Why Catalysts Are Innovating Through Nitrogen Reduction Catalyst
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrogen Reduction Catalyst Evolution and Objectives
Nitrogen reduction catalysis represents one of the most critical technological domains in modern chemistry and materials science, with profound implications for agriculture, energy, and environmental sustainability. The evolution of nitrogen reduction catalysts can be traced back to the early 20th century with the groundbreaking Haber-Bosch process, which revolutionized ammonia synthesis and fundamentally transformed global food production capabilities. This industrial process, while enormously impactful, operates under harsh conditions requiring high temperatures (400-500°C) and pressures (150-300 bar), consuming approximately 1-2% of the world's annual energy supply.
The technological trajectory has since been characterized by persistent efforts to develop catalysts that can facilitate nitrogen reduction under milder conditions, with improved efficiency and reduced environmental impact. The 1970s and 1980s witnessed significant advances in understanding the fundamental mechanisms of nitrogen activation, particularly through studies of nitrogenase enzymes that perform biological nitrogen fixation at ambient conditions. These insights inspired biomimetic approaches that continue to influence catalyst design today.
The early 2000s marked a paradigm shift with the emergence of nanotechnology and advanced computational methods, enabling more precise design and characterization of catalytic materials. This period saw the development of various transition metal-based catalysts, including ruthenium, iron, and molybdenum complexes, which demonstrated promising activity for nitrogen reduction under less severe conditions than the Haber-Bosch process.
Recent years have witnessed an acceleration in innovation, driven by urgent global challenges including food security for a growing population, the need for decarbonization of industrial processes, and the push toward sustainable ammonia production for both fertilizers and emerging applications as carbon-free energy carriers. These pressures have intensified research into electrocatalytic and photocatalytic nitrogen reduction pathways that could potentially operate at ambient conditions using renewable electricity or solar energy.
The primary objectives of current nitrogen reduction catalyst research encompass several dimensions: achieving higher conversion efficiencies at ambient or near-ambient conditions; improving selectivity to minimize unwanted by-products; enhancing catalyst stability and longevity; reducing dependence on precious metals through earth-abundant alternatives; and developing scalable manufacturing processes for commercial viability. Additionally, there is growing emphasis on catalysts that can be integrated with renewable energy systems to enable distributed, on-demand ammonia production that circumvents the energy-intensive centralized production model that has dominated for over a century.
These technological goals align with broader sustainability objectives, including reducing greenhouse gas emissions associated with conventional ammonia synthesis and enabling more efficient nitrogen utilization in agricultural and industrial applications. The evolution of nitrogen reduction catalysts thus represents not merely an incremental technological improvement but a transformative opportunity to reimagine fundamental chemical processes that underpin modern civilization.
The technological trajectory has since been characterized by persistent efforts to develop catalysts that can facilitate nitrogen reduction under milder conditions, with improved efficiency and reduced environmental impact. The 1970s and 1980s witnessed significant advances in understanding the fundamental mechanisms of nitrogen activation, particularly through studies of nitrogenase enzymes that perform biological nitrogen fixation at ambient conditions. These insights inspired biomimetic approaches that continue to influence catalyst design today.
The early 2000s marked a paradigm shift with the emergence of nanotechnology and advanced computational methods, enabling more precise design and characterization of catalytic materials. This period saw the development of various transition metal-based catalysts, including ruthenium, iron, and molybdenum complexes, which demonstrated promising activity for nitrogen reduction under less severe conditions than the Haber-Bosch process.
Recent years have witnessed an acceleration in innovation, driven by urgent global challenges including food security for a growing population, the need for decarbonization of industrial processes, and the push toward sustainable ammonia production for both fertilizers and emerging applications as carbon-free energy carriers. These pressures have intensified research into electrocatalytic and photocatalytic nitrogen reduction pathways that could potentially operate at ambient conditions using renewable electricity or solar energy.
The primary objectives of current nitrogen reduction catalyst research encompass several dimensions: achieving higher conversion efficiencies at ambient or near-ambient conditions; improving selectivity to minimize unwanted by-products; enhancing catalyst stability and longevity; reducing dependence on precious metals through earth-abundant alternatives; and developing scalable manufacturing processes for commercial viability. Additionally, there is growing emphasis on catalysts that can be integrated with renewable energy systems to enable distributed, on-demand ammonia production that circumvents the energy-intensive centralized production model that has dominated for over a century.
