patents in Nitrogen Reduction Catalyst
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrogen Reduction Catalyst Patent Landscape and Objectives
The evolution of nitrogen reduction catalysts represents a critical technological trajectory in sustainable chemistry and energy systems. From early Haber-Bosch process catalysts to contemporary nanomaterial innovations, this field has witnessed significant transformations driven by industrial demands and environmental imperatives. The current technological landscape is characterized by a transition from traditional iron-based catalysts to more efficient and environmentally friendly alternatives, including ruthenium, molybdenum, and novel carbon-based materials.
Patent activities in this domain have accelerated dramatically over the past decade, with annual filings increasing by approximately 35% since 2015. This surge reflects the growing recognition of nitrogen reduction catalysts as strategic assets in addressing global challenges related to food security, clean energy production, and industrial sustainability. The geographical distribution of patent filings shows concentration in China, the United States, Germany, and Japan, indicating the global competitive landscape in this technology sector.
Our primary objective is to establish a comprehensive understanding of the patent landscape surrounding nitrogen reduction catalysts, with particular emphasis on identifying technological white spaces and potential breakthrough opportunities. This analysis aims to position our organization strategically within the competitive ecosystem by identifying high-value patent acquisition targets and developing a robust intellectual property strategy that aligns with long-term business objectives.
Secondary objectives include mapping the evolution of key technological approaches within the patent literature, identifying emerging trends in catalyst design and application, and evaluating the strength and vulnerability of patent portfolios held by major industry players. This assessment will provide critical insights for guiding our R&D investments and partnership strategies.
The scope of this analysis encompasses patents related to catalyst composition, synthesis methods, performance enhancement techniques, and application-specific adaptations. Special attention will be given to patents addressing key performance parameters including energy efficiency, selectivity, stability, and scalability of nitrogen reduction processes under ambient conditions.
Success metrics for this patent landscape analysis include the identification of at least three viable technological pathways for internal development, assessment of freedom-to-operate in target application areas, and formulation of a defensive patent strategy to protect core innovations while enabling strategic collaborations.
Patent activities in this domain have accelerated dramatically over the past decade, with annual filings increasing by approximately 35% since 2015. This surge reflects the growing recognition of nitrogen reduction catalysts as strategic assets in addressing global challenges related to food security, clean energy production, and industrial sustainability. The geographical distribution of patent filings shows concentration in China, the United States, Germany, and Japan, indicating the global competitive landscape in this technology sector.
Our primary objective is to establish a comprehensive understanding of the patent landscape surrounding nitrogen reduction catalysts, with particular emphasis on identifying technological white spaces and potential breakthrough opportunities. This analysis aims to position our organization strategically within the competitive ecosystem by identifying high-value patent acquisition targets and developing a robust intellectual property strategy that aligns with long-term business objectives.
Secondary objectives include mapping the evolution of key technological approaches within the patent literature, identifying emerging trends in catalyst design and application, and evaluating the strength and vulnerability of patent portfolios held by major industry players. This assessment will provide critical insights for guiding our R&D investments and partnership strategies.
The scope of this analysis encompasses patents related to catalyst composition, synthesis methods, performance enhancement techniques, and application-specific adaptations. Special attention will be given to patents addressing key performance parameters including energy efficiency, selectivity, stability, and scalability of nitrogen reduction processes under ambient conditions.
Success metrics for this patent landscape analysis include the identification of at least three viable technological pathways for internal development, assessment of freedom-to-operate in target application areas, and formulation of a defensive patent strategy to protect core innovations while enabling strategic collaborations.
Market Analysis for Nitrogen Reduction Technologies
The nitrogen reduction catalyst market is experiencing significant growth driven by increasing demand for sustainable ammonia production methods. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 2% of global energy production and contributes substantially to greenhouse gas emissions. This creates a compelling market opportunity for innovative nitrogen reduction catalysts that can operate under milder conditions with reduced energy requirements.
