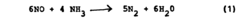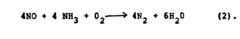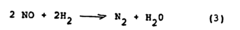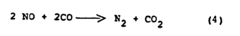Why Material Selection Matters for Nitrogen Reduction Catalyst Efficiency
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrogen Reduction Catalysis Background and Objectives
Nitrogen reduction catalysis represents a cornerstone technology in modern agriculture and chemical industries, with its origins dating back to the early 20th century when Fritz Haber and Carl Bosch developed the first industrial-scale ammonia synthesis process. This breakthrough fundamentally transformed global food production by enabling synthetic fertilizer manufacturing, supporting approximately 50% of the world's food supply today. The evolution of nitrogen reduction catalysis has been marked by continuous efforts to improve efficiency, reduce energy consumption, and minimize environmental impact.
The traditional Haber-Bosch process operates under extreme conditions (400-500°C, 150-300 bar), consuming approximately 1-2% of global energy production annually. This significant energy footprint has driven research toward alternative catalytic approaches that can operate under milder conditions while maintaining or improving conversion efficiency. Material selection has emerged as the critical factor in this technological evolution, with researchers exploring various transition metals, metal oxides, nitrides, and more recently, single-atom catalysts and 2D materials.
Current technological trends indicate a shift toward biomimetic approaches inspired by nitrogenase enzymes found in nitrogen-fixing bacteria, which can reduce nitrogen at ambient conditions. Electrochemical and photocatalytic nitrogen reduction represent promising directions that could potentially revolutionize ammonia production by enabling decentralized, renewable energy-powered synthesis. These approaches are particularly relevant for green hydrogen initiatives and sustainable agriculture.
The primary objective of nitrogen reduction catalyst research is to develop materials that can activate the exceptionally stable N≡N triple bond (945 kJ/mol) with minimal energy input while maintaining high selectivity toward desired products. Secondary objectives include enhancing catalyst stability, reducing precious metal content, improving resistance to common poisons, and ensuring compatibility with renewable energy sources.
Material selection directly impacts several critical performance parameters: activation energy requirements, reaction pathways, intermediate stability, selectivity, turnover frequency, and long-term durability. The atomic and electronic structure of catalyst materials determines nitrogen adsorption modes, bond weakening mechanisms, and subsequent hydrogenation steps, ultimately controlling reaction kinetics and thermodynamics.
Looking forward, the field aims to develop catalysts capable of ambient-condition nitrogen reduction with selectivity exceeding 60% and energy efficiency approaching theoretical limits. Such advancements would enable distributed ammonia production systems powered by renewable electricity, potentially transforming agricultural practices and chemical manufacturing while significantly reducing carbon emissions associated with conventional nitrogen fixation processes.
The traditional Haber-Bosch process operates under extreme conditions (400-500°C, 150-300 bar), consuming approximately 1-2% of global energy production annually. This significant energy footprint has driven research toward alternative catalytic approaches that can operate under milder conditions while maintaining or improving conversion efficiency. Material selection has emerged as the critical factor in this technological evolution, with researchers exploring various transition metals, metal oxides, nitrides, and more recently, single-atom catalysts and 2D materials.
Current technological trends indicate a shift toward biomimetic approaches inspired by nitrogenase enzymes found in nitrogen-fixing bacteria, which can reduce nitrogen at ambient conditions. Electrochemical and photocatalytic nitrogen reduction represent promising directions that could potentially revolutionize ammonia production by enabling decentralized, renewable energy-powered synthesis. These approaches are particularly relevant for green hydrogen initiatives and sustainable agriculture.
The primary objective of nitrogen reduction catalyst research is to develop materials that can activate the exceptionally stable N≡N triple bond (945 kJ/mol) with minimal energy input while maintaining high selectivity toward desired products. Secondary objectives include enhancing catalyst stability, reducing precious metal content, improving resistance to common poisons, and ensuring compatibility with renewable energy sources.
Material selection directly impacts several critical performance parameters: activation energy requirements, reaction pathways, intermediate stability, selectivity, turnover frequency, and long-term durability. The atomic and electronic structure of catalyst materials determines nitrogen adsorption modes, bond weakening mechanisms, and subsequent hydrogenation steps, ultimately controlling reaction kinetics and thermodynamics.