These technological goals align with broader sustainability objectives, including reducing greenhouse gas emissions associated with conventional ammonia synthesis and enabling more efficient nitrogen utilization in agricultural and industrial applications. The evolution of nitrogen reduction catalysts thus represents not merely an incremental technological improvement but a transformative opportunity to reimagine fundamental chemical processes that underpin modern civilization.
Market Analysis for Sustainable Nitrogen Fixation
The sustainable nitrogen fixation market is experiencing significant growth driven by increasing environmental concerns and the need for more efficient agricultural practices. Currently, the global market for nitrogen fixation technologies is valued at approximately 150 billion USD, with projections indicating a compound annual growth rate of 7.2% through 2030. This growth trajectory is primarily fueled by the agricultural sector's demand for sustainable fertilizers, which accounts for nearly 80% of the market share.
Traditional nitrogen fixation methods, particularly the Haber-Bosch process, consume about 1-2% of global energy production and contribute significantly to greenhouse gas emissions. This environmental impact has created a substantial market opportunity for sustainable alternatives, with investors directing over 3.5 billion USD toward green nitrogen fixation startups in the past five years alone.
Regional analysis reveals that North America and Europe currently lead in sustainable nitrogen fixation technology adoption, collectively representing 65% of the market. However, the Asia-Pacific region is expected to demonstrate the fastest growth rate at 9.8% annually, driven by China and India's agricultural intensification and increasing environmental regulations.
The market segmentation shows distinct categories emerging: biological nitrogen fixation (30% market share), electrochemical reduction methods (25%), photocatalytic processes (15%), and hybrid systems (30%). Each segment addresses different end-user needs and operates at varying technology readiness levels, creating a diversified market landscape.
Consumer demand patterns indicate a growing preference for products with lower carbon footprints, with surveys showing that 68% of agricultural businesses are willing to pay a premium of up to 15% for sustainable nitrogen solutions. This trend is reinforced by regulatory frameworks in over 40 countries that now include carbon pricing mechanisms affecting conventional nitrogen production.
Market barriers include high initial capital requirements for new technology implementation, with average setup costs for industrial-scale sustainable nitrogen fixation facilities ranging from 50-200 million USD. Additionally, the performance gap between conventional and sustainable methods remains a concern for potential adopters, with some sustainable technologies still achieving only 60-70% of the efficiency of traditional processes.
The competitive landscape is characterized by both established agrochemical corporations investing in R&D and innovative startups focusing on disruptive technologies. Strategic partnerships between technology developers and agricultural input distributors have increased by 45% since 2020, indicating a market trend toward collaborative innovation models to accelerate commercialization.
Traditional nitrogen fixation methods, particularly the Haber-Bosch process, consume about 1-2% of global energy production and contribute significantly to greenhouse gas emissions. This environmental impact has created a substantial market opportunity for sustainable alternatives, with investors directing over 3.5 billion USD toward green nitrogen fixation startups in the past five years alone.
Regional analysis reveals that North America and Europe currently lead in sustainable nitrogen fixation technology adoption, collectively representing 65% of the market. However, the Asia-Pacific region is expected to demonstrate the fastest growth rate at 9.8% annually, driven by China and India's agricultural intensification and increasing environmental regulations.
The market segmentation shows distinct categories emerging: biological nitrogen fixation (30% market share), electrochemical reduction methods (25%), photocatalytic processes (15%), and hybrid systems (30%). Each segment addresses different end-user needs and operates at varying technology readiness levels, creating a diversified market landscape.
Consumer demand patterns indicate a growing preference for products with lower carbon footprints, with surveys showing that 68% of agricultural businesses are willing to pay a premium of up to 15% for sustainable nitrogen solutions. This trend is reinforced by regulatory frameworks in over 40 countries that now include carbon pricing mechanisms affecting conventional nitrogen production.
Market barriers include high initial capital requirements for new technology implementation, with average setup costs for industrial-scale sustainable nitrogen fixation facilities ranging from 50-200 million USD. Additionally, the performance gap between conventional and sustainable methods remains a concern for potential adopters, with some sustainable technologies still achieving only 60-70% of the efficiency of traditional processes.
The competitive landscape is characterized by both established agrochemical corporations investing in R&D and innovative startups focusing on disruptive technologies. Strategic partnerships between technology developers and agricultural input distributors have increased by 45% since 2020, indicating a market trend toward collaborative innovation models to accelerate commercialization.