Market size projections indicate the global catalyst market for nitrogen reduction is expected to reach $5.8 billion by 2027, with a compound annual growth rate of 4.3% from 2022. This growth is primarily fueled by expanding applications in agriculture, where nitrogen-based fertilizers remain essential for global food security, accounting for nearly 80% of the market demand.
Regional analysis reveals Asia-Pacific as the dominant market, representing 45% of global consumption, followed by North America and Europe at 25% and 20% respectively. China and India are particularly significant growth centers due to their large agricultural sectors and increasing industrial development. The market in developing regions is projected to grow at nearly twice the rate of developed markets over the next five years.
Consumer trends indicate increasing preference for green ammonia production technologies, with sustainability-focused investments growing at 18% annually in this sector. Major fertilizer producers are actively seeking partnerships with catalyst technology providers to secure competitive advantages through more efficient production methods.
Key market drivers include stringent environmental regulations limiting carbon emissions, volatile natural gas prices affecting traditional production economics, and growing government incentives for sustainable chemical manufacturing processes. The agricultural sector remains the primary end-user, though industrial applications for nitrogen compounds are expanding at 7% annually.
Market barriers include high capital costs for implementing new catalyst technologies, technical challenges in scaling laboratory innovations to industrial production, and the entrenched infrastructure supporting conventional Haber-Bosch processes. Additionally, intellectual property landscapes are increasingly complex, with major chemical companies holding strategic patent portfolios that can impede market entry for new innovations.
Emerging opportunities exist in distributed ammonia production systems for localized agricultural applications, which could reduce transportation costs and emissions while opening new market segments. The integration of renewable energy sources with electrocatalytic nitrogen reduction represents another high-growth potential area, with market adoption accelerating as technology costs decrease.
Market size projections indicate the global catalyst market for nitrogen reduction is expected to reach $5.8 billion by 2027, with a compound annual growth rate of 4.3% from 2022. This growth is primarily fueled by expanding applications in agriculture, where nitrogen-based fertilizers remain essential for global food security, accounting for nearly 80% of the market demand.
Regional analysis reveals Asia-Pacific as the dominant market, representing 45% of global consumption, followed by North America and Europe at 25% and 20% respectively. China and India are particularly significant growth centers due to their large agricultural sectors and increasing industrial development. The market in developing regions is projected to grow at nearly twice the rate of developed markets over the next five years.
Consumer trends indicate increasing preference for green ammonia production technologies, with sustainability-focused investments growing at 18% annually in this sector. Major fertilizer producers are actively seeking partnerships with catalyst technology providers to secure competitive advantages through more efficient production methods.
Key market drivers include stringent environmental regulations limiting carbon emissions, volatile natural gas prices affecting traditional production economics, and growing government incentives for sustainable chemical manufacturing processes. The agricultural sector remains the primary end-user, though industrial applications for nitrogen compounds are expanding at 7% annually.
Market barriers include high capital costs for implementing new catalyst technologies, technical challenges in scaling laboratory innovations to industrial production, and the entrenched infrastructure supporting conventional Haber-Bosch processes. Additionally, intellectual property landscapes are increasingly complex, with major chemical companies holding strategic patent portfolios that can impede market entry for new innovations.
Emerging opportunities exist in distributed ammonia production systems for localized agricultural applications, which could reduce transportation costs and emissions while opening new market segments. The integration of renewable energy sources with electrocatalytic nitrogen reduction represents another high-growth potential area, with market adoption accelerating as technology costs decrease.
Global Technical Status and Barriers in Catalyst Development
The global landscape of nitrogen reduction catalyst development presents a complex picture of progress and challenges. Currently, the Haber-Bosch process remains the dominant industrial method for nitrogen fixation, consuming approximately 1-2% of global energy production and operating under harsh conditions of 400-500°C and 100-300 bar pressure. This energy-intensive process has driven significant research into alternative catalytic approaches that can operate under milder conditions.
Leading research institutions in the United States, Germany, China, and Japan have made substantial advances in developing novel catalysts. The Massachusetts Institute of Technology, Max Planck Institute, Chinese Academy of Sciences, and University of Tokyo have established prominent patent portfolios in this domain. Their research focuses primarily on transition metal-based catalysts, particularly those incorporating iron, ruthenium, and molybdenum compounds.