Looking forward, the field aims to develop catalysts capable of ambient-condition nitrogen reduction with selectivity exceeding 60% and energy efficiency approaching theoretical limits. Such advancements would enable distributed ammonia production systems powered by renewable electricity, potentially transforming agricultural practices and chemical manufacturing while significantly reducing carbon emissions associated with conventional nitrogen fixation processes.
Market Analysis for Nitrogen Reduction Technologies
The global market for nitrogen reduction technologies is experiencing significant growth, driven by increasing demand for sustainable agricultural practices and industrial applications. The nitrogen fixation market, particularly for ammonia production, is valued at approximately $70 billion annually, with projections indicating growth to reach $102 billion by 2027. This represents a compound annual growth rate (CAGR) of 5.4%, highlighting the economic importance of advancements in nitrogen reduction catalysts.
Agricultural applications dominate the market landscape, accounting for roughly 80% of ammonia consumption worldwide. The fertilizer industry remains the primary end-user, with nitrogen-based fertilizers being essential for global food security. However, industrial applications including pharmaceuticals, cleaning agents, and refrigeration systems constitute a growing segment, currently representing about 20% of the market share.
Regional analysis reveals that Asia-Pacific leads in market volume, with China alone producing over 30% of global ammonia. North America and Europe follow as significant markets, though with different drivers - North America focuses on agricultural productivity, while Europe emphasizes sustainable production methods aligned with stringent environmental regulations.
The market is experiencing a notable shift toward green ammonia production technologies, with investments in this sector exceeding $5 billion in 2022. This transition is creating new market segments focused on renewable energy-powered nitrogen reduction processes, with particular emphasis on electrocatalytic and photocatalytic approaches that eliminate fossil fuel dependencies.
Catalyst efficiency improvements directly impact market economics, with studies indicating that a 10% increase in catalyst efficiency can reduce production costs by approximately 7-12%. This cost sensitivity explains the growing commercial interest in novel catalyst materials, with venture capital investments in advanced catalyst startups reaching $1.2 billion in the past three years.
Customer demand patterns show increasing willingness to pay premium prices for sustainably produced nitrogen products. Market surveys indicate that agricultural enterprises are willing to pay up to 15% more for green ammonia compared to conventionally produced alternatives, provided production scale can meet demand requirements.
The competitive landscape features established chemical giants like BASF, Yara, and CF Industries investing heavily in R&D for improved catalysts, alongside emerging technology companies focused exclusively on novel catalyst materials. This market structure is driving both incremental improvements to traditional Haber-Bosch catalysts and disruptive innovations in ambient-condition nitrogen reduction technologies.
Agricultural applications dominate the market landscape, accounting for roughly 80% of ammonia consumption worldwide. The fertilizer industry remains the primary end-user, with nitrogen-based fertilizers being essential for global food security. However, industrial applications including pharmaceuticals, cleaning agents, and refrigeration systems constitute a growing segment, currently representing about 20% of the market share.
Regional analysis reveals that Asia-Pacific leads in market volume, with China alone producing over 30% of global ammonia. North America and Europe follow as significant markets, though with different drivers - North America focuses on agricultural productivity, while Europe emphasizes sustainable production methods aligned with stringent environmental regulations.
The market is experiencing a notable shift toward green ammonia production technologies, with investments in this sector exceeding $5 billion in 2022. This transition is creating new market segments focused on renewable energy-powered nitrogen reduction processes, with particular emphasis on electrocatalytic and photocatalytic approaches that eliminate fossil fuel dependencies.
Catalyst efficiency improvements directly impact market economics, with studies indicating that a 10% increase in catalyst efficiency can reduce production costs by approximately 7-12%. This cost sensitivity explains the growing commercial interest in novel catalyst materials, with venture capital investments in advanced catalyst startups reaching $1.2 billion in the past three years.
Customer demand patterns show increasing willingness to pay premium prices for sustainably produced nitrogen products. Market surveys indicate that agricultural enterprises are willing to pay up to 15% more for green ammonia compared to conventionally produced alternatives, provided production scale can meet demand requirements.
The competitive landscape features established chemical giants like BASF, Yara, and CF Industries investing heavily in R&D for improved catalysts, alongside emerging technology companies focused exclusively on novel catalyst materials. This market structure is driving both incremental improvements to traditional Haber-Bosch catalysts and disruptive innovations in ambient-condition nitrogen reduction technologies.