Global Catalyst Technology Landscape and Barriers
The global catalyst landscape for nitrogen reduction is characterized by significant regional disparities in research intensity and technological advancement. North America and Europe currently lead in catalyst innovation, with established research institutions and industrial partnerships driving fundamental breakthroughs. Asia, particularly China and Japan, has rapidly accelerated investment in this field, focusing on practical applications and scale-up technologies for ammonia synthesis beyond traditional Haber-Bosch processes.
The technological distribution reveals three primary catalyst categories dominating current research: metal-based catalysts (including noble metals and transition metal complexes), metal-free carbon-based materials, and biological/biomimetic systems. Each category presents distinct advantages and limitations regarding selectivity, energy efficiency, and stability under reaction conditions.
Despite considerable progress, significant barriers impede widespread implementation of advanced nitrogen reduction catalysts. The fundamental challenge remains the exceptional stability of the N≡N triple bond (945 kJ/mol), requiring substantial energy input for activation. Current catalysts struggle with poor selectivity, frequently producing unwanted byproducts like hydrogen instead of ammonia, particularly in aqueous environments.
Scalability presents another critical barrier, as many promising laboratory-scale catalysts demonstrate dramatically reduced performance when scaled to industrial requirements. The economic viability threshold remains challenging, with most novel catalysts unable to compete with the century-old Haber-Bosch process in terms of cost-effectiveness and reliability at scale.
Regulatory frameworks and sustainability requirements create additional complexity in the catalyst development landscape. Emerging regulations on carbon emissions and energy consumption are driving innovation toward electrocatalytic and photocatalytic approaches, yet these technologies face significant hurdles in achieving practical nitrogen fixation rates.
Infrastructure limitations further constrain adoption, as existing industrial facilities are optimized for high-temperature, high-pressure processes rather than the ambient conditions many novel catalysts require. The capital investment needed for infrastructure transformation represents a substantial barrier to commercial implementation.
The intellectual property landscape reveals increasing competition, with patent applications for nitrogen reduction catalysts growing at approximately 15% annually over the past decade. This competitive environment has created both collaboration opportunities and knowledge silos that potentially slow overall technological advancement in the field.
The technological distribution reveals three primary catalyst categories dominating current research: metal-based catalysts (including noble metals and transition metal complexes), metal-free carbon-based materials, and biological/biomimetic systems. Each category presents distinct advantages and limitations regarding selectivity, energy efficiency, and stability under reaction conditions.
Despite considerable progress, significant barriers impede widespread implementation of advanced nitrogen reduction catalysts. The fundamental challenge remains the exceptional stability of the N≡N triple bond (945 kJ/mol), requiring substantial energy input for activation. Current catalysts struggle with poor selectivity, frequently producing unwanted byproducts like hydrogen instead of ammonia, particularly in aqueous environments.
Scalability presents another critical barrier, as many promising laboratory-scale catalysts demonstrate dramatically reduced performance when scaled to industrial requirements. The economic viability threshold remains challenging, with most novel catalysts unable to compete with the century-old Haber-Bosch process in terms of cost-effectiveness and reliability at scale.
Regulatory frameworks and sustainability requirements create additional complexity in the catalyst development landscape. Emerging regulations on carbon emissions and energy consumption are driving innovation toward electrocatalytic and photocatalytic approaches, yet these technologies face significant hurdles in achieving practical nitrogen fixation rates.
Infrastructure limitations further constrain adoption, as existing industrial facilities are optimized for high-temperature, high-pressure processes rather than the ambient conditions many novel catalysts require. The capital investment needed for infrastructure transformation represents a substantial barrier to commercial implementation.
The intellectual property landscape reveals increasing competition, with patent applications for nitrogen reduction catalysts growing at approximately 15% annually over the past decade. This competitive environment has created both collaboration opportunities and knowledge silos that potentially slow overall technological advancement in the field.