A significant technical barrier remains the activation energy required to break the strong N≡N triple bond (945 kJ/mol), which necessitates either high energy input or highly efficient catalysts. Current catalytic systems struggle to achieve satisfactory conversion rates under ambient conditions, with most laboratory demonstrations showing nitrogen reduction rates below industrial viability thresholds.
Material stability presents another major challenge, as many promising catalysts degrade rapidly under reaction conditions or suffer from poisoning by reaction intermediates. This has limited the practical application of several theoretically promising catalyst designs, particularly those based on non-noble metals or metal-organic frameworks.
Selectivity issues also plague development efforts, with competing hydrogen evolution reactions often dominating over nitrogen reduction in aqueous environments. This parasitic reaction pathway significantly reduces Faradaic efficiency in electrochemical approaches, typically limiting it to below 30% in most reported systems.
Scale-up challenges further complicate the transition from laboratory success to industrial implementation. Many catalysts that demonstrate promising activity in controlled laboratory settings fail to maintain performance when scaled to industrially relevant dimensions, often due to mass transfer limitations or heat management issues.
The geographical distribution of technical expertise shows concentration in specific regions, with North America leading in fundamental catalyst design patents, East Asia dominating in application-specific implementations, and Europe focusing on sustainable and green chemistry approaches to nitrogen reduction catalysis. This regional specialization has created distinct patent landscapes that companies must navigate when developing global intellectual property strategies.
Leading research institutions in the United States, Germany, China, and Japan have made substantial advances in developing novel catalysts. The Massachusetts Institute of Technology, Max Planck Institute, Chinese Academy of Sciences, and University of Tokyo have established prominent patent portfolios in this domain. Their research focuses primarily on transition metal-based catalysts, particularly those incorporating iron, ruthenium, and molybdenum compounds.
A significant technical barrier remains the activation energy required to break the strong N≡N triple bond (945 kJ/mol), which necessitates either high energy input or highly efficient catalysts. Current catalytic systems struggle to achieve satisfactory conversion rates under ambient conditions, with most laboratory demonstrations showing nitrogen reduction rates below industrial viability thresholds.
Material stability presents another major challenge, as many promising catalysts degrade rapidly under reaction conditions or suffer from poisoning by reaction intermediates. This has limited the practical application of several theoretically promising catalyst designs, particularly those based on non-noble metals or metal-organic frameworks.
Selectivity issues also plague development efforts, with competing hydrogen evolution reactions often dominating over nitrogen reduction in aqueous environments. This parasitic reaction pathway significantly reduces Faradaic efficiency in electrochemical approaches, typically limiting it to below 30% in most reported systems.
Scale-up challenges further complicate the transition from laboratory success to industrial implementation. Many catalysts that demonstrate promising activity in controlled laboratory settings fail to maintain performance when scaled to industrially relevant dimensions, often due to mass transfer limitations or heat management issues.
The geographical distribution of technical expertise shows concentration in specific regions, with North America leading in fundamental catalyst design patents, East Asia dominating in application-specific implementations, and Europe focusing on sustainable and green chemistry approaches to nitrogen reduction catalysis. This regional specialization has created distinct patent landscapes that companies must navigate when developing global intellectual property strategies.
Current Patent Strategies and Technical Solutions
01 Metal-based catalysts for nitrogen reduction
Various metal-based catalysts have been developed for nitrogen reduction processes. These catalysts typically contain transition metals such as iron, nickel, cobalt, or noble metals that facilitate the breaking of the strong nitrogen-nitrogen triple bond. The catalysts are often supported on carrier materials to increase surface area and stability. These metal-based systems are crucial for industrial nitrogen fixation processes and can operate under different conditions depending on their composition.- Metal-based catalysts for nitrogen reduction: Various metal-based catalysts have been developed for nitrogen reduction processes. These catalysts typically contain metals such as iron, nickel, cobalt, or noble metals that facilitate the breaking of the strong nitrogen-nitrogen triple bond. The catalysts are often supported on materials like alumina or silica to increase surface area and stability. These metal-based systems are effective in converting nitrogen to ammonia or other nitrogen compounds under specific reaction conditions.