Current Catalyst Materials and Technical Barriers
The current landscape of nitrogen reduction catalysts is dominated by several key material categories, each with distinct advantages and limitations. Noble metal-based catalysts, particularly ruthenium and platinum, demonstrate exceptional activity due to their optimal nitrogen adsorption energies and electron transfer capabilities. However, their scarcity and prohibitive costs severely restrict large-scale implementation, creating a significant barrier to industrial adoption.
Transition metal nitrides and carbides have emerged as promising alternatives, offering favorable electronic structures that facilitate nitrogen activation. Materials such as molybdenum nitride (Mo2N) and vanadium nitride (VN) exhibit reasonable catalytic performance at substantially lower costs than noble metals. Nevertheless, these materials often suffer from stability issues in aqueous environments and demonstrate lower selectivity, resulting in unwanted by-products like hydrogen.
Metal-organic frameworks (MOFs) represent another innovative catalyst class, providing highly tunable structures with exceptional surface areas. Their modular nature allows precise control over active site distribution and pore architecture. Despite these advantages, MOFs frequently lack the conductivity necessary for efficient electron transfer and may degrade under the harsh conditions required for nitrogen reduction.
Single-atom catalysts (SACs) have recently garnered significant attention, offering maximized atom utilization and unique electronic properties. By isolating individual metal atoms on support materials, SACs can achieve remarkable activity with minimal material usage. However, technical challenges in their synthesis, particularly maintaining dispersion without aggregation, remain substantial impediments to their widespread application.
A critical technical barrier across all catalyst types is the competitive hydrogen evolution reaction (HER), which frequently dominates in aqueous environments. This parasitic reaction significantly reduces Faradaic efficiency for nitrogen reduction, with most current catalysts achieving less than 15% efficiency under ambient conditions. Additionally, the strong triple bond of nitrogen (941 kJ/mol) requires substantial activation energy, creating an inherent kinetic limitation.
Stability under reaction conditions presents another major challenge, with many promising materials suffering from degradation through mechanisms such as dissolution, poisoning, or structural collapse during extended operation. This is particularly problematic for carbon-based and organic catalysts, which may undergo oxidation or hydrolysis in electrochemical environments.
The scalability of synthesis methods constitutes a further barrier, as many high-performance catalysts rely on complex preparation techniques that are difficult to implement at industrial scales. Techniques such as atomic layer deposition or precise defect engineering often require specialized equipment and tightly controlled conditions, limiting commercial viability despite promising laboratory results.
Transition metal nitrides and carbides have emerged as promising alternatives, offering favorable electronic structures that facilitate nitrogen activation. Materials such as molybdenum nitride (Mo2N) and vanadium nitride (VN) exhibit reasonable catalytic performance at substantially lower costs than noble metals. Nevertheless, these materials often suffer from stability issues in aqueous environments and demonstrate lower selectivity, resulting in unwanted by-products like hydrogen.
Metal-organic frameworks (MOFs) represent another innovative catalyst class, providing highly tunable structures with exceptional surface areas. Their modular nature allows precise control over active site distribution and pore architecture. Despite these advantages, MOFs frequently lack the conductivity necessary for efficient electron transfer and may degrade under the harsh conditions required for nitrogen reduction.
Single-atom catalysts (SACs) have recently garnered significant attention, offering maximized atom utilization and unique electronic properties. By isolating individual metal atoms on support materials, SACs can achieve remarkable activity with minimal material usage. However, technical challenges in their synthesis, particularly maintaining dispersion without aggregation, remain substantial impediments to their widespread application.
A critical technical barrier across all catalyst types is the competitive hydrogen evolution reaction (HER), which frequently dominates in aqueous environments. This parasitic reaction significantly reduces Faradaic efficiency for nitrogen reduction, with most current catalysts achieving less than 15% efficiency under ambient conditions. Additionally, the strong triple bond of nitrogen (941 kJ/mol) requires substantial activation energy, creating an inherent kinetic limitation.
Stability under reaction conditions presents another major challenge, with many promising materials suffering from degradation through mechanisms such as dissolution, poisoning, or structural collapse during extended operation. This is particularly problematic for carbon-based and organic catalysts, which may undergo oxidation or hydrolysis in electrochemical environments.