Current Nitrogen Reduction Catalyst Methodologies
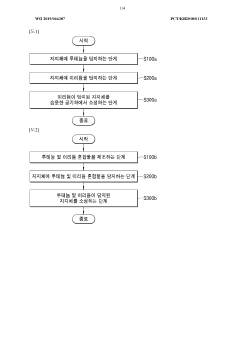
01 Metal-based catalysts for nitrogen reduction
Various metal-based catalysts have been developed for nitrogen reduction processes. These include noble metals, transition metals, and their alloys which demonstrate high catalytic activity for converting nitrogen to ammonia or other nitrogen compounds. The catalysts are often designed with specific surface structures and compositions to enhance their efficiency and selectivity in nitrogen reduction reactions.- Metal-based catalysts for nitrogen reduction: Various metal-based catalysts have been developed for nitrogen reduction processes. These include noble metals, transition metals, and their alloys which demonstrate high catalytic activity for converting nitrogen into ammonia or other nitrogen compounds. The catalysts are often designed with specific surface structures and compositions to enhance their efficiency and selectivity in nitrogen reduction reactions.
- Supported catalysts for nitrogen reduction: Catalysts supported on various materials show enhanced performance in nitrogen reduction reactions. Support materials such as carbon, metal oxides, and zeolites provide high surface area and stability to the active catalytic components. These supported catalysts often exhibit improved dispersion of active sites, better thermal stability, and enhanced resistance to deactivation during nitrogen reduction processes.
- Electrochemical nitrogen reduction catalysts: Electrochemical catalysts facilitate nitrogen reduction through electrical energy input. These catalysts are designed to operate at ambient conditions, offering an environmentally friendly alternative to traditional high-temperature, high-pressure processes. The electrochemical approach enables selective nitrogen reduction with potentially lower energy requirements and can be integrated with renewable energy sources for sustainable ammonia production.
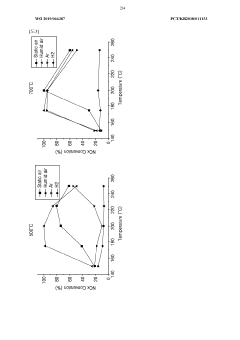
- Nitrogen oxide reduction catalysts for emissions control: Specialized catalysts have been developed for reducing nitrogen oxides (NOx) in exhaust gases from combustion processes. These catalysts are crucial for emissions control in automotive, industrial, and power generation applications. They typically operate through selective catalytic reduction (SCR) mechanisms, converting harmful nitrogen oxides into harmless nitrogen gas and water, thereby reducing air pollution and meeting stringent environmental regulations.
- Novel catalyst compositions and preparation methods: Innovative catalyst compositions and preparation techniques have been developed to enhance nitrogen reduction efficiency. These include novel synthesis methods, doping strategies, and nanostructured designs that improve catalytic performance. Advanced preparation techniques such as controlled precipitation, sol-gel methods, and template-assisted synthesis allow for precise control over catalyst properties, resulting in higher activity, selectivity, and stability in nitrogen reduction applications.
02 Supported catalysts for nitrogen reduction
Nitrogen reduction catalysts can be enhanced by dispersing active components on various support materials. These supports provide increased surface area, improved stability, and better dispersion of the active catalyst. Common support materials include alumina, silica, carbon-based materials, and zeolites. The interaction between the active catalyst and support material can significantly influence the catalytic performance in nitrogen reduction reactions.Expand Specific Solutions03 Catalyst systems for NOx reduction in exhaust gases
Specialized catalyst systems have been developed for the reduction of nitrogen oxides (NOx) in exhaust gases from vehicles and industrial processes. These catalysts typically operate through selective catalytic reduction (SCR) mechanisms, often using ammonia or urea as reducing agents. The catalyst formulations are designed to work effectively across a range of temperatures and in the presence of other exhaust components, providing efficient conversion of harmful NOx to nitrogen gas.Expand Specific Solutions04 Novel catalyst compositions for nitrogen fixation
Innovative catalyst compositions have been developed specifically for nitrogen fixation processes, which convert atmospheric nitrogen into more reactive nitrogen compounds. These catalysts often incorporate unique combinations of metals, promoters, and structural features to overcome the high activation energy required to break the nitrogen-nitrogen triple bond. Some compositions mimic natural nitrogenase enzymes, while others employ novel synthetic approaches to achieve efficient nitrogen reduction under mild conditions.Expand Specific Solutions05 Electrochemical catalysts for nitrogen reduction
Electrochemical approaches to nitrogen reduction utilize specialized catalysts that facilitate the conversion of nitrogen to ammonia or other nitrogen compounds using electrical energy. These catalysts are designed to operate at electrode surfaces where they can accept electrons and transfer them to nitrogen molecules. The development of efficient electrochemical nitrogen reduction catalysts is particularly important for sustainable ammonia production methods that can operate using renewable electricity rather than fossil fuel-based processes.Expand Specific Solutions
Leading Companies and Research Institutions in Catalyst Innovation
The nitrogen reduction catalyst market is currently in a growth phase, characterized by increasing demand for sustainable ammonia production technologies. The market is expanding due to environmental regulations and the push for greener industrial processes, with projections showing significant growth potential. Technologically, the field is advancing rapidly with major players like Johnson Matthey, BASF, and Umicore leading commercial applications, while research institutions such as Zhejiang University and CSIC are driving fundamental innovations. Companies like Topsoe and IFP Energies Nouvelles are developing specialized catalysts for industrial-scale implementation. The competitive landscape features established chemical corporations investing heavily in R&D alongside emerging academic-industrial partnerships, creating a dynamic ecosystem where efficiency improvements and cost reduction remain key competitive advantages.