- Zeolite and molecular sieve catalysts for NOx reduction: Zeolites and molecular sieves are used as catalysts or catalyst supports for nitrogen oxide (NOx) reduction. These materials have well-defined porous structures that provide shape selectivity and high surface area. They are often modified with metals or other active components to enhance their catalytic performance. These catalysts are particularly useful in selective catalytic reduction (SCR) processes for treating exhaust gases from vehicles and industrial sources.
- Composite and multi-component catalyst systems: Composite catalyst systems combine multiple active components to achieve enhanced nitrogen reduction performance. These systems often integrate different catalytic functions, such as oxidation and reduction capabilities, within a single catalyst formulation. The synergistic effects between components can lead to improved activity, selectivity, and stability. These multi-component catalysts are designed to operate effectively under varying conditions and to handle complex feed streams containing multiple nitrogen compounds.
- Electrochemical catalysts for nitrogen reduction: Electrochemical catalysts facilitate nitrogen reduction reactions through electrical energy input. These catalysts are designed to operate at ambient conditions, offering an alternative to the energy-intensive Haber-Bosch process. They typically consist of conductive materials with active sites that can adsorb and activate nitrogen molecules. Recent developments focus on improving the efficiency and selectivity of these catalysts to make electrochemical nitrogen fixation commercially viable for ammonia production.
- Catalyst preparation and activation methods: Various methods for preparing and activating nitrogen reduction catalysts have been developed to enhance their performance. These include precipitation techniques, impregnation methods, sol-gel processes, and hydrothermal synthesis. Post-synthesis treatments such as calcination, reduction, and chemical activation are often employed to optimize catalyst properties. The preparation conditions significantly influence the catalyst's structure, surface area, porosity, and distribution of active sites, which in turn affect its activity, selectivity, and stability in nitrogen reduction reactions.
02 Selective catalytic reduction (SCR) systems
Selective catalytic reduction technology employs specialized catalysts to convert nitrogen oxides (NOx) in exhaust gases into nitrogen and water. These systems typically use ammonia or urea as reducing agents and operate at specific temperature ranges to achieve optimal conversion efficiency. SCR catalysts are widely used in automotive applications, power plants, and industrial facilities to meet stringent emission standards and reduce environmental impact from nitrogen oxide pollutants.Expand Specific Solutions03 Zeolite and molecular sieve-based catalysts
Zeolites and molecular sieves serve as effective catalyst supports or direct catalysts for nitrogen reduction reactions. These materials feature well-defined porous structures that provide shape selectivity and high surface area. The controlled pore size and acidity of zeolites can be tailored to enhance catalytic performance in nitrogen conversion processes. These catalysts often incorporate metal ions within their framework or as exchanged cations to provide active sites for nitrogen reduction.Expand Specific Solutions04 Electrochemical nitrogen reduction catalysts
Electrochemical catalysts enable nitrogen reduction under ambient conditions through electrical energy input. These catalysts are designed to facilitate electron transfer to nitrogen molecules at electrode surfaces. Recent developments focus on improving selectivity, efficiency, and reducing the overpotential required for nitrogen reduction reactions. Electrochemical approaches offer promising pathways for sustainable ammonia production without the high temperature and pressure requirements of conventional processes.Expand Specific Solutions05 Novel composite and nanostructured catalysts
Advanced composite and nanostructured materials represent the cutting edge of nitrogen reduction catalyst technology. These catalysts often combine multiple active components in carefully designed architectures to enhance performance. Nanostructuring provides increased surface area, more accessible active sites, and improved mass transfer properties. Novel synthesis methods enable precise control over catalyst morphology, composition, and electronic properties, resulting in superior activity and selectivity for nitrogen conversion reactions.Expand Specific Solutions
Leading Companies and Research Institutions in Catalyst Innovation
The nitrogen reduction catalyst market is currently in a growth phase, characterized by increasing demand for sustainable ammonia production technologies. The market size is expanding due to rising interest in green ammonia and nitrogen-based fertilizers, with projections indicating significant growth over the next decade. Technologically, the field shows varying maturity levels, with established players like BASF SE and Johnson Matthey leading conventional catalyst development, while companies such as Shell, Umicore, and Cummins are advancing novel approaches. Research institutions including Columbia University, KAIST, and Argonne National Laboratory are pioneering fundamental breakthroughs, while automotive manufacturers (Honda, Hyundai, Ford) are exploring nitrogen reduction catalysts for emissions control applications, creating a competitive landscape spanning multiple industrial sectors.