The scalability of synthesis methods constitutes a further barrier, as many high-performance catalysts rely on complex preparation techniques that are difficult to implement at industrial scales. Techniques such as atomic layer deposition or precise defect engineering often require specialized equipment and tightly controlled conditions, limiting commercial viability despite promising laboratory results.
Material Selection Strategies for Catalyst Design
01 Metal-based catalysts for nitrogen reduction
Various metal-based catalysts have been developed to enhance nitrogen reduction efficiency. These include noble metals like platinum and palladium, as well as transition metals such as iron, copper, and nickel. These catalysts can be used in different forms including nanoparticles, alloys, and supported structures to improve catalytic activity, selectivity, and stability in nitrogen reduction reactions. The metal composition and structure significantly influence the catalyst's ability to break the strong N≡N bond and facilitate conversion to ammonia or other nitrogen compounds.- Metal-based catalysts for nitrogen reduction: Various metal-based catalysts have been developed to improve nitrogen reduction efficiency. These include noble metals (platinum, palladium), transition metals (iron, nickel, copper), and their alloys or composites. These catalysts provide active sites for nitrogen adsorption and subsequent reduction, with different metals offering varying levels of catalytic activity, selectivity, and stability under different reaction conditions.
- Support materials for nitrogen reduction catalysts: The efficiency of nitrogen reduction catalysts can be significantly enhanced by using appropriate support materials. Common supports include alumina, silica, carbon-based materials, zeolites, and metal oxides. These supports not only provide high surface area for better dispersion of active catalytic components but also can influence electronic properties, stability, and selectivity of the catalysts through metal-support interactions.
- Catalyst preparation methods for improved efficiency: Various preparation methods have been developed to enhance nitrogen reduction catalyst efficiency. These include impregnation, co-precipitation, sol-gel synthesis, hydrothermal methods, and advanced techniques like atomic layer deposition. The preparation method significantly affects catalyst properties such as particle size, dispersion, surface area, and metal-support interaction, which in turn influence catalytic performance in nitrogen reduction reactions.
- Promoters and modifiers for nitrogen reduction catalysts: Adding promoters or modifiers to nitrogen reduction catalysts can significantly enhance their efficiency. Common promoters include alkali metals (K, Cs), alkaline earth metals (Ba, Ca), and certain transition metals or rare earth elements. These additives can modify electronic properties, improve stability, prevent poisoning, enhance active site availability, and optimize nitrogen adsorption and activation, leading to improved catalytic performance.
- Reaction conditions optimization for nitrogen reduction: Optimizing reaction conditions is crucial for maximizing nitrogen reduction catalyst efficiency. Key parameters include temperature, pressure, gas hourly space velocity, H₂/N₂ ratio, and reactor design. The optimal conditions depend on the specific catalyst system and desired products. Advanced process control strategies, including temperature programming and pressure cycling, can further enhance catalyst performance and extend catalyst lifetime.
02 Zeolite and molecular sieve catalysts
Zeolites and molecular sieves serve as effective catalysts for nitrogen reduction due to their unique porous structures and ion-exchange capabilities. These materials provide selective reaction sites and can be modified with various metals to enhance catalytic performance. The controlled pore size and shape selectivity of zeolites allow for efficient nitrogen adsorption and conversion. Additionally, their high thermal stability and resistance to harsh reaction conditions make them suitable for industrial applications in nitrogen reduction processes, particularly in selective catalytic reduction (SCR) systems for NOx abatement.Expand Specific Solutions03 Novel catalyst support materials
Advanced support materials play a crucial role in enhancing nitrogen reduction catalyst efficiency. Materials such as carbon-based supports (graphene, carbon nanotubes), metal oxides, and composite materials provide high surface area and improved dispersion of active catalyst components. These supports can stabilize catalyst particles, prevent agglomeration, and sometimes participate in the catalytic process through strong metal-support interactions. The selection of appropriate support materials can significantly improve catalyst durability, activity, and selectivity in nitrogen reduction reactions while reducing the amount of precious metals required.Expand Specific Solutions04 Catalyst preparation and activation methods