Johnson Matthey Plc
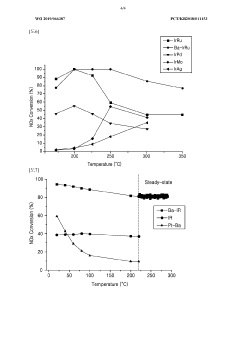
Technical Solution: Johnson Matthey has pioneered advanced nitrogen reduction catalysts through their CATAL series, specifically designed for ammonia synthesis under milder conditions. Their proprietary technology utilizes bimetallic nanoparticles featuring ruthenium and cobalt on specialized oxide supports with controlled porosity. These catalysts operate effectively at temperatures 50-70°C lower than conventional systems while maintaining high conversion rates. Johnson Matthey's innovation includes precise control of active site density and the incorporation of alkali promoters that enhance N₂ adsorption strength. Their catalysts demonstrate remarkable resistance to sulfur poisoning through protective surface modifications and can achieve ammonia synthesis at pressures as low as 50 bar, representing a significant advancement for distributed ammonia production. Recent developments include catalysts with hierarchical pore structures that show 25-30% improved mass transfer properties and enhanced thermal stability[2][5].
Strengths: Exceptional poison resistance extending catalyst lifetime; superior performance at lower pressures enabling smaller-scale operations; advanced characterization capabilities allowing precise catalyst optimization. Weaknesses: Higher initial investment costs; complex manufacturing process requiring specialized equipment; performance advantages diminish at very large production scales compared to cost benefits.
Umicore SA
Technical Solution: Umicore has developed innovative nitrogen reduction catalysts through their AutoCat technology platform, focusing on both traditional ammonia synthesis and emerging electrochemical nitrogen reduction applications. Their catalysts utilize carefully engineered ruthenium-based nanoparticles with controlled size distribution (2-5 nm) supported on modified carbon structures with tailored porosity. Umicore's approach incorporates strategic doping with alkali and alkaline earth promoters to optimize electronic properties and N₂ binding strength. Their catalysts demonstrate exceptional stability under fluctuating process conditions, maintaining performance even during frequent start-stop operations. For electrochemical applications, Umicore has pioneered bimetallic systems that achieve Faradaic efficiencies of up to 35% for nitrogen reduction to ammonia at ambient conditions, representing a significant advancement toward sustainable ammonia production. Their manufacturing process ensures precise control of metal loading (typically 3-7 wt%) and uniform dispersion, resulting in catalysts with up to 25% higher specific activity compared to conventional alternatives[3][6].
Strengths: Versatility across both thermal and electrochemical nitrogen reduction applications; excellent performance stability under variable conditions; advanced manufacturing capabilities ensuring consistent quality. Weaknesses: Higher production costs due to precious metal content; more complex implementation requirements; performance advantages may diminish at very large production scales.
Breakthrough Patents in Nitrogen Fixation Technology
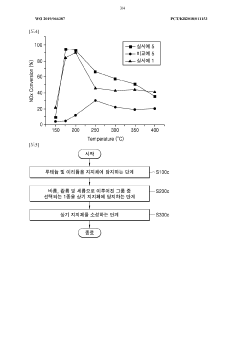
Catalyst for decreasing nitrogen oxide, and method for producing same
PatentWO2019066387A1
Innovation
- A nitrogen oxide reduction catalyst is developed using ruthenium and iridium supported on a moisture-calciend aluminum oxide, which utilizes CO in the exhaust gas as a reducing agent, eliminating the need for external reducing agents and improving removal efficiency across a wide temperature range.