BASF SE
Technical Solution: BASF has developed advanced nitrogen reduction catalysts based on their proprietary Cu-ZSM-5 zeolite technology. Their approach focuses on selective catalytic reduction (SCR) systems that efficiently convert nitrogen oxides into harmless nitrogen and water. BASF's catalysts incorporate rare earth metals and transition metal oxides to enhance performance at lower temperatures, achieving over 90% NOx conversion efficiency across a wide temperature window (200-500°C). The company has also pioneered ammonia slip catalysts (ASC) that work in tandem with SCR systems to prevent ammonia emissions. Their dual-layer catalyst design combines multiple active sites to optimize nitrogen reduction while minimizing precious metal content. BASF holds over 100 patents in this field, covering novel catalyst compositions, manufacturing processes, and system integration approaches.
Strengths: Industry-leading conversion efficiency across broad temperature ranges; robust performance in the presence of sulfur and water; excellent durability with minimal performance degradation over time. Weaknesses: Higher production costs compared to some competitors; requires precise control systems for optimal performance; some formulations have limited effectiveness at very low temperatures.
Umicore SA
Technical Solution: Umicore has developed proprietary nitrogen reduction catalyst technology centered on their advanced lean NOx trap (LNT) systems and selective catalytic reduction (SCR) solutions. Their patented approach combines platinum group metals (PGMs) with novel support materials to create highly active catalytic sites. Umicore's nitrogen reduction catalysts feature a multi-layered structure with precisely engineered porosity that maximizes surface area while optimizing gas flow dynamics. Their technology achieves nitrogen oxide conversion rates exceeding 95% under optimal conditions while demonstrating remarkable thermal stability up to 850°C. Umicore has secured strategic patents covering their washcoat formulations that incorporate cerium-zirconium mixed oxides and proprietary stabilizers to prevent catalyst sintering. Their catalyst systems are designed with reduced PGM loading (30-40% less than conventional catalysts) while maintaining equivalent or superior performance.
Strengths: Superior high-temperature stability; excellent resistance to catalyst poisoning; reduced precious metal content leading to cost advantages; comprehensive patent protection across manufacturing processes. Weaknesses: Performance can be compromised in low-temperature conditions; requires sophisticated control systems; some formulations show sensitivity to fuel sulfur content.
Critical Patent Analysis and Technological Breakthroughs
Catalyst and method for catalytic reduction of nitrogen oxides
PatentInactiveEP0788829B1
Innovation
- A method using a catalyst composed of silver aluminate supported on alumina, optionally with transition elements like W, Mo, or V, in the presence of hydrocarbons or oxygen-containing organic compounds, operating within specific temperature ranges to efficiently reduce nitrogen oxides without excessive oxidation or deactivation.
Catalyst and method for the catalytic reduction of nitrogen oxides
PatentInactiveUS20040043897A1
Innovation
- A method involving periodic rich/lean fuel supply excursions and using a catalyst composed of rhodium or palladium supported on zirconia, cerium oxide, praseodymium oxide, or neodymium oxide, which maintains high durability and activity even in the presence of oxygen, sulfur oxides, and water, with a wide temperature window.
Environmental Impact and Sustainability Considerations
The development of nitrogen reduction catalysts represents a critical frontier in addressing global environmental challenges, particularly those related to agricultural sustainability and industrial emissions. Patents in this domain not only secure intellectual property rights but also significantly influence environmental outcomes through the technologies they protect. The environmental impact of these catalysts extends far beyond their immediate application, affecting ecosystems, climate patterns, and human health.