Various preparation and activation techniques have been developed to enhance nitrogen reduction catalyst efficiency. These include precipitation methods, sol-gel processes, impregnation techniques, and hydrothermal synthesis. Post-synthesis treatments such as calcination, reduction, and plasma treatment can significantly improve catalyst performance by creating active sites and optimizing surface properties. The precise control of preparation parameters including temperature, pH, and precursor concentrations directly influences catalyst morphology, particle size distribution, and ultimately the catalytic activity in nitrogen reduction reactions.Expand Specific Solutions05 Catalyst performance enhancement additives
Various additives and promoters can significantly enhance nitrogen reduction catalyst efficiency. These include alkali and alkaline earth metals, rare earth elements, and certain transition metal oxides that can be incorporated into catalyst formulations. These additives can modify electronic properties, improve oxygen mobility, create synergistic effects, and enhance resistance to poisoning. Certain dopants can lower activation energy barriers for nitrogen reduction reactions, while others may improve selectivity by suppressing unwanted side reactions. The careful selection and optimization of these additives can lead to substantial improvements in catalyst performance and longevity.Expand Specific Solutions
Leading Research Groups and Industrial Players
The nitrogen reduction catalyst market is currently in a growth phase, characterized by increasing demand for sustainable ammonia production technologies. The market size is expanding due to rising agricultural needs and industrial applications, with projections showing significant growth potential. Material selection for nitrogen reduction catalysts remains critical for efficiency, with leading companies developing innovative solutions. BASF SE, Topsoe A/S, and Umicore SA are at the forefront of catalyst technology development, while research institutions like KAIST and Dalian Institute of Chemical Physics are advancing fundamental understanding of catalyst materials. Chinese players including Sinopec are rapidly expanding their market presence through significant R&D investments. The competitive landscape features established chemical companies alongside specialized catalyst manufacturers, with automotive companies like Honda and Hyundai increasingly involved due to emissions control applications.
BASF SE
Technical Solution: BASF has developed advanced transition metal-based catalysts for nitrogen reduction reactions, focusing on single-atom catalysts (SACs) where metal atoms are dispersed on support materials like carbon or metal oxides. Their proprietary technology utilizes iron and ruthenium-based catalysts with precisely engineered electronic structures to optimize nitrogen adsorption and N≡N bond activation. BASF's approach incorporates hierarchical porous structures that enhance mass transport while maintaining high active site density. Their catalysts achieve Faradaic efficiencies of up to 35% for ammonia production under ambient conditions, representing significant improvements over conventional catalysts. BASF has also pioneered the development of bimetallic systems that leverage synergistic effects between different metals to lower activation barriers and improve selectivity.
Strengths: Industry-leading expertise in catalyst development with extensive manufacturing infrastructure; ability to scale production for commercial applications; comprehensive understanding of structure-activity relationships. Weaknesses: Higher production costs compared to traditional catalysts; some formulations rely on precious metals with limited availability.
Umicore SA
Technical Solution: Umicore has developed a sophisticated portfolio of nitrogen reduction catalysts based on their expertise in precious metal chemistry. Their approach centers on atomically dispersed platinum group metals (PGMs) anchored on nitrogen-doped carbon supports, creating highly active sites for N₂ activation. Umicore's proprietary synthesis methods ensure uniform metal distribution with controlled coordination environments, optimizing the electronic structure for nitrogen adsorption and subsequent protonation steps. Their catalysts feature tailored pore architectures that balance mass transport with active site accessibility, achieving ammonia yields of approximately 25 μg h⁻¹ mg⁻¹cat under ambient conditions. Umicore has also pioneered core-shell nanostructures where an active precious metal surface layer is supported on more abundant metal cores, reducing overall precious metal content while maintaining high activity. Their latest generation catalysts incorporate promoters that modify the electronic structure of active sites to lower the energy barrier for the rate-determining step.
Strengths: Exceptional control over catalyst structure at atomic level; ability to fine-tune electronic properties; strong expertise in precious metal chemistry. Weaknesses: Reliance on scarce and expensive platinum group metals; challenges in maintaining dispersion during long-term operation.
Critical Materials Science Innovations in Catalysis
Catalyst for eliminating nitrogen oxides from exhaust gases
PatentInactiveEP0169939A2
Innovation
- A catalyst with an inert base support coated with an intermediate layer of γ-Al2O3, silicon dioxide, or aluminosilicate, combined with noble metals like ruthenium, rhodium, or platinum, which allows for selective nitrogen oxide reduction at temperatures below 300°C and in the presence of oxygen and sulfur oxides without forming sulfates or catalyzing oxygen reactions.