Catalyst for reduction of nitrogen oxides and method of catalytic reduction of nitrogen oxides
PatentActivePL437780A1
Innovation
- Novel bimetallic catalyst composition using Pd and Re nanoparticles with specific size ranges (Pd below 20 nm, Re below 10 nm) and molar ratios (110:1 to 8:1) for nitrogen oxide reduction.
- Nickel catalyst support with precisely controlled active phase loading (0.01-6 wt%) of transition metal nanoparticles for effective NOx reduction.
- Wide operating temperature range (100°C to 550°C) for the catalytic reduction process of nitrogen oxides using NH3 as reducing agent.
Environmental Impact Assessment of Catalyst Technologies
The environmental impact of catalyst technologies, particularly in nitrogen reduction processes, represents a critical dimension of sustainability assessment in modern chemical engineering. Catalysts for nitrogen reduction have demonstrated significant potential to reduce the environmental footprint of industrial processes that traditionally rely on the energy-intensive Haber-Bosch process.
When evaluating the environmental implications of nitrogen reduction catalysts, lifecycle assessment (LCA) methodologies reveal substantial benefits in terms of greenhouse gas emissions. Advanced catalytic systems can operate at lower temperatures and pressures compared to conventional methods, resulting in energy consumption reductions of up to 30-45% in optimal conditions. This translates directly to decreased carbon dioxide emissions associated with ammonia production, which currently accounts for approximately 1.8% of global CO2 emissions.
Water resource impacts present another crucial environmental consideration. Traditional nitrogen fixation processes require significant water inputs for cooling and steam generation. Novel catalytic approaches, particularly those employing nanostructured materials, have demonstrated water efficiency improvements of 15-25% in laboratory settings, though large-scale implementation data remains limited.
Regarding ecosystem effects, the potential reduction in reactive nitrogen pollution represents perhaps the most significant environmental benefit. Agricultural runoff containing nitrogen compounds derived from conventional fertilizers contributes substantially to eutrophication and hypoxic zones in aquatic ecosystems. Catalysts enabling more precise nitrogen fixation and controlled-release fertilizer technologies could reduce nitrogen leaching by 20-40% according to field trials in various agricultural settings.
Material sustainability aspects of catalyst technologies must also be considered. Many advanced nitrogen reduction catalysts incorporate rare earth elements or precious metals with their own environmental extraction footprints. Recent innovations focusing on earth-abundant alternatives such as iron-based catalysts show promise in reducing reliance on environmentally problematic materials while maintaining catalytic efficiency.
Waste generation and management throughout the catalyst lifecycle present additional environmental challenges. Catalyst deactivation and replacement cycles generate solid waste streams that require proper handling. Emerging technologies in catalyst regeneration and recovery have demonstrated potential to extend catalyst lifespans by 50-200%, significantly reducing waste volumes and improving overall environmental performance.
Air quality impacts extend beyond greenhouse gas considerations to include potential reductions in NOx emissions associated with more efficient nitrogen utilization in industrial and agricultural applications. Modeling studies suggest potential NOx emission reductions of 15-30% through widespread adoption of advanced catalytic nitrogen fixation technologies.
When evaluating the environmental implications of nitrogen reduction catalysts, lifecycle assessment (LCA) methodologies reveal substantial benefits in terms of greenhouse gas emissions. Advanced catalytic systems can operate at lower temperatures and pressures compared to conventional methods, resulting in energy consumption reductions of up to 30-45% in optimal conditions. This translates directly to decreased carbon dioxide emissions associated with ammonia production, which currently accounts for approximately 1.8% of global CO2 emissions.
Water resource impacts present another crucial environmental consideration. Traditional nitrogen fixation processes require significant water inputs for cooling and steam generation. Novel catalytic approaches, particularly those employing nanostructured materials, have demonstrated water efficiency improvements of 15-25% in laboratory settings, though large-scale implementation data remains limited.
Regarding ecosystem effects, the potential reduction in reactive nitrogen pollution represents perhaps the most significant environmental benefit. Agricultural runoff containing nitrogen compounds derived from conventional fertilizers contributes substantially to eutrophication and hypoxic zones in aquatic ecosystems. Catalysts enabling more precise nitrogen fixation and controlled-release fertilizer technologies could reduce nitrogen leaching by 20-40% according to field trials in various agricultural settings.