Nitrogen reduction catalysts offer substantial environmental benefits by potentially reducing reliance on traditional Haber-Bosch ammonia synthesis, which currently consumes approximately 1-2% of global energy production and generates significant greenhouse gas emissions. Patents that protect more energy-efficient catalytic processes could contribute to reducing the carbon footprint of fertilizer production, addressing one of agriculture's most energy-intensive inputs.
Water pollution from nitrogen runoff represents another critical environmental concern that patented catalyst technologies may help mitigate. By enabling more precise nitrogen fixation or more efficient fertilizer formulations, these innovations could reduce excess nitrogen application, subsequently decreasing eutrophication in aquatic ecosystems and protecting biodiversity. The economic value of such environmental services, though often unquantified in patent valuations, represents a significant sustainability benefit.
Life cycle assessment (LCA) considerations are increasingly important in evaluating the true environmental impact of patented nitrogen reduction technologies. Patents that account for catalyst recyclability, reduced rare earth metal dependency, or decreased toxic byproduct formation demonstrate heightened environmental awareness and potentially greater long-term market value. Companies holding such patents may gain competitive advantages as regulatory frameworks increasingly prioritize sustainability metrics.
The circular economy potential of patented nitrogen reduction catalysts deserves particular attention. Innovations that enable nitrogen recovery from waste streams or atmospheric capture represent transformative opportunities for creating closed-loop systems. Such patents may become increasingly valuable as resource scarcity and environmental regulations intensify, potentially creating new market opportunities beyond traditional agricultural applications.
Climate change mitigation represents perhaps the most significant environmental consideration for nitrogen reduction catalyst patents. Technologies that reduce nitrous oxide emissions—a greenhouse gas approximately 300 times more potent than carbon dioxide—could qualify for carbon credits or other climate-related financial incentives, enhancing their market value while delivering substantial environmental benefits.
Nitrogen reduction catalysts offer substantial environmental benefits by potentially reducing reliance on traditional Haber-Bosch ammonia synthesis, which currently consumes approximately 1-2% of global energy production and generates significant greenhouse gas emissions. Patents that protect more energy-efficient catalytic processes could contribute to reducing the carbon footprint of fertilizer production, addressing one of agriculture's most energy-intensive inputs.
Water pollution from nitrogen runoff represents another critical environmental concern that patented catalyst technologies may help mitigate. By enabling more precise nitrogen fixation or more efficient fertilizer formulations, these innovations could reduce excess nitrogen application, subsequently decreasing eutrophication in aquatic ecosystems and protecting biodiversity. The economic value of such environmental services, though often unquantified in patent valuations, represents a significant sustainability benefit.
Life cycle assessment (LCA) considerations are increasingly important in evaluating the true environmental impact of patented nitrogen reduction technologies. Patents that account for catalyst recyclability, reduced rare earth metal dependency, or decreased toxic byproduct formation demonstrate heightened environmental awareness and potentially greater long-term market value. Companies holding such patents may gain competitive advantages as regulatory frameworks increasingly prioritize sustainability metrics.
The circular economy potential of patented nitrogen reduction catalysts deserves particular attention. Innovations that enable nitrogen recovery from waste streams or atmospheric capture represent transformative opportunities for creating closed-loop systems. Such patents may become increasingly valuable as resource scarcity and environmental regulations intensify, potentially creating new market opportunities beyond traditional agricultural applications.
Climate change mitigation represents perhaps the most significant environmental consideration for nitrogen reduction catalyst patents. Technologies that reduce nitrous oxide emissions—a greenhouse gas approximately 300 times more potent than carbon dioxide—could qualify for carbon credits or other climate-related financial incentives, enhancing their market value while delivering substantial environmental benefits.
IP Portfolio Management and Competitive Advantage
In the competitive landscape of nitrogen reduction catalyst development, effective IP portfolio management represents a critical strategic advantage. Companies that systematically build and leverage their patent portfolios can establish dominant market positions while creating significant barriers to entry for competitors. The strategic value of patents in this field extends beyond mere legal protection to become a fundamental business asset that drives innovation leadership and commercial success.