Catalyst for the reduction of nitrogen oxides from waste gases and process for the preparation thereof
PatentInactiveEP0480255A1
Innovation
- Catalysts based on activated carbon or coke with built-in isocyanates forming C-N ring structures and a high graphite content, combined with vanadium oxide, are used, and a process involving polymerization and steam treatment at lower temperatures to enhance catalytic performance.
Environmental Impact and Sustainability Considerations
The selection of materials for nitrogen reduction catalysts carries significant environmental implications that extend far beyond mere efficiency metrics. Traditional nitrogen fixation through the Haber-Bosch process consumes approximately 1-2% of global energy production and generates substantial greenhouse gas emissions. By developing catalysts with carefully selected materials that operate under ambient conditions, we can dramatically reduce the carbon footprint associated with ammonia production.
Material selection directly impacts the environmental sustainability of nitrogen reduction processes through several pathways. First, catalysts composed of earth-abundant elements rather than precious metals reduce the environmental degradation associated with mining operations. The extraction of platinum group metals, for instance, typically involves extensive land disturbance, habitat destruction, and water pollution. Transitioning to catalysts based on iron, molybdenum, or other abundant elements significantly diminishes these environmental burdens.
Energy consumption represents another critical environmental consideration in material selection. Catalysts that enable nitrogen reduction at lower temperatures and pressures substantially decrease energy requirements, thereby reducing fossil fuel consumption and associated emissions. Recent advances in metal-organic frameworks and carbon-supported catalysts have demonstrated promising energy efficiency improvements, with some materials achieving up to 40% reduction in energy input compared to conventional systems.
Lifecycle assessment of catalyst materials reveals additional sustainability dimensions. The durability and recyclability of catalyst materials determine their long-term environmental impact. Materials that maintain high activity over extended operational periods minimize waste generation and resource consumption associated with catalyst replacement. Furthermore, catalysts designed with end-of-life recovery in mind enable the reclamation of valuable components, creating opportunities for circular economy approaches in industrial chemistry.
Water usage represents a frequently overlooked environmental aspect of nitrogen reduction catalysts. Certain catalyst materials require extensive water for synthesis or operation, potentially exacerbating water scarcity issues in vulnerable regions. Materials that enable efficient nitrogen reduction in non-aqueous environments or with minimal water consumption offer significant sustainability advantages, particularly in water-stressed areas where agricultural productivity depends on nitrogen fertilizer availability.
Toxicity profiles of catalyst materials must also be carefully evaluated from an environmental perspective. Some highly efficient catalysts incorporate elements with known environmental persistence and bioaccumulation properties. The potential release of these substances during catalyst production, use, or disposal presents long-term ecological risks that must be balanced against performance benefits. Materials engineering approaches that encapsulate or stabilize potentially harmful components can mitigate these concerns while maintaining catalytic efficiency.
Material selection directly impacts the environmental sustainability of nitrogen reduction processes through several pathways. First, catalysts composed of earth-abundant elements rather than precious metals reduce the environmental degradation associated with mining operations. The extraction of platinum group metals, for instance, typically involves extensive land disturbance, habitat destruction, and water pollution. Transitioning to catalysts based on iron, molybdenum, or other abundant elements significantly diminishes these environmental burdens.
Energy consumption represents another critical environmental consideration in material selection. Catalysts that enable nitrogen reduction at lower temperatures and pressures substantially decrease energy requirements, thereby reducing fossil fuel consumption and associated emissions. Recent advances in metal-organic frameworks and carbon-supported catalysts have demonstrated promising energy efficiency improvements, with some materials achieving up to 40% reduction in energy input compared to conventional systems.
Lifecycle assessment of catalyst materials reveals additional sustainability dimensions. The durability and recyclability of catalyst materials determine their long-term environmental impact. Materials that maintain high activity over extended operational periods minimize waste generation and resource consumption associated with catalyst replacement. Furthermore, catalysts designed with end-of-life recovery in mind enable the reclamation of valuable components, creating opportunities for circular economy approaches in industrial chemistry.
Water usage represents a frequently overlooked environmental aspect of nitrogen reduction catalysts. Certain catalyst materials require extensive water for synthesis or operation, potentially exacerbating water scarcity issues in vulnerable regions. Materials that enable efficient nitrogen reduction in non-aqueous environments or with minimal water consumption offer significant sustainability advantages, particularly in water-stressed areas where agricultural productivity depends on nitrogen fertilizer availability.