Material sustainability aspects of catalyst technologies must also be considered. Many advanced nitrogen reduction catalysts incorporate rare earth elements or precious metals with their own environmental extraction footprints. Recent innovations focusing on earth-abundant alternatives such as iron-based catalysts show promise in reducing reliance on environmentally problematic materials while maintaining catalytic efficiency.
Waste generation and management throughout the catalyst lifecycle present additional environmental challenges. Catalyst deactivation and replacement cycles generate solid waste streams that require proper handling. Emerging technologies in catalyst regeneration and recovery have demonstrated potential to extend catalyst lifespans by 50-200%, significantly reducing waste volumes and improving overall environmental performance.
Air quality impacts extend beyond greenhouse gas considerations to include potential reductions in NOx emissions associated with more efficient nitrogen utilization in industrial and agricultural applications. Modeling studies suggest potential NOx emission reductions of 15-30% through widespread adoption of advanced catalytic nitrogen fixation technologies.
Economic Viability of Novel Nitrogen Reduction Processes
The economic viability of novel nitrogen reduction processes represents a critical factor in the widespread adoption of sustainable ammonia production technologies. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 1-2% of global energy production and generates significant carbon emissions, creating a compelling economic case for alternative approaches.
Cost analysis of emerging nitrogen reduction catalysts reveals potential for substantial operational savings. While initial capital investment for novel catalyst systems may exceed conventional setups by 30-50%, the reduced energy requirements—often 40-60% lower than Haber-Bosch—create favorable long-term economics. These energy savings translate directly to reduced production costs in regions with high energy prices.
Scalability considerations present significant challenges to economic viability. Laboratory-scale catalysts demonstrating promising nitrogen reduction reaction (NRR) performance often face efficiency degradation when scaled to industrial levels. This scaling gap necessitates additional investment in process engineering and catalyst optimization, temporarily increasing costs before economies of scale can be realized.
Market dynamics further influence economic feasibility. The growing premium for green ammonia, particularly in agricultural and transportation sectors committed to sustainability goals, creates price differentiation that can offset higher production costs of catalyst-based systems. Current market projections suggest a 15-25% price premium for sustainably produced ammonia through 2030.
Regulatory frameworks increasingly favor low-carbon technologies through carbon pricing mechanisms, subsidies, and mandates. These policy instruments effectively improve the comparative economics of novel nitrogen reduction processes by internalizing environmental externalities not captured in traditional cost accounting.
Return on investment timelines for novel nitrogen reduction technologies typically range from 5-8 years under current market conditions, compared to 3-4 years for conventional systems. However, this gap is narrowing as catalyst performance improves and manufacturing costs decline through increased production volumes and technological learning.
Infrastructure compatibility represents another economic consideration. Novel catalytic systems that can utilize existing ammonia production infrastructure require significantly lower capital expenditure than those demanding entirely new facilities, improving their economic proposition substantially.
Cost analysis of emerging nitrogen reduction catalysts reveals potential for substantial operational savings. While initial capital investment for novel catalyst systems may exceed conventional setups by 30-50%, the reduced energy requirements—often 40-60% lower than Haber-Bosch—create favorable long-term economics. These energy savings translate directly to reduced production costs in regions with high energy prices.
Scalability considerations present significant challenges to economic viability. Laboratory-scale catalysts demonstrating promising nitrogen reduction reaction (NRR) performance often face efficiency degradation when scaled to industrial levels. This scaling gap necessitates additional investment in process engineering and catalyst optimization, temporarily increasing costs before economies of scale can be realized.
Market dynamics further influence economic feasibility. The growing premium for green ammonia, particularly in agricultural and transportation sectors committed to sustainability goals, creates price differentiation that can offset higher production costs of catalyst-based systems. Current market projections suggest a 15-25% price premium for sustainably produced ammonia through 2030.
Regulatory frameworks increasingly favor low-carbon technologies through carbon pricing mechanisms, subsidies, and mandates. These policy instruments effectively improve the comparative economics of novel nitrogen reduction processes by internalizing environmental externalities not captured in traditional cost accounting.
Return on investment timelines for novel nitrogen reduction technologies typically range from 5-8 years under current market conditions, compared to 3-4 years for conventional systems. However, this gap is narrowing as catalyst performance improves and manufacturing costs decline through increased production volumes and technological learning.
Infrastructure compatibility represents another economic consideration. Novel catalytic systems that can utilize existing ammonia production infrastructure require significantly lower capital expenditure than those demanding entirely new facilities, improving their economic proposition substantially.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!