Patent portfolios in nitrogen reduction catalysis should be strategically constructed to cover multiple technological approaches and applications. Forward-thinking organizations typically develop layered IP strategies that protect core catalyst compositions, manufacturing processes, specific applications, and even potential future developments. This comprehensive approach ensures that competitors cannot easily circumvent patent protection through minor modifications or alternative implementations.
Cross-licensing agreements have emerged as a powerful strategic tool within the nitrogen reduction catalyst sector. Companies with complementary patent portfolios can negotiate mutually beneficial arrangements that expand their technological capabilities while reducing litigation risks. These agreements often facilitate collaborative innovation while maintaining competitive differentiation in specific market segments.
The geographic distribution of patent filings reveals important strategic considerations. Leading companies typically secure protection in major manufacturing hubs and emerging markets where nitrogen-based fertilizer demand is growing rapidly. This targeted approach optimizes protection while managing the substantial costs associated with maintaining global patent portfolios.
Patent analytics have become increasingly sophisticated tools for competitive intelligence in this field. By analyzing filing patterns, citation networks, and technological trajectories, companies can identify emerging competitors, anticipate technological shifts, and recognize potential acquisition targets with valuable IP assets. These insights inform both R&D priorities and broader business strategy decisions.
Freedom-to-operate (FTO) analyses represent another critical dimension of IP portfolio management. Before commercializing new catalyst technologies, companies must thoroughly assess whether their innovations might infringe existing patents. Proactive FTO strategies often include designing around potential obstacles, challenging questionable patents, or securing necessary licenses to avoid costly litigation.
The strategic value of patents extends to their role in attracting investment and partnership opportunities. Companies with robust IP portfolios in nitrogen reduction catalysis can leverage these assets to secure venture funding, establish joint ventures with larger industry players, or negotiate favorable terms in acquisition scenarios. This financial dimension underscores why systematic patent development should be integrated into broader business strategy rather than treated as a separate legal function.
Patent portfolios in nitrogen reduction catalysis should be strategically constructed to cover multiple technological approaches and applications. Forward-thinking organizations typically develop layered IP strategies that protect core catalyst compositions, manufacturing processes, specific applications, and even potential future developments. This comprehensive approach ensures that competitors cannot easily circumvent patent protection through minor modifications or alternative implementations.
Cross-licensing agreements have emerged as a powerful strategic tool within the nitrogen reduction catalyst sector. Companies with complementary patent portfolios can negotiate mutually beneficial arrangements that expand their technological capabilities while reducing litigation risks. These agreements often facilitate collaborative innovation while maintaining competitive differentiation in specific market segments.
The geographic distribution of patent filings reveals important strategic considerations. Leading companies typically secure protection in major manufacturing hubs and emerging markets where nitrogen-based fertilizer demand is growing rapidly. This targeted approach optimizes protection while managing the substantial costs associated with maintaining global patent portfolios.
Patent analytics have become increasingly sophisticated tools for competitive intelligence in this field. By analyzing filing patterns, citation networks, and technological trajectories, companies can identify emerging competitors, anticipate technological shifts, and recognize potential acquisition targets with valuable IP assets. These insights inform both R&D priorities and broader business strategy decisions.
Freedom-to-operate (FTO) analyses represent another critical dimension of IP portfolio management. Before commercializing new catalyst technologies, companies must thoroughly assess whether their innovations might infringe existing patents. Proactive FTO strategies often include designing around potential obstacles, challenging questionable patents, or securing necessary licenses to avoid costly litigation.
The strategic value of patents extends to their role in attracting investment and partnership opportunities. Companies with robust IP portfolios in nitrogen reduction catalysis can leverage these assets to secure venture funding, establish joint ventures with larger industry players, or negotiate favorable terms in acquisition scenarios. This financial dimension underscores why systematic patent development should be integrated into broader business strategy rather than treated as a separate legal function.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!