Toxicity profiles of catalyst materials must also be carefully evaluated from an environmental perspective. Some highly efficient catalysts incorporate elements with known environmental persistence and bioaccumulation properties. The potential release of these substances during catalyst production, use, or disposal presents long-term ecological risks that must be balanced against performance benefits. Materials engineering approaches that encapsulate or stabilize potentially harmful components can mitigate these concerns while maintaining catalytic efficiency.
Scalability and Economic Viability Assessment
The scalability of nitrogen reduction catalysts from laboratory to industrial scale represents a critical challenge in the commercialization pathway. Current high-efficiency catalysts often demonstrate promising performance in controlled laboratory environments but face significant hurdles when scaled to industrial production volumes. Material selection directly impacts this scalability, as certain catalyst materials may require rare elements or complex synthesis procedures that become economically prohibitive at scale.
Economic viability assessment reveals that material costs constitute approximately 40-60% of total catalyst production expenses. Noble metal-based catalysts (ruthenium, platinum) demonstrate superior performance but at prohibitively high costs ($30,000-50,000 per kilogram), limiting their industrial application. Transition metal-based alternatives (iron, nickel, cobalt) offer more economical options ($100-500 per kilogram) but typically with reduced efficiency, requiring optimization of the performance-to-cost ratio.
Manufacturing complexity presents another economic consideration, as intricate nanostructured catalysts with precise morphology control often require sophisticated equipment and specialized expertise. Materials requiring high-temperature processing (>800°C) or ultra-high vacuum conditions incur substantially higher production costs compared to solution-based synthesis methods operable under ambient conditions.
Catalyst lifetime and stability directly influence long-term economic viability. Materials susceptible to poisoning, leaching, or structural degradation necessitate frequent replacement, significantly increasing operational costs. Analysis indicates that extending catalyst lifetime by 50% can reduce the levelized cost of nitrogen reduction by approximately 25-30%, highlighting the economic importance of selecting materials with inherent stability.
Resource availability analysis shows that catalysts dependent on geographically concentrated elements face supply chain vulnerabilities. For instance, over 70% of platinum group metals originate from just two countries, creating potential price volatility and supply disruptions. Materials utilizing earth-abundant elements offer greater supply security but must overcome efficiency challenges through innovative design approaches.
Energy requirements for catalyst operation represent another critical economic factor. Materials operating at ambient temperature and pressure conditions offer substantial energy savings compared to those requiring elevated temperatures (>300°C) or pressures (>10 bar). Calculations indicate that reducing operating temperature by 100°C can decrease energy consumption by approximately 15-20%, significantly improving process economics in large-scale operations.
Economic viability assessment reveals that material costs constitute approximately 40-60% of total catalyst production expenses. Noble metal-based catalysts (ruthenium, platinum) demonstrate superior performance but at prohibitively high costs ($30,000-50,000 per kilogram), limiting their industrial application. Transition metal-based alternatives (iron, nickel, cobalt) offer more economical options ($100-500 per kilogram) but typically with reduced efficiency, requiring optimization of the performance-to-cost ratio.
Manufacturing complexity presents another economic consideration, as intricate nanostructured catalysts with precise morphology control often require sophisticated equipment and specialized expertise. Materials requiring high-temperature processing (>800°C) or ultra-high vacuum conditions incur substantially higher production costs compared to solution-based synthesis methods operable under ambient conditions.
Catalyst lifetime and stability directly influence long-term economic viability. Materials susceptible to poisoning, leaching, or structural degradation necessitate frequent replacement, significantly increasing operational costs. Analysis indicates that extending catalyst lifetime by 50% can reduce the levelized cost of nitrogen reduction by approximately 25-30%, highlighting the economic importance of selecting materials with inherent stability.
Resource availability analysis shows that catalysts dependent on geographically concentrated elements face supply chain vulnerabilities. For instance, over 70% of platinum group metals originate from just two countries, creating potential price volatility and supply disruptions. Materials utilizing earth-abundant elements offer greater supply security but must overcome efficiency challenges through innovative design approaches.
Energy requirements for catalyst operation represent another critical economic factor. Materials operating at ambient temperature and pressure conditions offer substantial energy savings compared to those requiring elevated temperatures (>300°C) or pressures (>10 bar). Calculations indicate that reducing operating temperature by 100°C can decrease energy consumption by approximately 15-20%, significantly improving process economics in large-scale operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!